Effect of Expandable Graphite Flakes on the Flame Resistance of Oak Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Treatment
2.2. Sample Analyses
The Radiant Heat Source Test
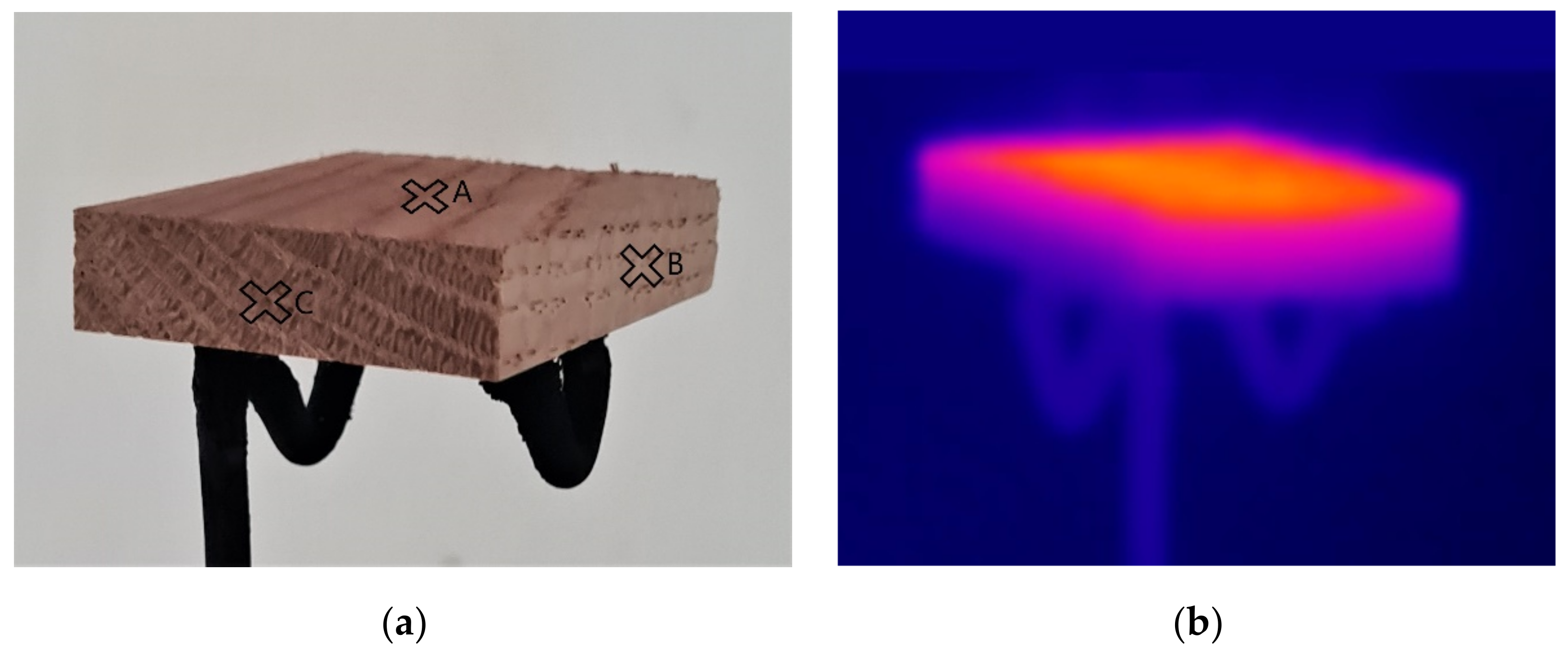
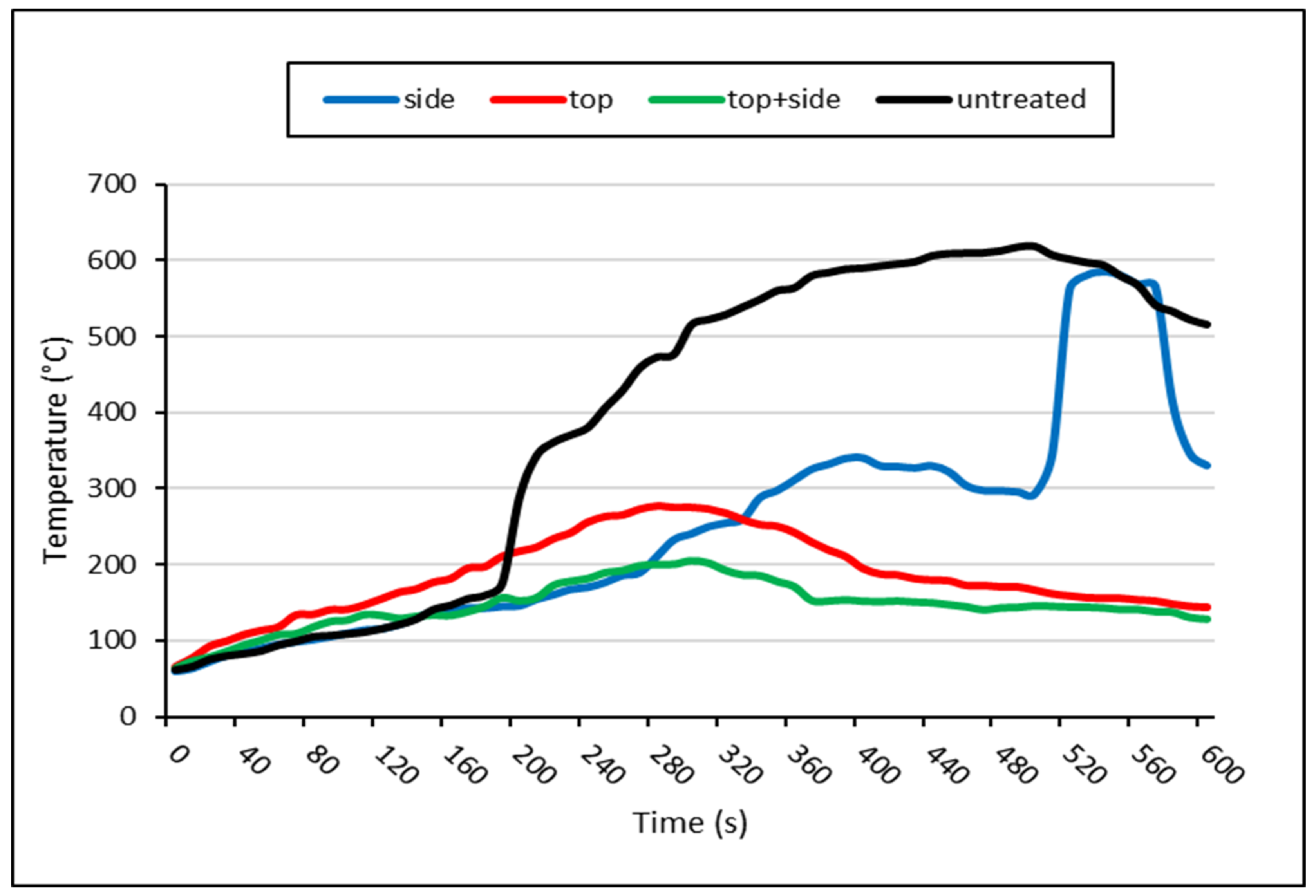
2.3. Surface Temperature Measurement
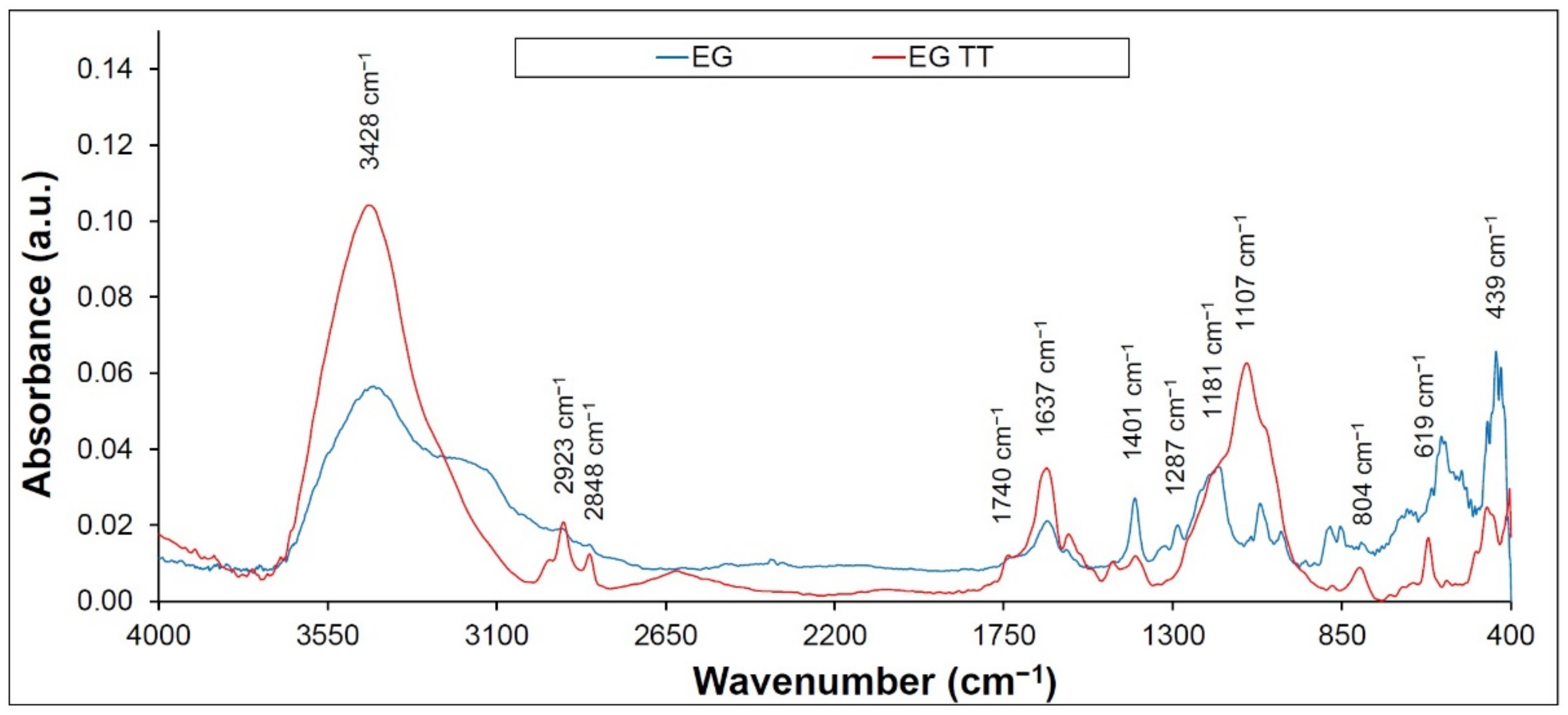
2.4. FTIR Spectroscopy
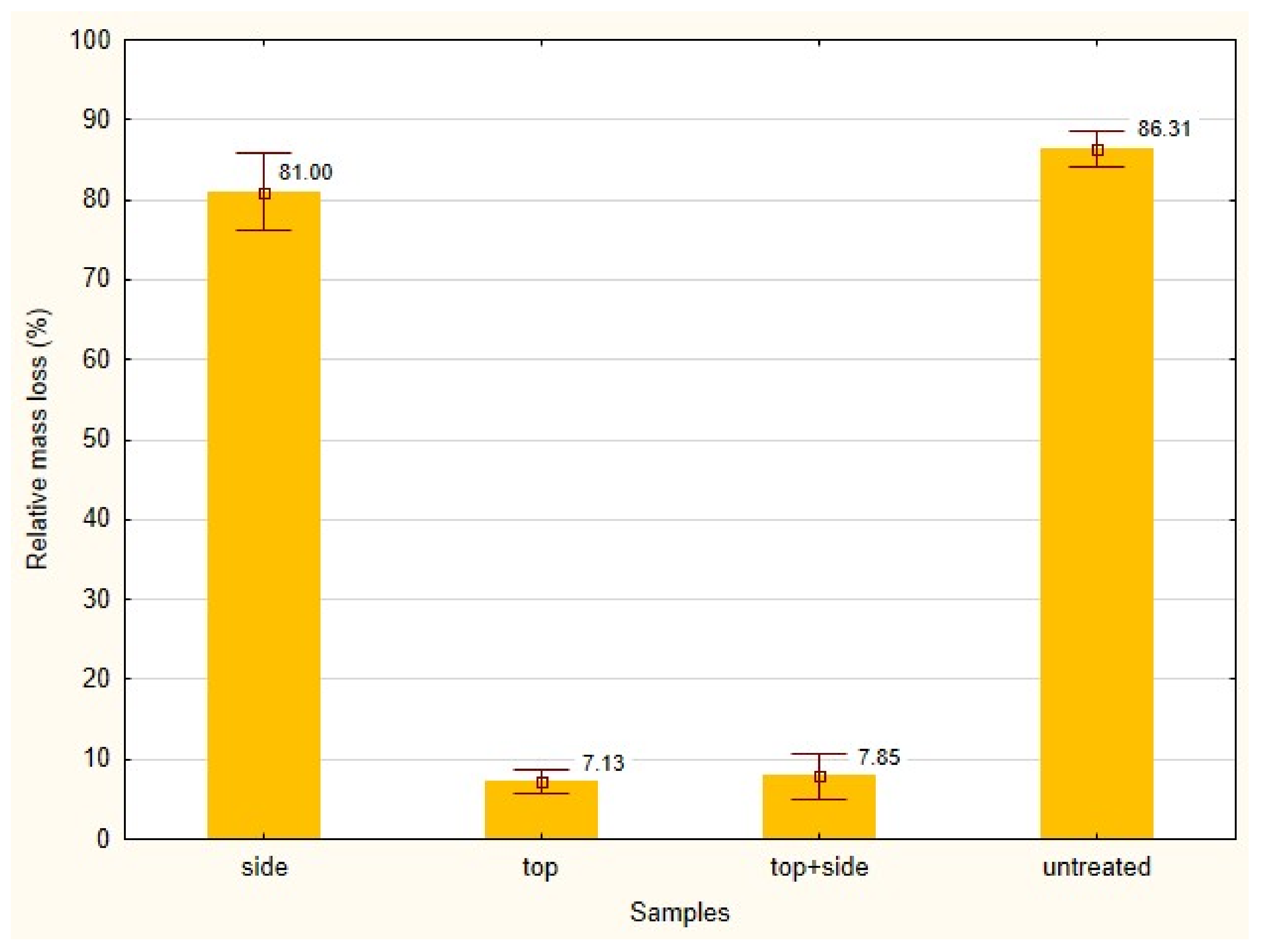
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Recent developments in different types of flame retardants and effect on fire retardancy of epoxy composite. Polym. Plast. Technol. Eng. 2016, 55, 1512–1535. [Google Scholar] [CrossRef]
- Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Gebke, S.; Thümmler, K.; Sonnier, R.; Tech, S.; Wagenführ, A.; Fischer, S. Flame Retardancy of Wood Fiber Materials Using Phosphorus-Modified Wheat Starch. Molecules 2020, 25, 335. [Google Scholar] [CrossRef] [PubMed]
- Kačíková, D.; Kubovský, I.; Eštoková, A.; Kačík, F.; Kmeťová, E.; Kováč, J.; Ďurkovič, J. The Influence of Nanoparticles on Fire Retardancy of Pedunculate Oak Wood. Nanomaterials 2021, 11, 3405. [Google Scholar] [CrossRef] [PubMed]
- Taib, M.N.A.M.; Antov, P.; Savov, V.; Fatriasari, W.; Madyaratri, E.W.; Wirawan, R.; Makovická Osvaldová, L.; Hua, L.S.; Ghani, M.A.A.; Edrus, S.S.A.O.A.; et al. Current progress of biopolymer-based flame retardant. Polym. Degrad. Stab. 2022, 205, 110153. [Google Scholar] [CrossRef]
- Mazela, B.; Batista, A.; Grześkowiak, W. Expandable Graphite as a Fire Retardant for Cellulosic Materials—A Review. Forests 2020, 11, 755. [Google Scholar] [CrossRef]
- Wladyka-Przybylak, M. Combustion characteristics of wood protected by intumescent coatings and the influence of different additives on fire retardant effectiveness of the coatings. Mol. Cryst. Liq. Cryst. A 2000, 354, 449–456. [Google Scholar] [CrossRef]
- Mariappan, T. Chapter 6. Fire retardant coatings. In New Technologies in Protective Coatings; Giudice, C., Canosa, G., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Elvira-León, J.C.; Chimenos, J.M.; Isábal, C.; Monton, J.; Formosa, J.; Haurie, L. Epsomite as flame retardant treatment for wood: Preliminary study. Constr. Build. Mater. 2016, 126, 936–942. [Google Scholar] [CrossRef]
- Vahidi, G.; Bajwa, D.S.; Shojaeiarani, J.; Stark, N.; Darabi, A. Advancements in traditional and nanosized flame retardants for polymers—A review. J. Appl. Polym. Sci. 2021, 138, 50050. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, W.; Feng, Y.; Tang, X.; Yan, L. Flame-retardant, heat-insulating and char formation properties of densified Pinus sylvestris treated with different compression pressures. Eur. J. Wood Wood Prod. 2022, 80, 1321–1331. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Han, E.H.; Ke, W. Influence of expandable graphite on fire resistance and water resistance of flame retardant coatings. Corros. Sci. 2007, 49, 2237–2253. [Google Scholar] [CrossRef]
- Tomiak, F.; Schoeffel, A.; Rathberger, K.; Drummer, D. A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-) Polyphospinate and Melamine Polyphosphate for Polyamide 6. Polymers 2021, 13, 2712. [Google Scholar] [CrossRef]
- Tomiak, F.; Schoeffel, A.; Rathberger, K.; Drummer, D. Expandable Graphite, Aluminum Diethylphospinate and Melamine Polyphosphate as Flame Retarding System in Glass Fiber-Reinforced PA6. Polymers 2022, 14, 1263. [Google Scholar] [CrossRef]
- Bai, G.; Guo, C.; Li, L. Synergistic effect of intumescent flame retardant and expandable graphite on mechanical and flame-retardant properties of wood flour-polypropylene composites. Constr. Build. Mater. 2014, 50, 148–153. [Google Scholar] [CrossRef]
- Börcsök, Z.; Pásztory, Z. The role of lignin in wood working processes using elevated temperatures: An abbreviated literature survey. Eur. J. Wood Wood Prod. 2021, 79, 511–526. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Franke, T.; Volkmer, T. Mineralization treatment of European oak heartwood with calcium oxalate for im-proved fire retardancy. Holzforschung 2022, 76, 77–88. [Google Scholar] [CrossRef]
- Santos, L.P.; da Silva, D.S.; Morari, T.H.; Galembeck, F. Environmentally Friendly, High-Performance Fire Retardant Made from Cellulose and Graphite. Polymers 2021, 13, 2400. [Google Scholar] [CrossRef]
- Chun, K.; Kim, J.; Rie, D. Thermal Characteristics of Expandable Graphite–Wood Particle Composites. Materials 2020, 13, 2732. [Google Scholar] [CrossRef]
- Anielkis, B.; Wojciech, G.; Bartłomiej, M. Expandable Graphite Flakes as an Additive for a New Fire Retardant Coating for Wood and Cellulose Materials–Comparison Analysis. In Wood & Fire Safety; Makovicka Osvaldova, L., Markert, F., Zelinka, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Kmeťová, E.; Mitterová, I.; Kačíková, E. Evaluation of selected coniferous and deciduous trees species after radiant heat loading by the method of mass loss. Delta 2020, 14, 16–29. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jiang, L.; Renner, J.S.; Xu, Q. Characterisation of the fire behaviour of wood: From pyrolysis to fire retardant mechanisms. J. Therm. Anal. Calorim. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Su, W.; Li, J. Preparation and Catalytic Performance of Expanded Graphite for Oxidation of Organic Pollutant. Catalysts 2019, 9, 280. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon Nanostructures Derived through Hypergolic Reaction of Conductive Polymers with Fuming Nitric Acid at Ambient Conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.C.; Giannakopoulou, T.; Romanos, G.E.; Boukos, N.; Giannouri, M.; Lei, C.; Lekakou, C.; Trapalis, C. Non-activated high surface area expanded graphite oxide for supercapacitors. Appl. Surf. Sci. 2015, 358, 110–121. [Google Scholar] [CrossRef]
- Polovka, M.; Polovková, J.; Vizárová, K.; Kirschnerová, S.; Bieliková, L.; Vrška, M. The application of FTIR spectroscopy on characterization of paper samples, modified by Bookkeeper process. Vib. Spectrosc. 2006, 41, 112–117. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds. C 2020, 6, 61. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Xiao, T.; Zhang, Y.; Zheng, D. Promoting effect of ammonia modification on activated carbon catalyzed peroxy-monosulfate oxidation. Sep. Purif. Technol. 2016, 160, 81–88. [Google Scholar] [CrossRef]
- Hashmi, A.; Singh, A.K.; Jain, B.; Carabineiro, S.A.C. Chloramine-T/N-Bromosuccinimide/FeCl3/KIO3 Decorated Graphene Oxide Nanosheets and Their Antibacterial Activity. Nanomaterials 2020, 10, 105. [Google Scholar] [CrossRef]
- Xie, R.; Qu, B. Thermo-oxidative degradation behaviors of expandable graphite-based intumescent halogen-free flame retardant LLDPE blends. Polym. Degrad. Stabil. 2001, 71, 395–402. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Park, J.K.; Kim, H.K. Influence of Solvent Exchange on the Physical Properties of Sodium Silicate Based Aerogel Prepared at Ambient Pressure. Aerosol Air Qual. Res. 2006, 6, 93–105. [Google Scholar] [CrossRef]
- Chao, C.; Gao, M.; Chen, S. Expanded graphite. J. Therm. Anal. Calorim. 2018, 131, 71–79. [Google Scholar] [CrossRef]
- Wang, S.; Qian, L.; Xin, F. The synergistic flame—Retardant behaviors of pentaerythritol phosphate and expandable graphite in rigid polyurethane foams. Polym. Compos. 2018, 39, 329–336. [Google Scholar] [CrossRef]
- Strąkowska, A.; Członka, S.; Konca, P.; Strzelec, K. New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams. Appl. Sci. 2020, 10, 5817. [Google Scholar] [CrossRef]



| Samples | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean ± SD | |
| side | 281 | 188 | 291 | 168 | 136 | 213 ± 69 |
| top | - | - | - | - | - | - |
| top + side | - | - | - | - | - | - |
| untreated | 169 | 188 | 157 | 139 | 136 | 158 ± 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmeťová, E.; Kačík, F.; Kubovský, I.; Kačíková, D. Effect of Expandable Graphite Flakes on the Flame Resistance of Oak Wood. Coatings 2022, 12, 1908. https://doi.org/10.3390/coatings12121908
Kmeťová E, Kačík F, Kubovský I, Kačíková D. Effect of Expandable Graphite Flakes on the Flame Resistance of Oak Wood. Coatings. 2022; 12(12):1908. https://doi.org/10.3390/coatings12121908
Chicago/Turabian StyleKmeťová, Elena, František Kačík, Ivan Kubovský, and Danica Kačíková. 2022. "Effect of Expandable Graphite Flakes on the Flame Resistance of Oak Wood" Coatings 12, no. 12: 1908. https://doi.org/10.3390/coatings12121908
APA StyleKmeťová, E., Kačík, F., Kubovský, I., & Kačíková, D. (2022). Effect of Expandable Graphite Flakes on the Flame Resistance of Oak Wood. Coatings, 12(12), 1908. https://doi.org/10.3390/coatings12121908







