TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs) Decorated with Au Nanoparticles and Au Nanorods for Efficient Photoelectrochemical Water Splitting and Photocatalytic Degradation of Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs)
2.2. Synthesis of Au Nanoparticles and Au Nanorods
2.3. Preparation of Au NPs-, Au NRs-, and Au-NRs-NPs-Decorated TNWs/TNAs
2.4. Characterization Methods
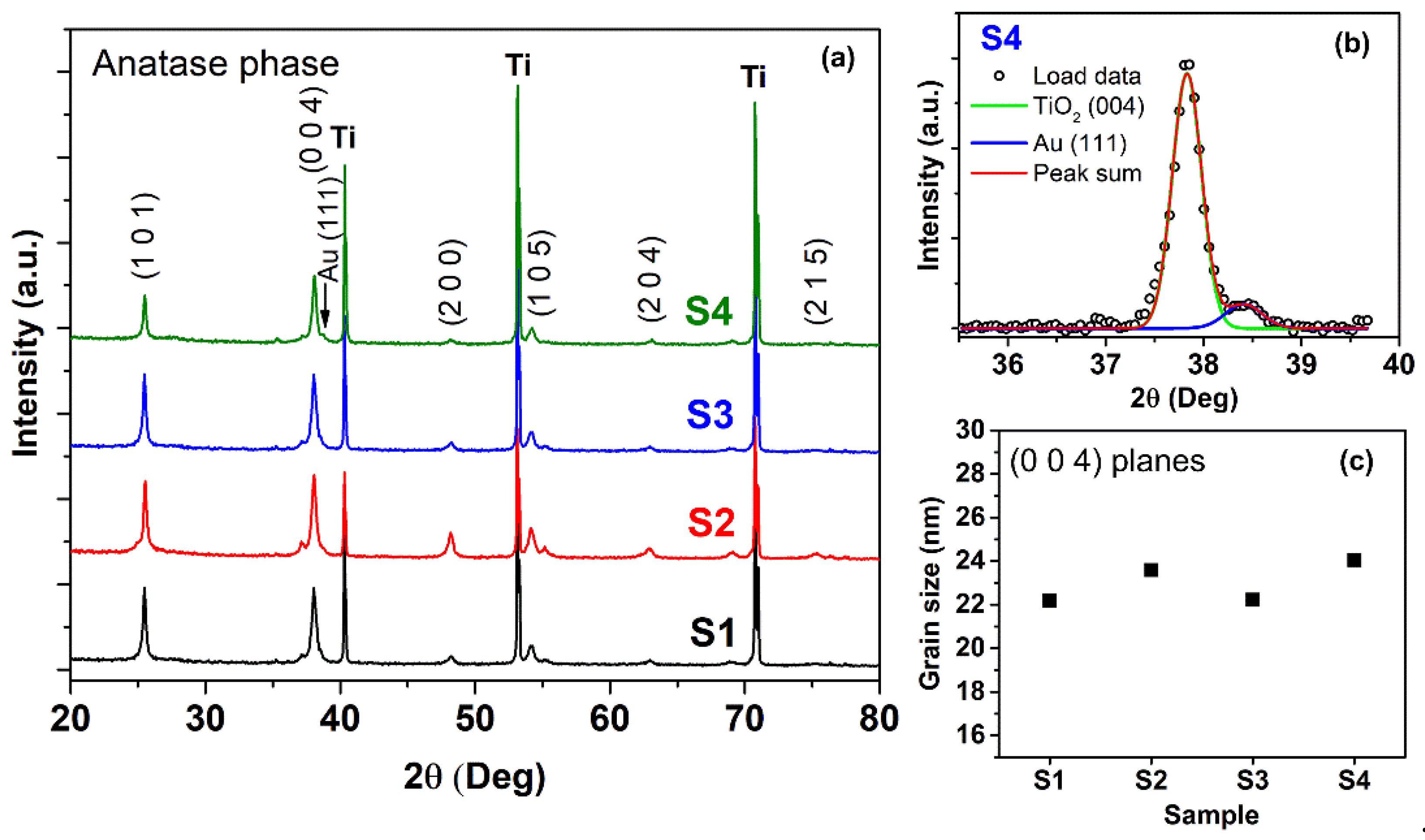
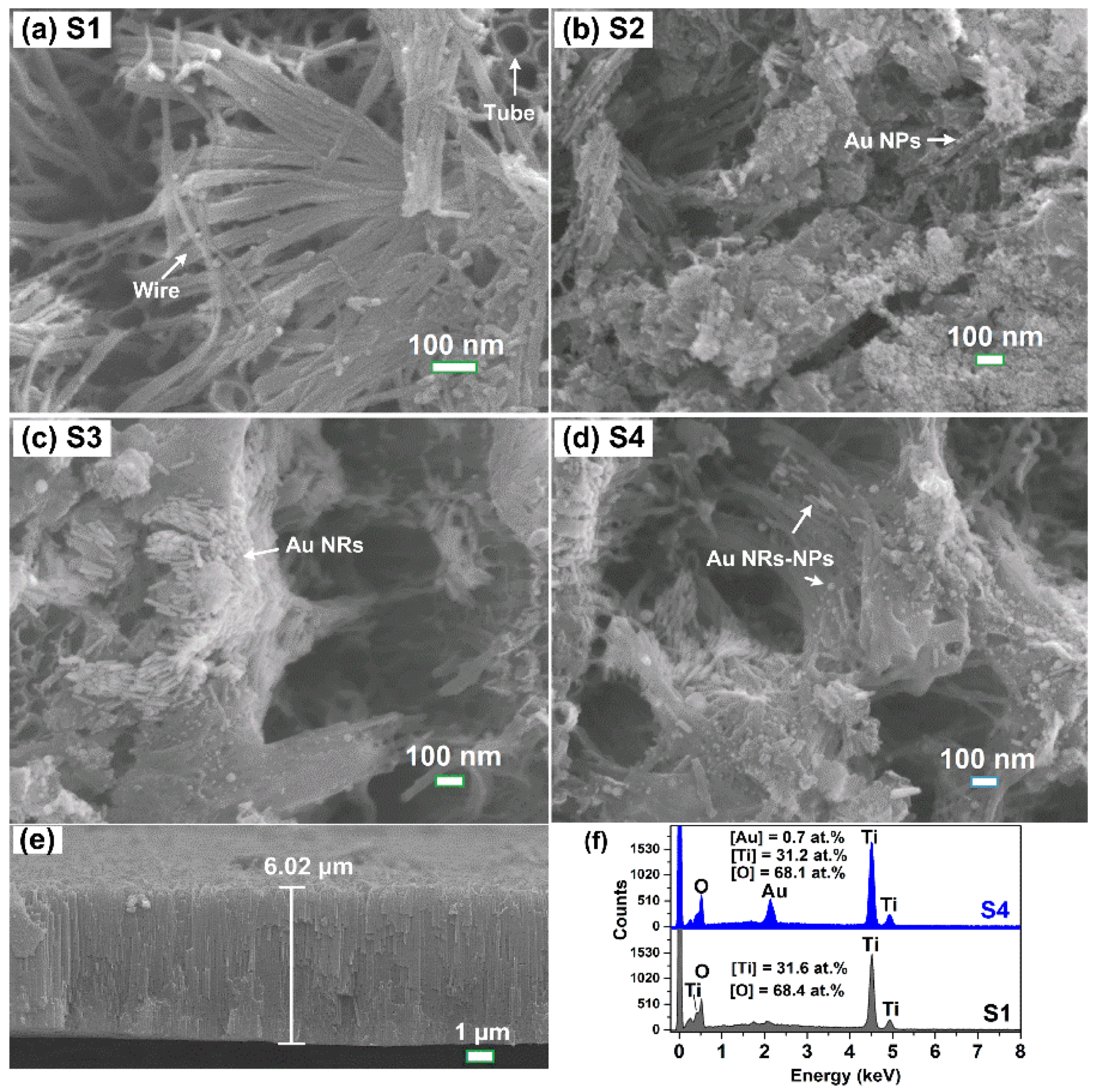
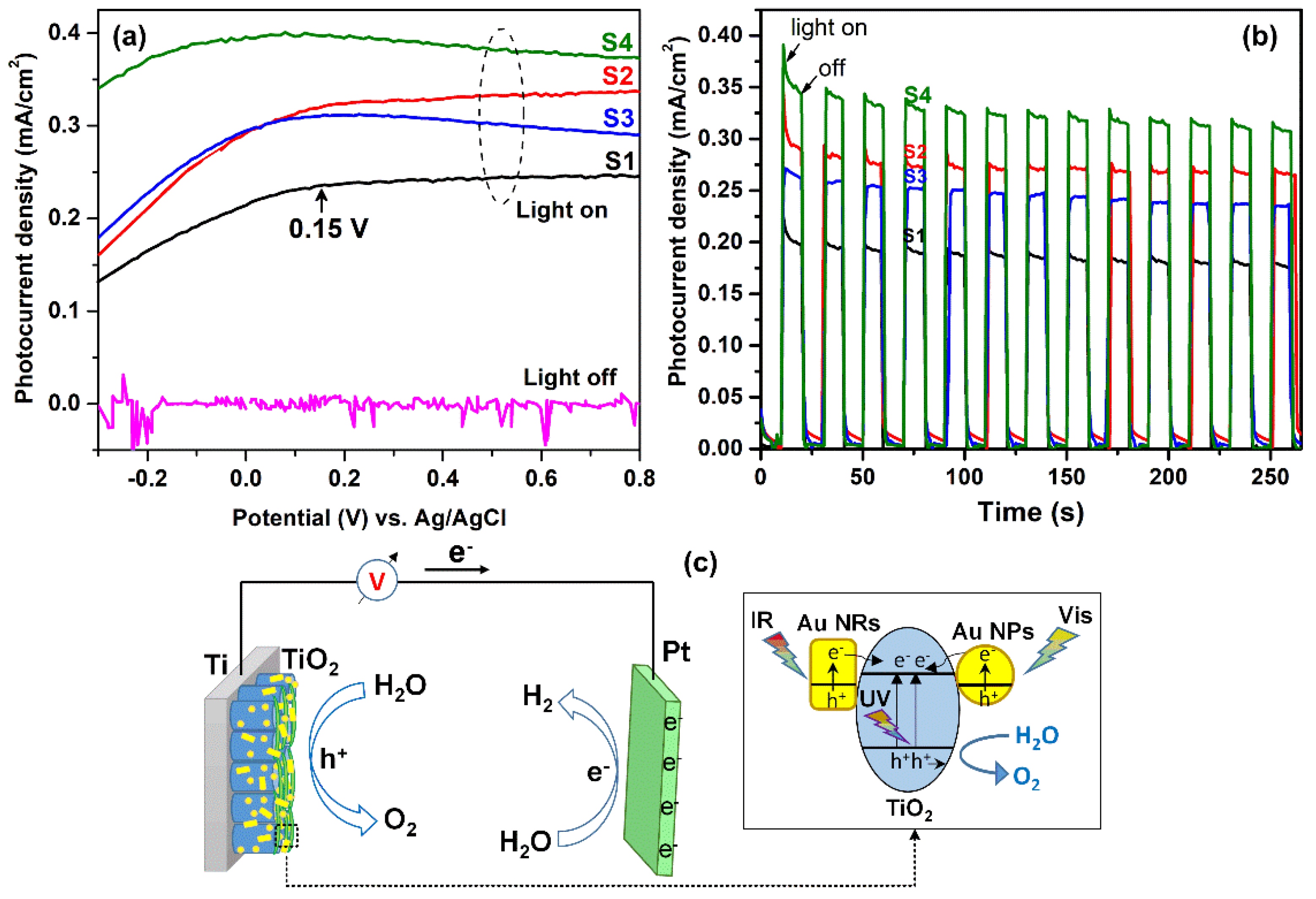
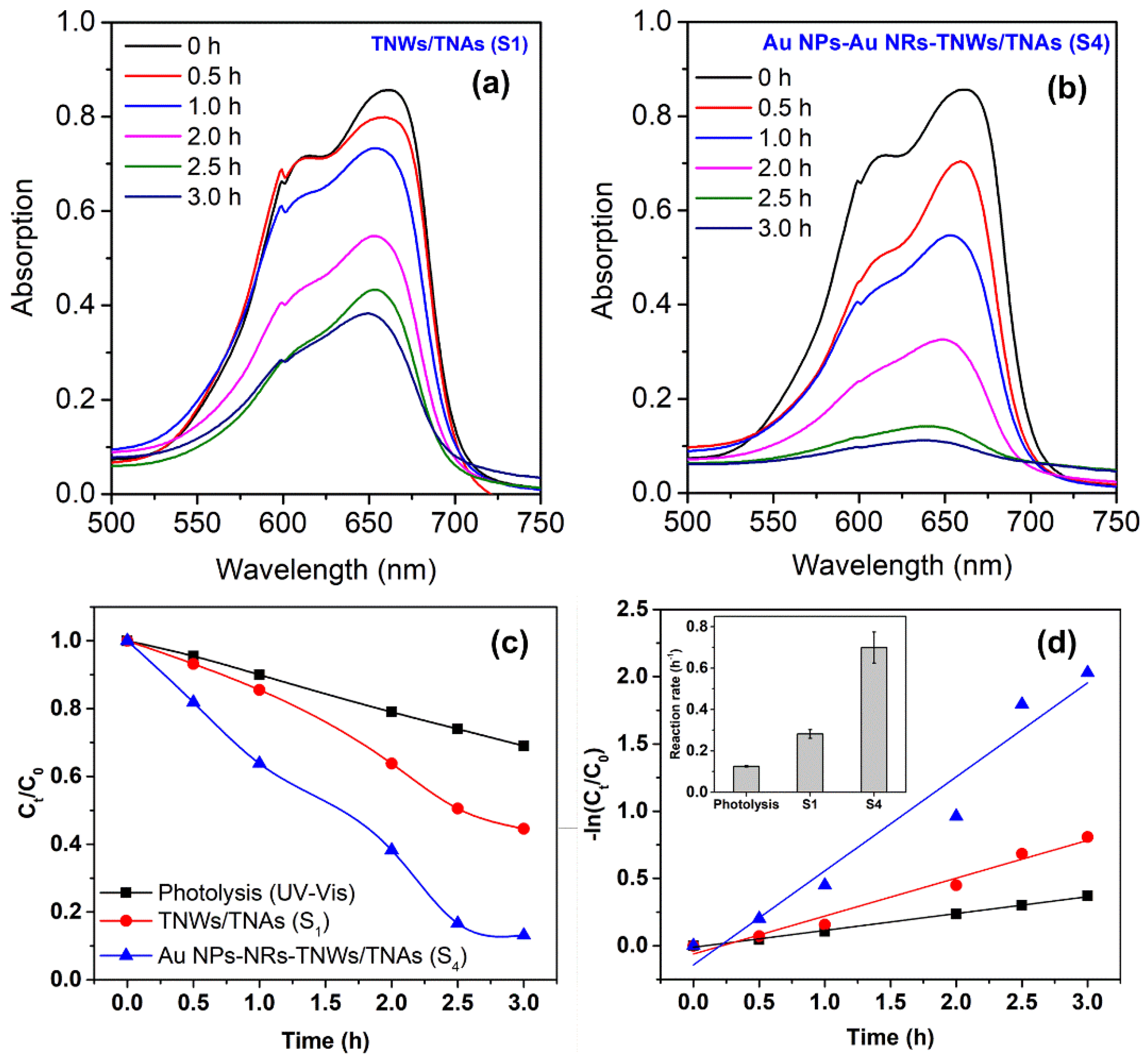
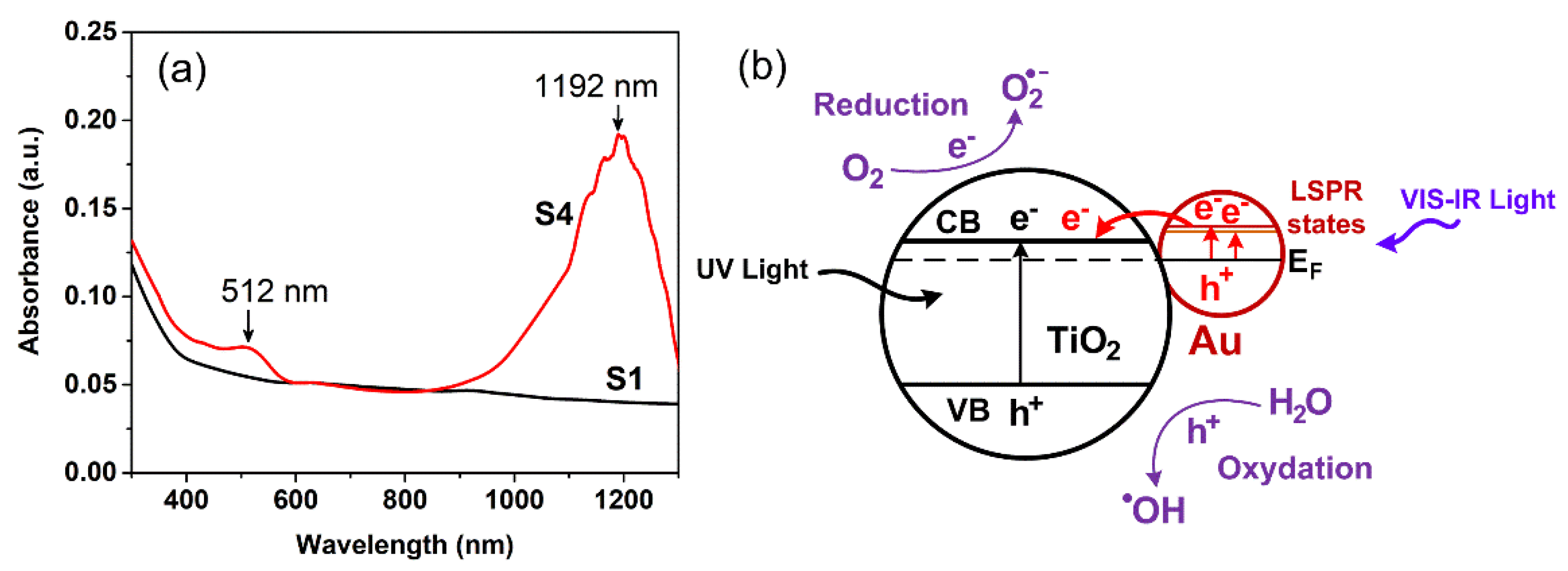
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Wang, T.; Lv, R.; Zhang, J.; Zhang, P.; Lu, J.; Gong, J. Dendritic Au/TiO2 nanorod arrays for visible-light driven photoelectrochemical water splitting. Nanoscale 2013, 5, 9001. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Manna, A.K.; Soni, R.K. Bifunctional Au—TiO2 thin films with enhanced photocatalytic activity and SERS based multiplexed detection of organic pollutant. J. Mater. Sci. Mater. Electron. 2019, 30, 16478–16493. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, S.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Visible Light Driven Photoelectrochemical Water Oxidation on Nitrogen-Modified TiO2 Nanowires. Nano Lett. 2012, 12, 26–32. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Photosensitization of TiO2 Nanostructures with CdS Quantum Dots: Particulate versus Tubular Support Architectures. Adv. Funct. Mater. 2009, 46556, 805–811. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsu, H.-L.; Leu, J. TiO2 Nanowires on Anodic TiO2 Nanotube Arrays (TNWs/TNAs): Formation Mechanism and Photocatalytic Performance. J. Electrochem. Soc. 2012, 159, H722–H727. [Google Scholar] [CrossRef]
- Pu, Y.C.; Wang, G.; Chang, K.-D.; Ling, Y.; Lin, Y.K.; Fitzmorris, B.C.; Liu, C.M.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-D.; Ching, W.Y. Electronic and optical properties ofthree phases oftitanium dioxide: Rutile, anatase, and brookite. Phys. Rev. B. 1995, 51, 13023. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.M.; Siwach, R.; Kumar, S.; Alhazaa, A.N. Role of Fe doping in tuning photocatalytic and photoelectrochemical properties of TiO2 for photodegradation of methylene blue. Opt. Laser Technol. 2019, 118, 170–178. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, X.; Morales-Guzman, P.I.; Yu, X.; Liu, H.; Zhang, H.; Razzari, L.; Claverie, J.P. Engineering the Absorption and Field Enhancement Properties of Au−TiO2 Nanohybrids via Whispering Gallery Mode Resonances for Photocatalytic Water Splitting. ACS Nano 2016, 10, 4496–4503. [Google Scholar] [CrossRef]
- Luo, J.; Ma, L.; He, T.; Ng, C.F.; Wang, S.; Sun, H.; Fan, H.J. TiO2/(CdS, CdSe, CdSeS) Nanorod Heterostructures and Photoelectrochemical Properties. J. Phys. Chem. C 2012, 116, 11956–11963. [Google Scholar] [CrossRef]
- Lin, H.; Mao, Z.; Zhou, N.; Wang, M.; Li, L.; Li, Q. Fabrication of CdS quantum dots sensitized TiO2 nanowires/nanotubes arrays and their photoelectrochemical properties. SN Appl. Sci. 2019, 1, 391. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B. 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chou, C.-H.; Chen, C.-D.; Wang, C.R.C. Highly Efficient, Wavelength-Tunable, Gold Nanoparticle Based Optothermal Nanoconvertors. J. Phys. Chem. B. 2005, 109, 11135–11138. [Google Scholar] [CrossRef]
- Li, C.; Shuford, K.L.; Chen, M.; Lee, E.J.; Cho, S.O. A Facile Polyol Route to Uniform Gold Octahedra with Tailorable Size and Their Optical Properties. ACS Nano 2008, 2, 1760. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon Resonant Enhancement of Photocatalytic Water Splitting Under Visible Illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.K.; Giri, P.K. Role of Surface Plasmons and Hot Electrons on the Multi-Step Photocatalytic Decay by Defect Enriched Ag@TiO2 Nanorods Under Visible Light Illumination. J. Phys. Chem. C 2017, 121, 20016–20030. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sahu, K.; Satpati, B.; Shah, J.; Kotnala, R.K.; Mohapatra, S. Facile synthesis, structural and optical properties of Au-TiO2 plasmonic nanohybrids for photocatalytic applications. J. Phys. Chem. Solids 2019, 135, 109100. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Zhang, Z.; Zhang, X.; Chen, Y.; Zhang, Y. The preparation and performance of Au loads TiO2 nanomaterials. Mater. Res. Express 2019, 6, 095041. [Google Scholar] [CrossRef]
- Veziroglu, S.; Ullrich, M.; Hussain, M.; Drewes, J.; Shondo, J.; Strunskus, T.; Adam, J.; Faupel, F.; Aktas, O.C. Plasmonic and non-plasmonic contributions on photocatalytic activity of Au-TiO2 thin film under mixed UV—Visible light. Surf. Coat. Technol. 2020, 389, 125613. [Google Scholar] [CrossRef]
- Link, S.; Mohamed, M.B.; El-Sayed, M.A. Simulation of the Optical Absorption Spectra of Gold Nanorods as a Function of Their Aspect Ratio and the Effect of the Medium Dielectric Constant. J. Phys. Chem. B 1999, 103, 3073–3077. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef]
- Kumar, R.; Binetti, L.; Nguyen, T.H.; Alwis, L.S.M.; Agrawal, A.; Sun, T.; Grattan, K.T.V. Determination of the Aspect-ratio Distribution of Gold Nanorods in a Colloidal Solution using UV-visible absorption spectroscopy. Sci. Rep. 2019, 9, 17469. [Google Scholar] [CrossRef] [PubMed]
- Enustun, B.V.; Turkevich, J. Coagulation of Colloidal Gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B. 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Wu, Q.; Huang, P.; Su, Y.; Lin, C. N-doped TiO2 nanotube array photoelectrode for visible-light-induced photoelectrochemical and photoelectrocatalytic activities. Electrochim. Acta 2013, 108, 525–531. [Google Scholar] [CrossRef]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; Walczak, L.; Gopinath, C.S. A Study on Doped Heterojunctions in TiO2 Nanotubes: An Efficient Photocatalyst for Solar Water Splitting. Sci. Rep. 2017, 7, 14314. [Google Scholar] [CrossRef] [PubMed]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, T.D.; Le, P.H. Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials. Catalysts 2020, 10, 356. [Google Scholar] [CrossRef]
- Tuyen, L.T.C.; Jian, S.-R.; Tien, N.T.; Le, P.H. Nanomechanical and Material Properties of Fluorine-Doped Tin Oxide Thin Films Prepared by Ultrasonic Spray Pyrolysis: Effects of F-Doping. Materials 2019, 12, 1665. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, J.; Qi, L.; Liu, E.; Fan, J. Efficient Degradation of Methylene Blue over Two-Dimensional Au/TiO2 Nanosheet Films with Overlapped Light Harvesting Nanostructures. J. Nanomater. 2015, 2015, 905259. [Google Scholar] [CrossRef]
- Padikkaparambil, S.; Narayanan, B.; Yaakob, Z.; Viswanathan, S.; Tasirin, S.M. Au/TiO2 Reusable Photocatalysts for Dye Degradation. Inter. J. Photoenergy 2013, 2013, 752605. [Google Scholar] [CrossRef]
- Liu, W.; Duan, W.; Jia, L.; Wang, S.; Guo, Y.; Zhang, G.; Zhu, B.; Huang, W.; Zhang, S. Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes. Nanomaterials 2022, 12, 2058. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A. 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Yoshiiri, K.; Wang, K.; Kowalska, E. TiO2/Au/TiO2 Plasmonic Photocatalysts: The Influence of Titania Matrix and Gold Properties. Inventions 2022, 7, 54. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X.; Zhao, Q. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid Film. 2000, 379, 7–14. [Google Scholar] [CrossRef]
- Xu, F.; Mei, J.; Li, X.; Sun, Y.; Wu, D.; Gao, Z.; Zhang, Q.; Jiang, K. Heterogeneous three-dimensional TiO2/ZnO nanorod array for enhanced photoelectrochemical water splitting properties. J. Nanopart Res. 2017, 19, 297. [Google Scholar] [CrossRef]
- Luo, J.; Li, D.; Yang, Y.; Liu, H.; Chen, J.; Wang, H. Preparation of Au/reduced graphene oxide/hydrogenated TiO2 nanotube arrays ternary composites for visible-light-driven photoelectrochemical water splitting. J. Alloys Compd. 2016, 661, 380–388. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zhang, C.; Fu, L.; Li, G.; Shao, Z.; Yi, B. Enhancement of photoelectrochemical response by Au modified in TiO2 nanorods. Int. J. Hydrogen Energy 2013, 38, 13023–13030. [Google Scholar] [CrossRef]
- Xu, F.; Bai, D.; Mei, J.; Wu, D.; Gao, Z.; Jiang, K.; Liu, B. Enhanced photoelectrochemical performance with in-situ Au modified TiO2 nanorod arrays as photoanode. J. Alloys Compd. 2016, 688, 914–920. [Google Scholar] [CrossRef]
- Xu, F.; Mei, J.; Zheng, M.; Bai, D.; Wu, D.; Gao, Z.; Jiang, K. Au nanoparticles modified branched TiO2 nanorod array arranged with ultrathin nanorods for enhanced photoelectrochemical water splitting. J. Alloys Compd. 2017, 693, 1124–1132. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Yu, Y.; Ma, Y.; Yang, W.; Wang, F.; Yin, X.; Wang, X. Surface-Plasmon-Resonance-Enhanced Photoelectrochemical Water Splitting from Au-Nanoparticle-Decorated 3D TiO2 Nanorod Architectures. J. Phys. Chem. C 2017, 121, 12071–12079. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Chen, Y.-W. Facile Synthesis of Ag/TiO2 by Photoreduction Method and Its Degradation Activity of Methylene Blue under UV and Visible Light Irradiation. Mod. Res. Catal. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Mishra, S.; Chakinala, N.; Chakinala, A.G.; Surolia, P.K. Photocatalytic degradation of methylene blue using monometallic and bimetallic Bi-Fe doped TiO2. Catal. Commun. 2022, 171, 106518. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Hu, X.; Li, Z.; Fan, J.; Liu, E. Facile fabrication of SnO2/TiO2 nanotube arrays for efficient degradation of pollutants. Opt. Mater. 2022, 127, 112252. [Google Scholar] [CrossRef]
- Kang, X.; Chen, S. Photocatalytic reduction of methylene blue by TiO2 nanotube arrays: Effects of TiO2 crystalline phase. J. Mater. Sci. 2010, 45, 2696–2702. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Pan, K.; Tian, C.; Zhou, J.; Zhou, W.; Ren, Z.; Fu, H. In situ controlled growth of well-dispersed gold nanoparticles in TiO2 nanotube arrays as recyclable substrates for surface-enhanced Raman scattering. Dalt. Trans. 2012, 41, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 2013, 13, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2010, 1, 356–359. [Google Scholar] [CrossRef]




| Photoanode Nanomaterial | Electrolyte/Potential (V) vs. RHE | Illumination | Photocurrent Density (mA/cm2) | Ref. |
|---|---|---|---|---|
| Au NPs-NRs/TNWs/TNAs | 0.5 M Na2SO4/0.15 V | 100 W Xe lamp | 0.40 | This study |
| Au/RGO/H-TNTs | 1 M KOH/1.23 V | Xe lamp, AM 1.5G filter, λ > 400 nm | 0.22 | [45] |
| Au/TiO2/Au heterostructure | 1 M KOH/0.2 V | 300 W Xenon Lamp, 100 mW/cm2 UV–100 mW/cm2 Vis | 0.94 | [46] |
| Au/TiO2 nanorod arrays | 0.5 M Na2SO4/1.0 V | 500 W Xe lamp, 100 mW/cm2 | 1.1 | [47] |
| Au NPs-NRs/TiO2 nanowires | 1 M KOH/0 V | AM 1.5G, 100 mW/cm2 | 1.49 | [11] |
| Au NPs-branched TiO2 | 0.5 M Na2SO4/1.23 V | 500 W Xe lamp, 100 mW/cm2 | 2.5 | [48] |
| Au NPs/3D TiO2 nanorods | 1 M NaOH/0 V | 150 W Xe lamp, AM 1.5G, 100 mW/cm2 | 2.7 | [49] |
| Photocatalyst | Synthesis Methods | Reaction Rate (h−1) | Ref. |
|---|---|---|---|
| TNWs/TNAs | Anodization | 0.28 ± 0.02 | This study |
| Au NPs-NRs/TNWs/TNAs | Turkevick method–anodization–drop casting method | 0.70 ± 0.07 | This study |
| 40 nm-TNWs/20 nm-TNAs | Anodic oxidation | 0.13 | [10] |
| TiO2 nanoparticles P25 film | - | 0.14 | [10] |
| TNAs | Anodization | 1.45 | [53] |
| Ag/TiO2 P25 | Photo-reduction method | 0.65 | [50] |
| Bi-Fe doped TiO2 | Wet impregnation technique | 0.78 | [51] |
| SnO2 NPs-decorated TNAs | Anodization–solvothermal process | 1.86 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyen, N.N.; Tuyen, L.T.C.; Hieu, L.T.; Nguyen, T.T.T.; Thao, H.P.; Do, T.C.M.V.; Nguyen, K.T.; Hang, N.T.N.; Jian, S.-R.; Tu, L.A.; et al. TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs) Decorated with Au Nanoparticles and Au Nanorods for Efficient Photoelectrochemical Water Splitting and Photocatalytic Degradation of Methylene Blue. Coatings 2022, 12, 1957. https://doi.org/10.3390/coatings12121957
Uyen NN, Tuyen LTC, Hieu LT, Nguyen TTT, Thao HP, Do TCMV, Nguyen KT, Hang NTN, Jian S-R, Tu LA, et al. TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs) Decorated with Au Nanoparticles and Au Nanorods for Efficient Photoelectrochemical Water Splitting and Photocatalytic Degradation of Methylene Blue. Coatings. 2022; 12(12):1957. https://doi.org/10.3390/coatings12121957
Chicago/Turabian StyleUyen, Ngo Ngoc, Le Thi Cam Tuyen, Le Trung Hieu, Thi Thu Tram Nguyen, Huynh Phuong Thao, Tho Chau Minh Vinh Do, Kien Trung Nguyen, Nguyen Thi Nhat Hang, Sheng-Rui Jian, Ly Anh Tu, and et al. 2022. "TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs) Decorated with Au Nanoparticles and Au Nanorods for Efficient Photoelectrochemical Water Splitting and Photocatalytic Degradation of Methylene Blue" Coatings 12, no. 12: 1957. https://doi.org/10.3390/coatings12121957
APA StyleUyen, N. N., Tuyen, L. T. C., Hieu, L. T., Nguyen, T. T. T., Thao, H. P., Do, T. C. M. V., Nguyen, K. T., Hang, N. T. N., Jian, S.-R., Tu, L. A., Le, P. H., & Luo, C.-W. (2022). TiO2 Nanowires on TiO2 Nanotubes Arrays (TNWs/TNAs) Decorated with Au Nanoparticles and Au Nanorods for Efficient Photoelectrochemical Water Splitting and Photocatalytic Degradation of Methylene Blue. Coatings, 12(12), 1957. https://doi.org/10.3390/coatings12121957









