Improvement of Electrical Properties of Silver Nanowires Transparent Conductive by Metal Oxide Nanoparticles Modification
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
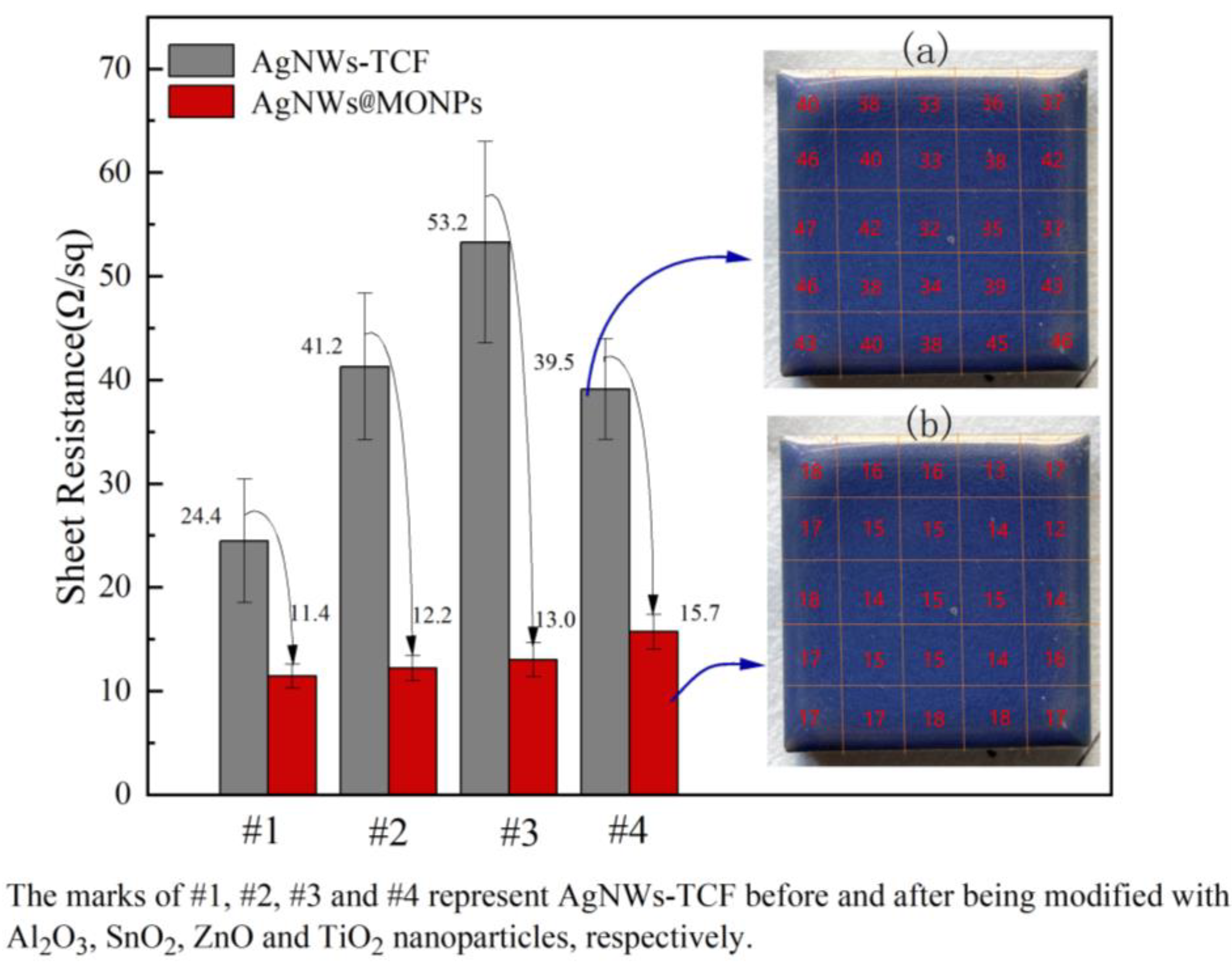
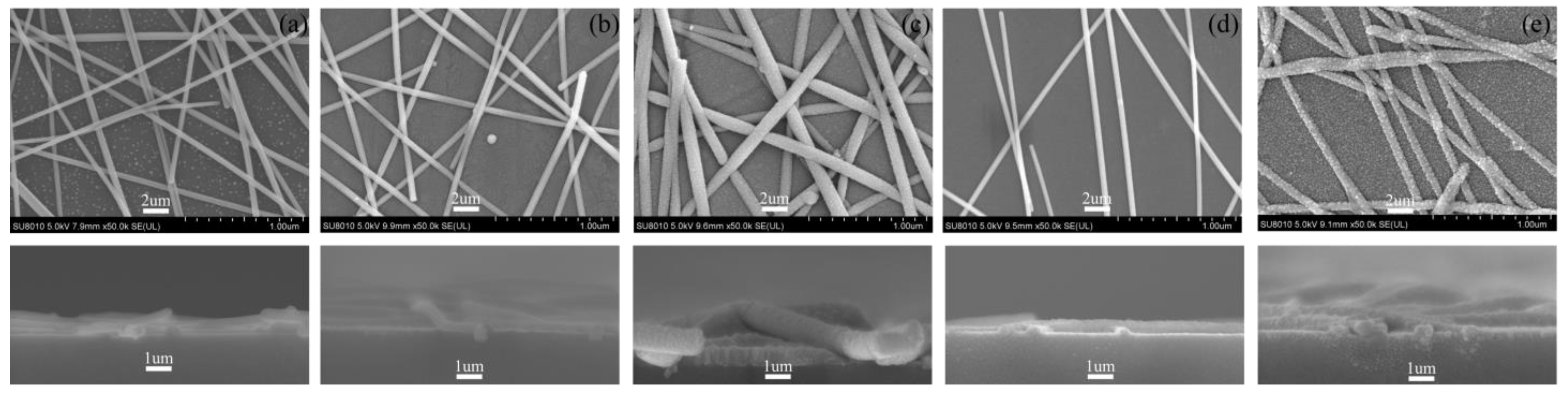
3.1. Electrical Properties of AgNWs Transparent Conductive Films
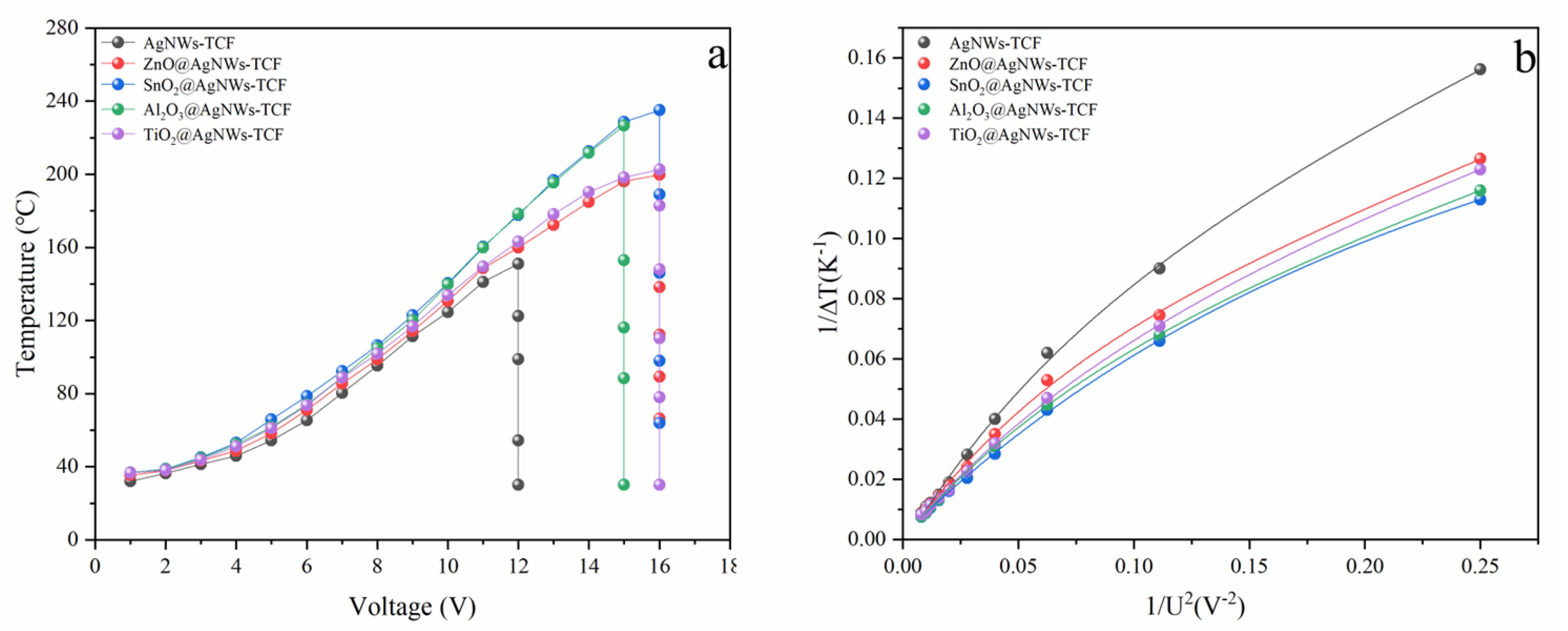
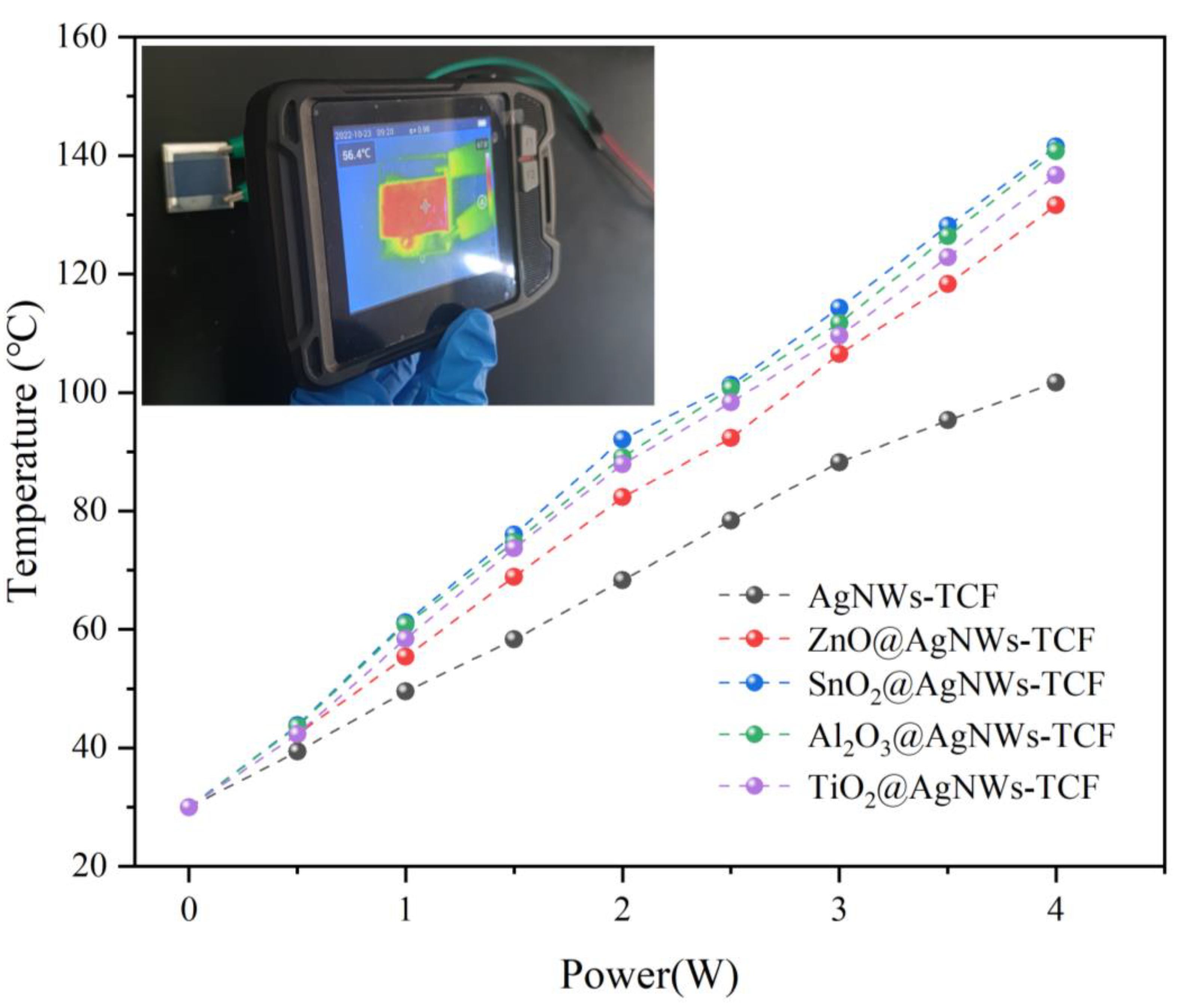
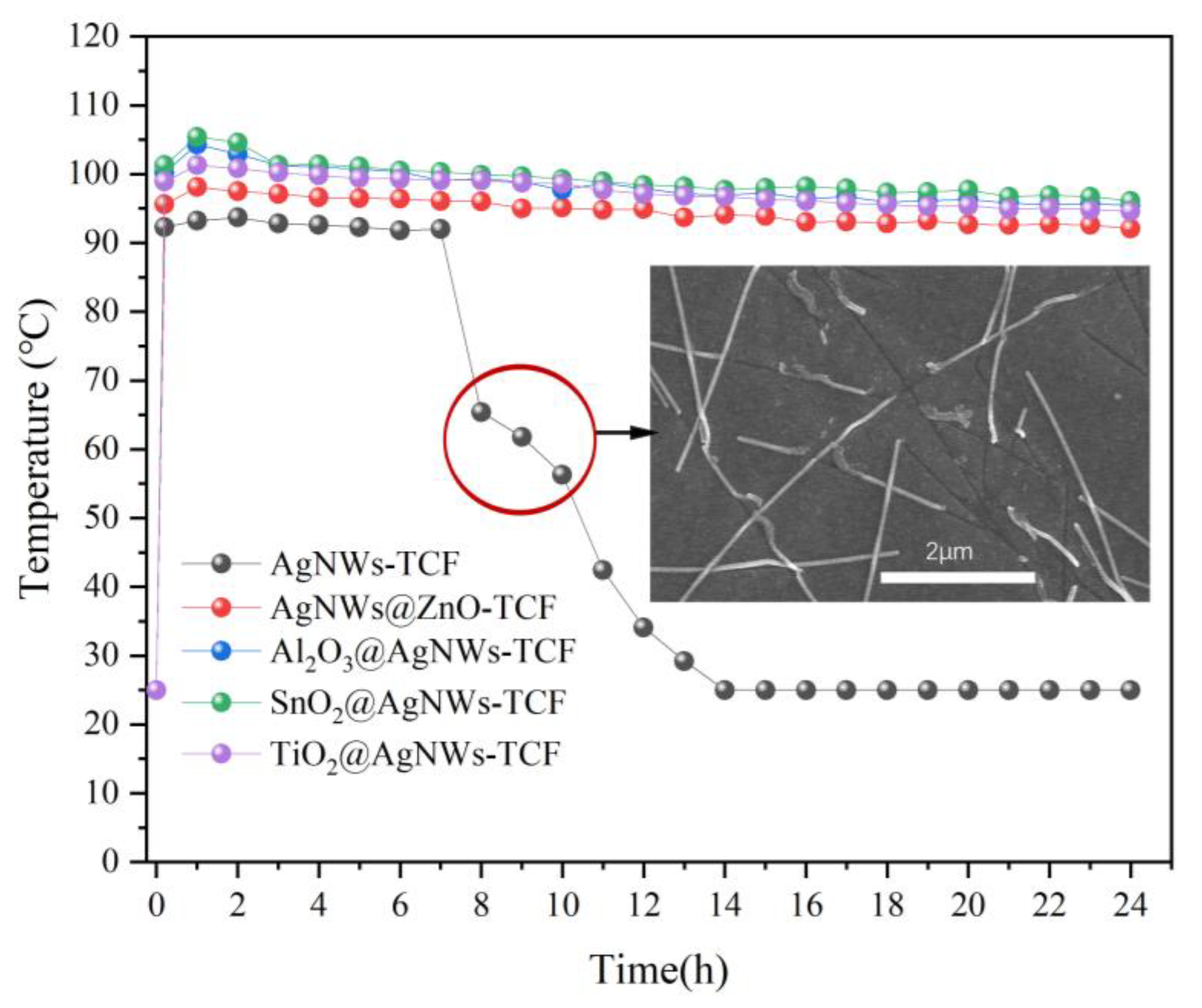
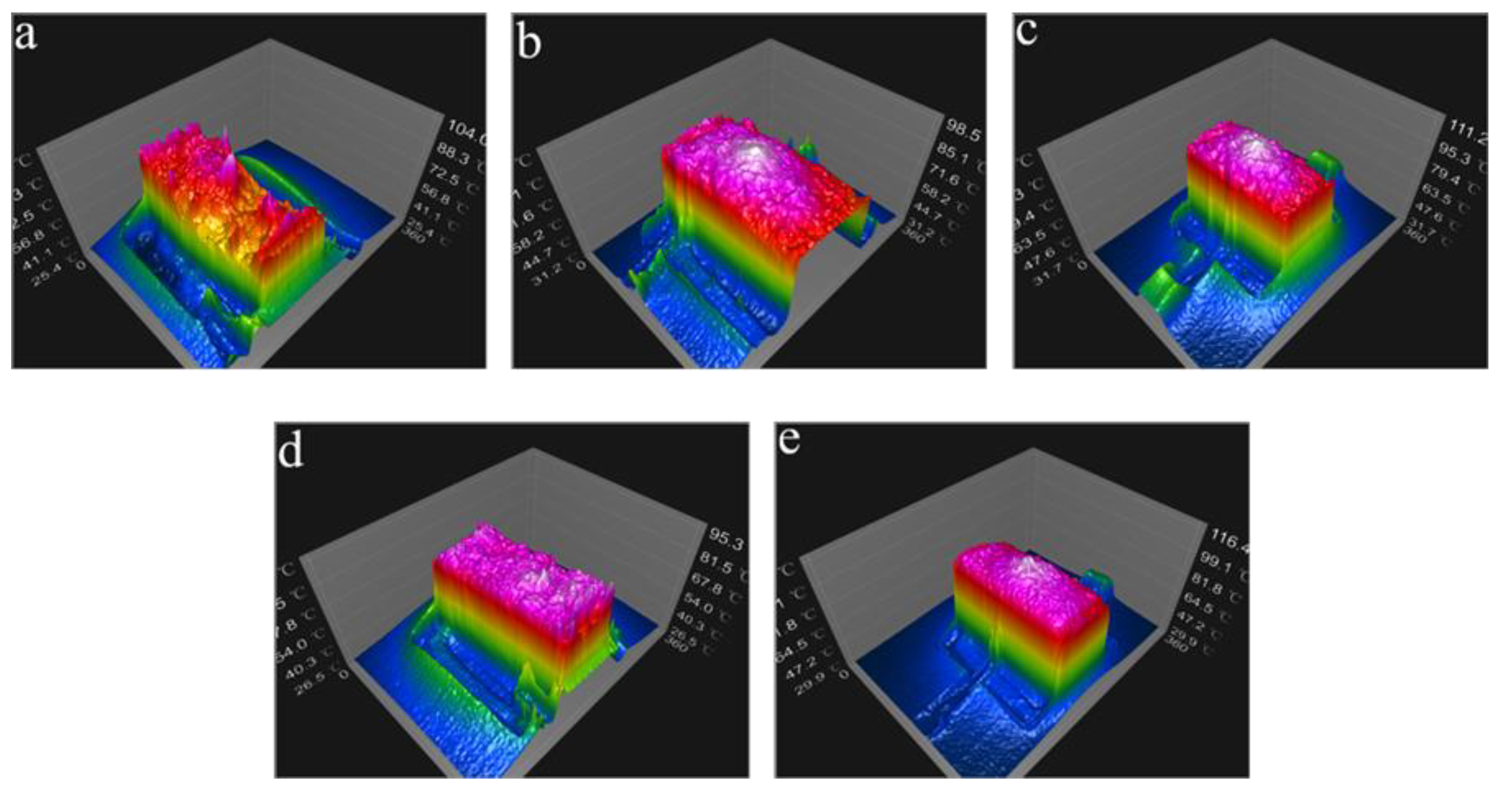
3.2. Electrothermal Properties of AgNWs Transparent Conductive Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, J.; Lee, J.; Jeong, S.; Yang, S.; Ko, J.-H.; Im, H.-G.; Baek, S.-W.; Lee, J.-Y.; Bae, B.-S. High-performance hybrid plastic films: A robust electrode platform for thin-film optoelectronics. Energy Environ. Sci. 2013, 6, 1811–1817. [Google Scholar] [CrossRef]
- Chen, R.; Das, S.R.; Jeong, C.; Khan, M.R.; Janes, D.B.; Alam, M.A. Co-percolating graphene-wrapped silver nanowire network for high performance, highly stable, transparent conducting electrodes. Adv. Funct. Mater. 2013, 23, 5150–5158. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, S.-W.; Han, J.I. Solution-processed indium–tin-oxide nanoparticle transparent conductors on flexible glass substrate with high optical transmittance and high thermal stability. Jpn. J. Appl. Phys. 2014, 53, 08NF04. [Google Scholar] [CrossRef]
- Azani, M.R.; Hassanpour, A.; Torres, T. Benefits, problems, and solutions of silver nanowire transparent conductive electrodes in indium tin oxide (ITO)-free flexible solar cells. Adv. Energy Mater. 2020, 10, 2002536. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, A.; Bai, S.; Ma, Y.; Bridges, D. Anisotropic optical properties of large-scale aligned silver nanowire films via controlled coffee ring effects. RSC Adv. 2015, 5, 39103–39109. [Google Scholar] [CrossRef]
- Zhou, W.; Bai, S.; Hu, A. Self-assembly and laser sintering of silver nanowire films prepared by the modified coffee-ring effect for surface enhanced Raman scattering. In International Congress on Applications of Lasers & Electro-Optics; Laser Institute of America: Orlando, FL, USA, 2014; pp. 1026–1034. [Google Scholar]
- Woo, J.S.; Han, J.T.; Jung, S.; Jang, J.I.; Kim, H.Y.; Jeong, H.J.; Jeong, S.Y.; Baeg, K.-J.; Lee, G.-W. Electrically robust metal nanowire network formation by in-situ interconnection with single-walled carbon nanotubes. Sci. Rep. 2014, 4, 4804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Zhang, J.; Dong, L.; Pan, Y.; Yang, C.; Li, M.; Ruan, Y.; Ma, J.; Lu, H. Coating-free, air-stable silver nanowires for high-performance transparent conductive film. Nanotechnology 2018, 29, 375601. [Google Scholar] [CrossRef]
- Wang, Y.; Du, D.; Yang, X.; Zhang, X.; Zhao, Y. Optoelectronic and electrothermal properties of transparent conductive silver nanowires films. Nanomaterials 2019, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, J.; Fang, Y.; Luo, B.; Zhang, Y.; Li, Y.; Zhou, J.; Hu, B. Unraveling the solvent induced welding of silver nanowires for high performance flexible transparent electrodes. Nanoscale 2018, 10, 12981–12990. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, Y.W.; Lee, H.S.; Kim, H.; Yang, W.S.; Suh, K.S. Uniformly interconnected silver-nanowire networks for transparent film heaters. Adv. Funct. Mater. 2013, 23, 1250–1255. [Google Scholar] [CrossRef]
- Shi, X.; Xu, W.; Shen, W.; Wang, G.; Wang, R.; Li, X.; Song, W. Improving the stability of silver nanowire/polyimide composite films for transparent film heaters. J. Mater. Sci. Mater. Electron. 2019, 30, 2089–2095. [Google Scholar] [CrossRef]
- Tan, D.; Jiang, C.; Li, Q.; Bi, S.; Song, J. Silver nanowire networks with preparations and applications: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 15669–15696. [Google Scholar] [CrossRef]
- Kwon, J.; Suh, Y.D.; Lee, J.; Lee, P.; Han, S.; Hong, S.; Yeo, J.; Lee, H.; Ko, S.H. Recent progress in silver nanowire based flexible/wearable optoelectronics. J. Mater. Chem. C 2018, 6, 7445–7461. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, M.; Meng, L.; Wan, X.; Gao, H.; Zhang, Y.; Zhao, K.; Sun, Z.; Li, C.; Liu, S. Flexible organic photovoltaics based on water-processed silver nanowire electrodes. Nat. Electron. 2019, 2, 513–520. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Shi, S.; Xu, J.; Qin, X.; He, Q.; Yang, K.; Dai, W.; Liu, G.; Zhou, Q. Recent progress in silver nanowire networks for flexible organic electronics. J. Mater. Chem. C 2020, 8, 4636–4674. [Google Scholar] [CrossRef]
- Sohn, H.; Park, C.; Oh, J.-M.; Kang, S.W.; Kim, M.-J. Silver nanowire networks: Mechano-electric properties and applications. Materials 2019, 12, 2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, P.; Zhu, R.; Zhu, Y.; Chen, F.; Wan, T.; Xu, Z.; Joshi, R.; Han, Z.; Hu, L.; Wu, T. Performance degradation and mitigation strategies of silver nanowire networks: A review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 435–459. [Google Scholar] [CrossRef]
- Zhang, R.; Engholm, M. Recent progress on the fabrication and properties of silver nanowire-based transparent electrodes. Nanomaterials 2018, 8, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Sun, Y.; Wang, K.; Chen, Y.; Liang, Z.; Xu, Y.; Xiao, F. Long-term stable silver nanowire transparent composite as bottom electrode for perovskite solar cells. Nano Res. 2018, 11, 1998–2011. [Google Scholar] [CrossRef]
- Aghazadehchors, S.; Nguyen, V.H.; Munoz-Rojas, D.; Jiménez, C.; Rapenne, L.; Nguyen, N.D.; Bellet, D. Versatility of bilayer metal oxide coatings on silver nanowire networks for enhanced stability with minimal transparency loss. Nanoscale 2019, 11, 19969–19979. [Google Scholar] [CrossRef] [PubMed]
- Aghazadehchors, S. Metallic Nanowire Networks: Silver Nanowire Network Stability Enhancement Using Metal Oxide Coatings, Percolation Onset of Nano-Object Network; Université Grenoble Alpes [2020-....]; Université de Liège: Liège, Belgium, 2021. [Google Scholar]
- Cheuk, K.W.; Pei, K.; Chan, P.K. Degradation mechanism of a junction-free transparent silver network electrode. RSC Adv. 2016, 6, 73769–73775. [Google Scholar] [CrossRef]
- Han, B.; Huang, Y.; Li, R.; Peng, Q.; Luo, J.; Pei, K.; Herczynski, A.; Kempa, K.; Ren, Z.; Gao, J. Bio-inspired networks for optoelectronic applications. Nat. Commun. 2014, 5, 5674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, L.; Li, J.; Yu, Y.; Bi, X. Gold nanoparticles decorated silver-nanowire films for transparent electrode with excellent thermal stability. Mater. Lett. 2018, 217, 52–55. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.-S.; Sharma, A. Transparent AgNW-CoNPs conducting film for heat sensor. Microelectron. Eng. 2019, 205, 37–43. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Y.; Gao, H.; Gao, Y.; Zhu, W.; Zhan, L.; Liu, H.; Chen, Y.; Hu, K.; Wang, P. Moisture-assisted formation of high-quality silver nanowire transparent conductive films with low junction resistance. Coatings 2021, 11, 671. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, C.; Jia, D.; Li, S.; Ji, S.; Ye, C. Silver nanowire transparent conductive films with high uniformity fabricated via a dynamic heating method. ACS Appl. Mater. Interfaces 2016, 8, 9865–9871. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, W.; Xu, F.; Shen, W.; Song, W. AgNW/Chinese Xuan paper film heaters for electro-thermochromic paper display. Mater. Res. Express 2017, 4, 116405. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Kim, M.K.; Hudaya, C.; Park, J.H.; Byun, D.; Lim, J.C.; Lee, J.K. Oxidation-resistant hybrid metal oxides/metal nanodots/silver nanowires for high performance flexible transparent heaters. Nanoscale 2016, 8, 3307–3313. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Hu, Y.; Chen, Y.; Hu, Z.; Zhou, K.; Min, Z.; Liu, H.; Zhan, L.; Dai, Y. Improvement of Electrical Properties of Silver Nanowires Transparent Conductive by Metal Oxide Nanoparticles Modification. Coatings 2022, 12, 1816. https://doi.org/10.3390/coatings12121816
Liu W, Hu Y, Chen Y, Hu Z, Zhou K, Min Z, Liu H, Zhan L, Dai Y. Improvement of Electrical Properties of Silver Nanowires Transparent Conductive by Metal Oxide Nanoparticles Modification. Coatings. 2022; 12(12):1816. https://doi.org/10.3390/coatings12121816
Chicago/Turabian StyleLiu, Wei, Yuehui Hu, Yichuan Chen, Zhiqiang Hu, Ke Zhou, Zhijian Min, Huiwen Liu, Lilin Zhan, and Yinjie Dai. 2022. "Improvement of Electrical Properties of Silver Nanowires Transparent Conductive by Metal Oxide Nanoparticles Modification" Coatings 12, no. 12: 1816. https://doi.org/10.3390/coatings12121816




