Chemical Stability of Sputter Deposited Silver Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results
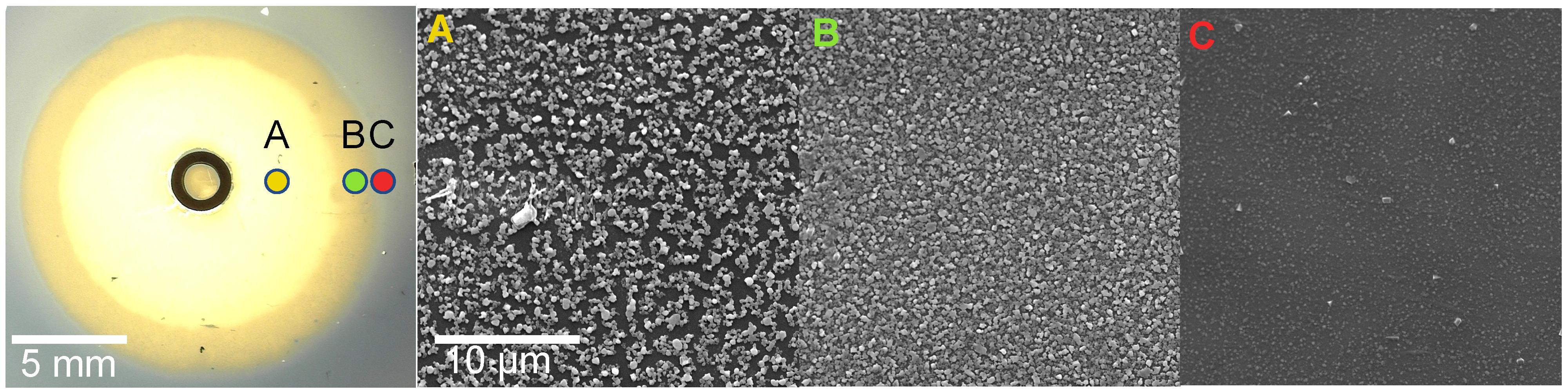
3.1. Image Analysis
3.2. Influence of HCl Concentration
3.3. Influence of Film Thickness
3.4. Influence of the Seed Layer
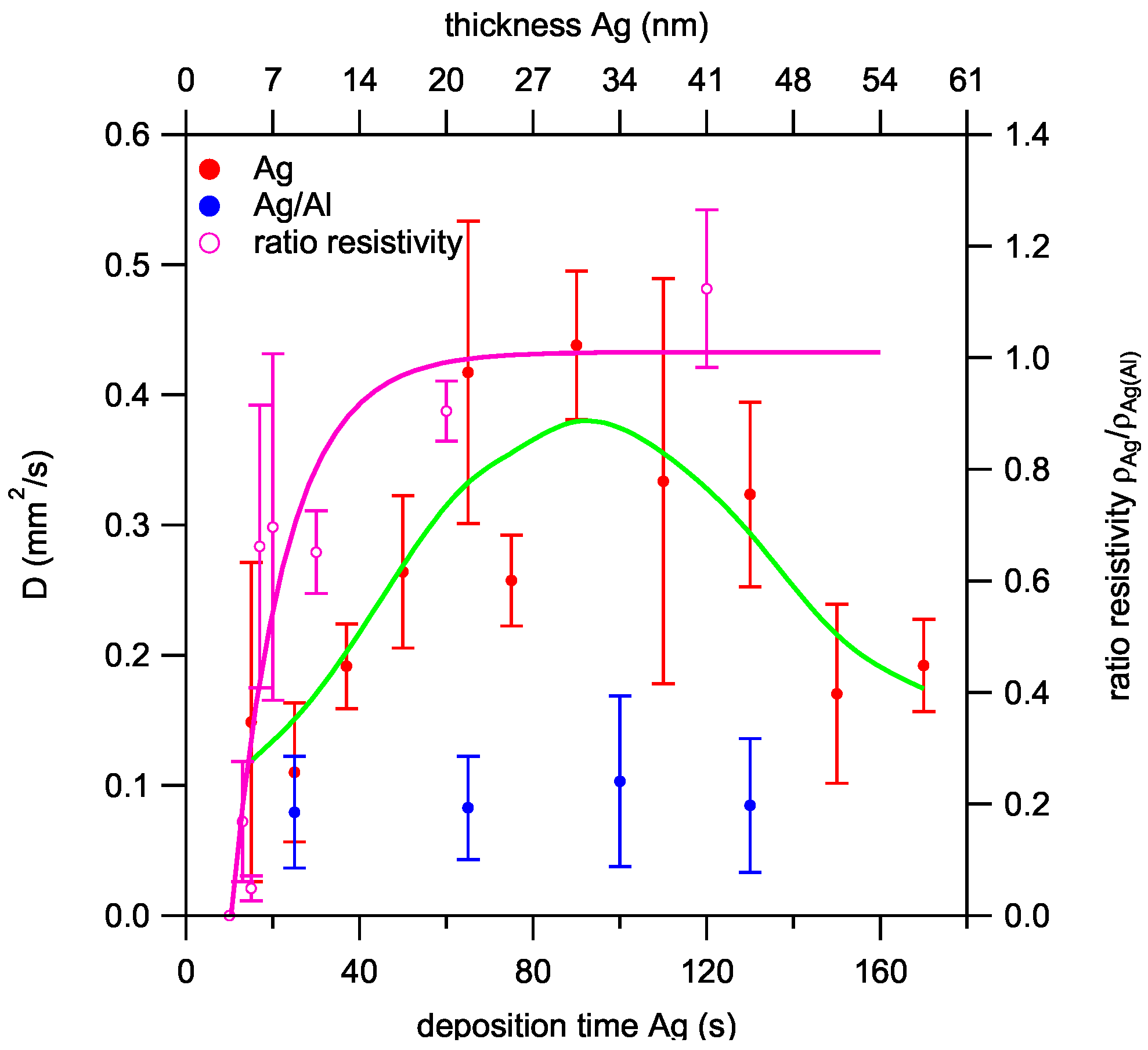
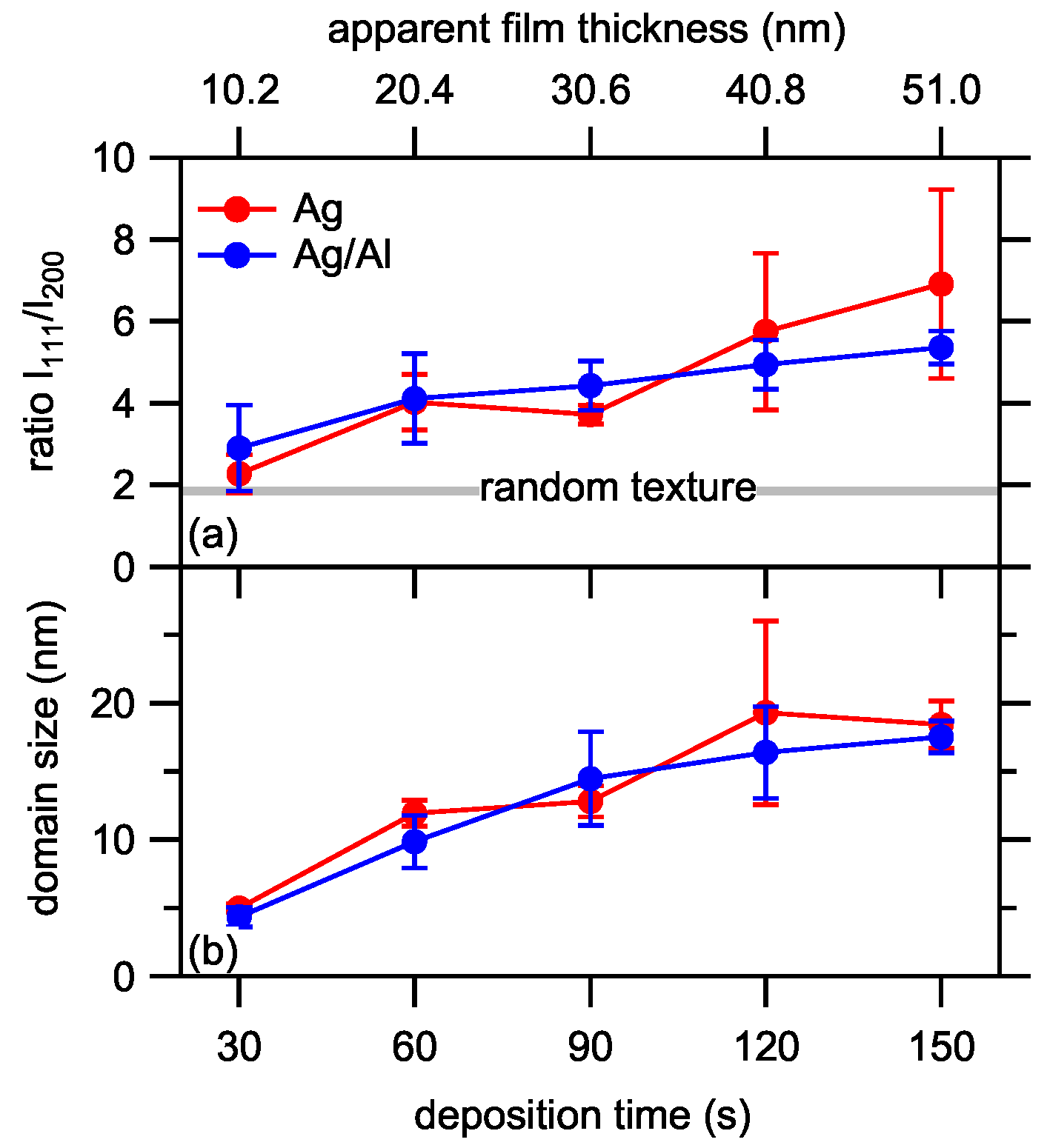
3.5. Film Characterization
3.5.1. As-Deposited Films
3.5.2. Exposed Films
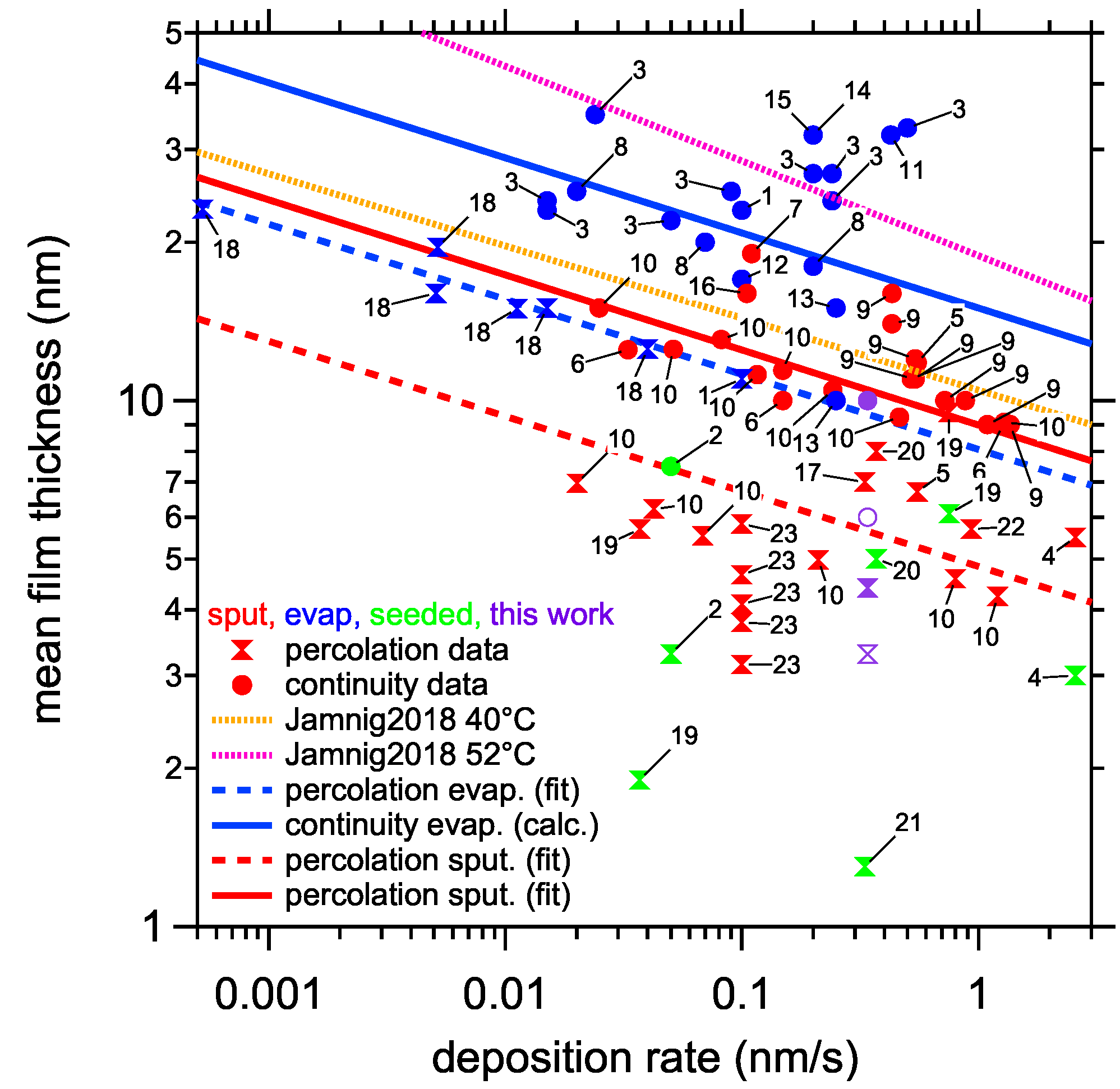
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vitelaru, C.; Parau, A.C.; Kiss, A.E.; Pana, I.; Dinu, M.; Constantin, L.R.; Vladescu, A.; Tonofrei, L.E.; Adochite, C.S.; Costinas, S.; et al. Silver-containing thin films on transparent polymer foils for antimicrobial applications. Coatings 2022, 12, 170. [Google Scholar] [CrossRef]
- Abbas, N.; Shad, M.R.; Hussain, M.; Mehdi, S.M.Z.; Sajjad, U. Fabrication and characterization of silver thin films using physical vapor deposition, and the investigation of annealing effects on their structures. Mater. Res. Express 2019, 6, 116437. [Google Scholar] [CrossRef]
- Martín-Palma, R.J.; Vázquez, L.; Martínez-Duart, J.M.; Malats-Riera, A. Silver-based low-emissivity coatings for architectural windows: Optical and structural properties. Sol. Energy Mater. Sol. Cells 1998, 53, 55–66. [Google Scholar] [CrossRef]
- Aguilar-Santana, J.L.; Jarimi, H.; Velasco-Carrasco, M.; Riffat, S. Review on window-glazing technologies and future prospects. Int. J. Low-Carbon Technol. 2019, 15, 112–120. [Google Scholar] [CrossRef]
- Park, H.K.; Kang, J.W.; Na, S.I.; Kim, D.Y.; Kim, H.K. Characteristics of indium-free GZO/Ag/GZO and AZO/Ag/AZO multilayer electrode grown by dual target DC sputtering at room temperature for low-cost organic photovoltaics. Sol. Energy Mater. Sol. Cells 2009, 93, 1994–2002. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, C.; Park, Y.B.; Guo, L.J. Thin-metal-film-based transparent conductors: Material preparation, optical design, and device applications. Adv. Opt. Mater. 2020, 9, 2001298. [Google Scholar] [CrossRef]
- Lee, E.S. Spectrally Selective Glazings; Technical Report; New Technology Energy Management Program, Federal Energy Management Program: Washington, DC, USA, 1998. [Google Scholar]
- Ding, G.; Clavero, C. Silver based low emissivity coating technology for energy saving window applications. In Modern Technologies for Creating the Thin-Film Systems and Coatings; InTech: Vienna, Austria, 2017. [Google Scholar] [CrossRef]
- American Institute of Architects Continuing Education Systems. In The Value-Added Performance of Coated Glass; Technical Report; AIACES: New York, NY, USA, 2020.
- Bender, M.; Seelig, W.; Daube, C.; Frankenberger, H.; Ocker, B.; Stollenwerk, J. Dependence of film composition and thicknesses on optical and electrical properties of ITO–metal–ITO multilayers. Thin Solid Film. 1998, 326, 67–71. [Google Scholar] [CrossRef]
- Gu, D.; Zhang, C.; Wu, Y.K.; Guo, L.J. Ultrasmooth and thermally stable silver-based thin films with subnanometer roughness by aluminum doping. ACS Nano 2014, 8, 10343–10351. [Google Scholar] [CrossRef]
- Zhao, G.; Jeong, E.; Choi, E.A.; Yu, S.M.; Bae, J.S.; Lee, S.G.; Han, S.Z.; Lee, G.H.; Yun, J. Strategy for improving Ag wetting on oxides: Coalescence dynamics versus nucleation density. Appl. Surf. Sci. 2020, 510, 145515. [Google Scholar] [CrossRef]
- Jamnig, A.; Pliatsikas, N.; Abadias, G.; Sarakinos, K. Manipulation of thin metal film morphology on weakly interacting substrates via selective deployment of alloying species. J. Vac. Sci. Technol. A 2022, 40, 033407. [Google Scholar] [CrossRef]
- Shamsuddin, W.N.S.W.; Roccisano, A.; Zuber, K.; Murphy, P.J.; Jane, M.L. Environmentally robust Ag–Cu based low-E coatings. Sol. Energy Mater. Sol. Cells 2022, 248, 112033. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Kato, K.; Omoto, H.; Yonekura, M. Correlation between microstructure and salt-water durability of Ag thin films deposited by magnetron sputtering. Thin Solid Film. 2013, 532, 141–146. [Google Scholar] [CrossRef]
- Jamnig, A.; Pliatsikas, N.; Konpan, M.; Lu, J.; Kehagias, T.; Kotanidis, A.N.; Kalfagiannis, N.; Bellas, D.V.; Lidorikis, E.; Kovac, J.; et al. 3D-to-2D morphology manipulation of sputter-deposited nanoscale silver films on weakly interacting substrates via selective nitrogen deployment for multifunctional metal contacts. ACS Appl. Nano Mater. 2020, 3, 4728–4738. [Google Scholar] [CrossRef]
- Hill, R.J.; Nadel, S.N. Coated Glass: Applications and Markets; BOC Coating Technology: Fairfield, CA, USA, 1999. [Google Scholar]
- Park, H.S.; Day, D.E. Corrosion of evaporated Ag films on glass by saturated water vapor. Sol. Energy Mater. 1986, 13, 351–365. [Google Scholar] [CrossRef]
- Ross, R.C. Observations on humidity-induced degradation of Ag-based low-emissivity films. Sol. Energy Mater. 1990, 21, 25–42. [Google Scholar] [CrossRef]
- Ando, E.; Miyazaki, M. Moisture degradation mechanism of silver-based low-emissivity coatings. Thin Solid Film. 1999, 351, 308–312. [Google Scholar] [CrossRef]
- Ando, E.; Miyazaki, M. Moisture resistance of the low-emissivity coatings with a layer structure of Al-doped ZnO/Ag/Al-doped ZnO. Thin Solid Film. 2001, 392, 289–293. [Google Scholar] [CrossRef]
- Wang, X.; Santschi, C.; Martin, O.J.F. Strong improvement of long-term chemical and thermal stability of plasmonic silver nanoantennas and films. Small 2017, 13, 1700044. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Shao, Y.; Jin, Y.; Yi, M. Studying corrosion of silver thin film by surface plasmon resonance technique. Opt. Quantum Electron. 2019, 52, 31. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kawamura, M.; Kiba, T.; Abe, Y.; Kim, K.H.; Murotani, H. Improved durability of Ag thin films under high humidity environment by deposition of surface Al nanolayer. Appl. Surf. Sci. 2020, 506, 144929. [Google Scholar] [CrossRef]
- Chen, S.W.; Bai, C.Y.; Jain, C.C.; Zhan, C.J.; Koo, C.H. Durability of indium tin oxide-silver-indium tin oxide films against moisture investigated through the wettability of the top oxide layer. Mater. Trans. 2007, 48, 2230–2234. [Google Scholar] [CrossRef]
- Han, G.C.; Wu, Y.H.; Luo, P.; Qiu, J.J.; Chong, T.C. Dewetting observations of ultrathin metallic films. Solid State Commun. 2003, 126, 479–484. [Google Scholar] [CrossRef]
- Srolovitz, D.J.; Goldiner, M.G. The thermodynamics and kinetics of film agglomeration. JOM 1995, 47, 31–36. [Google Scholar] [CrossRef]
- Agrawal, P.M.; Rice, B.M.; Thompson, D.L. Predicting trends in rate parameters for self-diffusion on FCC metal surfaces. Surf. Sci. 2002, 515, 21–35. [Google Scholar] [CrossRef]
- Srolovitz, D.J.; Safran, S.A. Capillary instabilities in thin films. II. Kinetics. J. Appl. Phys. 1986, 60, 255–260. [Google Scholar] [CrossRef]
- Koike, K.; Yamazaki, F.; Okamura, T.; Fukuda, S. Improvement of corrosion resistance of transparent conductive multilayer coating consisting of silver layers and transparent metal oxide layers. J. Vac. Sci. Technol. A Vac. Surf. Film. 2007, 25, 527–531. [Google Scholar] [CrossRef]
- Koike, K.; Shimada, K.; Fukuda, S. Aggregation in thin-film silver: Induced by chlorine and inhibited by alloying with two dopants. Corros. Sci. 2009, 51, 2557–2564. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, I.; Edler, F.; Pfnür, H.; Tegenkamp, C. The 100th anniversary of the four-point probe technique: The role of probe geometries in isotropic and anisotropic systems. J. Phys. Condens. Matter 2015, 27, 223201. [Google Scholar] [CrossRef]
- Maaroof, A.I.; Evans, B.L. Onset of electrical conduction in Pt and Ni films. J. Appl. Phys. 1994, 76, 1047–1054. [Google Scholar] [CrossRef]
- Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Axelevitch, A.; Gorenstein, B.; Golan, G. Investigation of optical transmission in thin metal films. Phys. Procedia 2012, 32, 1–13. [Google Scholar] [CrossRef]
- Wei, H.; Eilers, H. From silver nanoparticles to thin films: Evolution of microstructure and electrical conduction on glass substrates. J. Phys. Chem. Solids 2009, 70, 459–465. [Google Scholar] [CrossRef]
- Hoffman, R.W. Stresses in thin films: The relevance of grain boundaries and impurities. Thin Solid Film. 1976, 34, 185–190. [Google Scholar] [CrossRef]
- Abermann, R.; Koch, R. The internal stress in thin silver, copper and gold films. Thin Solid Film. 1985, 129, 71–78. [Google Scholar] [CrossRef]
- Chen, W.; Thoreson, M.D.; Ishii, S.; Kildishev, A.V.; Shalaev, V.M. Ultra-thin ultra-smooth and low-loss silver films on a germanium wetting layer. Opt. Express 2010, 18, 5124. [Google Scholar] [CrossRef]
- Del Vecchio, A.L.; Spaepen, F. The effect of deposition rate on the intrinsic stress in copper and silver thin films. J. Appl. Phys. 2007, 101, 063518. [Google Scholar] [CrossRef]
- Formica, N.; Ghosh, D.S.; Carrilero, A.; Chen, T.L.; Simpson, R.E.; Pruneri, V. Ultrastable and atomically smooth ultrathin silver films grown on a copper seed layer. ACS Appl. Mater. Interfaces 2013, 5, 3048–3053. [Google Scholar] [CrossRef] [PubMed]
- Grachev, S.; Hérault, Q.; Wang, J.; Balestrieri, M.; Montigaud, H.; Lazzari, R.; Gozhyk, I. A new method for high resolution curvature measurement applied to stress monitoring in thin films. Nanotechnology 2022, 33, 185701. [Google Scholar] [CrossRef]
- Jamnig, A.; Pliatsikas, N.; Sarakinos, K.; Abadias, G. The effect of kinetics on intrinsic stress generation and evolution in sputter-deposited films at conditions of high atomic mobility. J. Appl. Phys. 2020, 127, 045302. [Google Scholar] [CrossRef]
- Pliatsikas, N.; Jamnig, A.; Konpan, M.; Delimitis, A.; Abadias, G.; Sarakinos, K. Manipulation of thin silver film growth on weakly interacting silicon dioxide substrates using oxygen as a surfactant. J. Vac. Sci. Technol. A 2020, 38, 043406. [Google Scholar] [CrossRef]
- Kinosita, K.; Maki, K.; Nakamizo, K.; Takeuchi, K. Stress in vacuum deposited films of silver. Jpn. J. Appl. Phys. 1967, 6, 42–53. [Google Scholar] [CrossRef]
- Hérault, Q.; Gozhyk, I.; Balestrieri, M.; Montigaud, H.; Grachev, G.; Lazzari, R. Kinetics and mechanisms of stress relaxation in sputtered silver thin films. Acta Mater. 2021, 221, 117385. [Google Scholar] [CrossRef]
- Jamnig, A.; Sangiovanni, D.G.; Abadias, G.; Sarakinos, K. Atomic-scale diffusion rates during growth of thin metal films on weakly-interacting substrates. Sci. Rep. 2019, 9, 6640. [Google Scholar] [CrossRef]
- Wilcock, J.D.; Campbell, D.S.; Anderson, J.C. The internal stress in evaporated silver and gold films. Thin Solid Film. 1969, 3, 13–34. [Google Scholar] [CrossRef]
- Koch, R.; Winau, D.; Führmann, A.; Rieder, K.H. Growth-mode-specific intrinsic stress of thin silver films. Phys. Rev. B 1991, 44, 3369–3372. [Google Scholar] [CrossRef]
- Abermann, R.; Koch, R.; Kramer, R. Electron microscope structure and internal stress in thin silver and gold films deposited onto MgF2 and SiO substrates. Thin Solid Film. 1979, 58, 365–370. [Google Scholar] [CrossRef]
- Floro, J.A.; Hearne, S.J.; Hunter, J.A.; Kotula, P.; Chason, E.; Seel, S.C.; Thompson, C.V. The dynamic competition between stress generation and relaxation mechanisms during coalescence of Volmer-Weber thin films. J. Appl. Phys. 2001, 89, 4886–4897. [Google Scholar] [CrossRef]
- Seel, S.C. Stress and Structure Evolution during Volmer-Weber Growth of Thin Films. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2002. [Google Scholar]
- Abadias, G.; Fillon, A.; Colin, J.J.; Michel, A.; Jaouen, C. Real-time stress evolution during early growth stages of sputter-deposited metal films: Influence of adatom mobility. Vacuum 2014, 100, 36–40. [Google Scholar] [CrossRef]
- Castillo, R.H.; Peñuñuri, F.; Canto-Reyes, D.; Borges Pool, A.; Mendez-Gamboa, J.A.; Acosta, M. Electrical percolation threshold evaluation of silver thin films for multilayer WO3/Ag/WO3 transparent conductive oxide. Mater. Lett. 2020, 260, 126913. [Google Scholar] [CrossRef]
- Lewowski, T. Photoelectron spectroscopy of thin discontinuous silver films deposited onto a sapphire substrate, near the percolation threshold. Thin Solid Film. 1995, 259, 53–58. [Google Scholar] [CrossRef]
- Byon, E.; Oates, T.W.H.; Anders, A. Coalescence of nanometer silver islands on oxides grown by filtered cathodic arc deposition. Appl. Phys. Lett. 2003, 82, 1634–1636. [Google Scholar] [CrossRef]
- Ko, R.H.H.; Khalatpour, A.; Clark, J.K.D.; Kherani, N.P. Ultrasmooth ultrathin Ag films by AlN seeding and Ar/N2 sputtering for transparent conductive and heating applications. APL Mater. 2018, 6, 121112. [Google Scholar] [CrossRef]
- Martínez-Cercós, D.; Paulillo, B.; Maniyara, R.A.; Rezikyan, A.; Bhattacharyya, I.; Mazumder, P.; Pruneri, V. Ultrathin metals on a transparent seed and application to infrared reflectors. ACS Appl. Mater. Interfaces 2021, 13, 46990–46997. [Google Scholar] [CrossRef]
- Santoro, G.; Yu, S.; Schwartzkopf, M.; Zhang, P.; Vayalil, S.K.; Risch, J.F.H.; Rübhausen, M.A.; Hernández, M.; Domingo, C.; Roth, S.V. Silver substrates for surface enhanced Raman scattering: Correlation between nanostructure and Raman scattering enhancement. Appl. Phys. Lett. 2014, 104, 243107. [Google Scholar] [CrossRef]
- Hafezian, S. Growth Control and Study of Ultrathin Silver Films for Energy-Saving Coatings. Ph.D. Thesis, Polytechnique Montréal, Montreal, QC, Canada, 2019. [Google Scholar]
- Šmilauer, P. Thin metal films and percolation theory. Contemp. Phys. 1991, 32, 89–102. [Google Scholar] [CrossRef]
- Goetz, S.; Bauch, M.; Dimopoulos, T.; Trassl, S. Ultrathin sputter-deposited plasmonic silver nanostructures. Nanoscale Adv. 2020, 2, 869–877. [Google Scholar] [CrossRef]
- Fukuda, K.; Lim, S.H.N.; Anders, A. Coalescence of magnetron-sputtered silver islands affected by transition metal seeding (Ni, Cr, Nb, Zr, Mo, W, Ta) and other parameters. Thin Solid Film. 2008, 516, 4546–4552. [Google Scholar] [CrossRef]
- Logeeswaran, V.J.; Kobayashi, N.P.; Islam, M.S.; Wu, W.; Chaturvedi, P.; Fang, N.X.; Wang, S.Y.; Williams, R.S. Ultrasmooth silver thin films deposited with a germanium nucleation layer. Nano Lett. 2009, 9, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, B.; Leong, E.S.P.; Yang, P.; Zong, Y.; Si, G.; Teng, J.; Maier, S.A. Enhanced surface plasmon resonance on a smooth silver film with a seed growth layer. ACS Nano 2010, 4, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Colenso, H.R.; Rafealov, E.Z.; Maddah, M.; Plank, N.O.V.; Chen, W.T.; Waterhouse, G.I.N.; Hao, J.; Gouws, G.J.; Moore, C.P. Comparison of seed layers for smooth, low loss silver films used in ultraviolet-visible plasmonic imaging devices. Thin Solid Film. 2018, 656, 68–74. [Google Scholar] [CrossRef]
- Sonmez, N.A.; Donmez, M.; Comert, B.; Ozcelik, S. Ag/M-seed/AZO/glass structures for low-E glass: Effects of metal seeds. Int. J. Appl. Glass Sci. 2017, 9, 383–391. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Szoplik, T. Controlling the optical parameters of self-assembled silver films with wetting layers and annealing. Appl. Surf. Sci. 2017, 421, 349–356. [Google Scholar] [CrossRef]
- Cazabat, A.M. How does a droplet spread? Contemp. Phys. 1987, 28, 347–364. [Google Scholar] [CrossRef]
- Joanny, J.F.; de Gennes, P.G. Upward creep of a wetting fluid: A scaling analysis. J. Phys. 1986, 47, 121–127. [Google Scholar] [CrossRef]
- Shiomoto, S.; Higuchi, H.; Yamaguchi, K.; Takaba, H.; Kobayashi, M. Spreading dynamics of a precursor film of ionic liquid or water on a micropatterned polyelectrolyte brush surface. Langmuir 2021, 37, 3049–3056. [Google Scholar] [CrossRef]
- Pinchuk, A.O. Size-dependent Hamaker constant for silver nanoparticles. J. Phys. Chem. C 2012, 116, 20099–20102. [Google Scholar] [CrossRef]
- Demirdjian, B.; Bedu, F.; Ranguis, A.; Ozerov, I.; Henry, C.R. Water adsorption by a sensitive calibrated gold plasmonic nanosensor. Langmuir 2018, 34, 5381–5385. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Q.; Zhao, X.M.; Bai, Y.; Qiao, L.J. Water adsorption behavior on metal surfaces and its influence on surface potential studied by in situ SPM. Appl. Surf. Sci. 2012, 258, 9087–9091. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Hamada, H. Reactive wetting by liquid sodium on thin Au plating. J. Nucl. Sci. Technol. 2013, 51, 201–207. [Google Scholar] [CrossRef]
- Porter, J.D.; Robinson, T.O. Surface diffusion of silver at the silver (111)/liquid-water interface from electrocrystallization measurements. J. Phys. Chem. 1993, 97, 6696–6709. [Google Scholar] [CrossRef]
- Sanders, D.E.; DePristo, A.E. Predicted diffusion rates on fcc (001) metal surfaces for adsorbate/substrate combinations of Ni, Cu, Rh, Pd, Ag, Pt, Au. Surf. Sci. 1992, 260, 116–128. [Google Scholar] [CrossRef]
- Han, H.; Zoo, Y.; Mayer, J.W.; Alford, T.L. Improved surface morphology and texture of Ag films on indium tin oxide via Cu additions. J. Appl. Phys. 2007, 102, 036101. [Google Scholar] [CrossRef]
- Baburin, A.S.; Ivanov, A.I.; Lotkov, E.S.; Sorokina, O.S.; Boginskaya, I.A.; Sergeev, E.V.; Buzaverov, K.A.; Konstantinova, T.G.; Moskalev, D.O.; Issabayeva, Z.; et al. Epitaxial silver films morphology and optical properties evolution over two years. Coatings 2020, 10, 911. [Google Scholar] [CrossRef]
- Flötotto, D.; Wang, Z.M.; Jeurgens, L.P.H.; Mittemeijer, E.J. Kinetics and magnitude of the reversible stress evolution during polycrystalline film growth interruptions. J. Appl. Phys. 2015, 118, 055305. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depla, D. Chemical Stability of Sputter Deposited Silver Thin Films. Coatings 2022, 12, 1915. https://doi.org/10.3390/coatings12121915
Depla D. Chemical Stability of Sputter Deposited Silver Thin Films. Coatings. 2022; 12(12):1915. https://doi.org/10.3390/coatings12121915
Chicago/Turabian StyleDepla, Diederik. 2022. "Chemical Stability of Sputter Deposited Silver Thin Films" Coatings 12, no. 12: 1915. https://doi.org/10.3390/coatings12121915
APA StyleDepla, D. (2022). Chemical Stability of Sputter Deposited Silver Thin Films. Coatings, 12(12), 1915. https://doi.org/10.3390/coatings12121915





