Mechanical and Anticorrosive Properties of TiNbTa and TiNbTaZr Films on Ti-6Al-4V Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
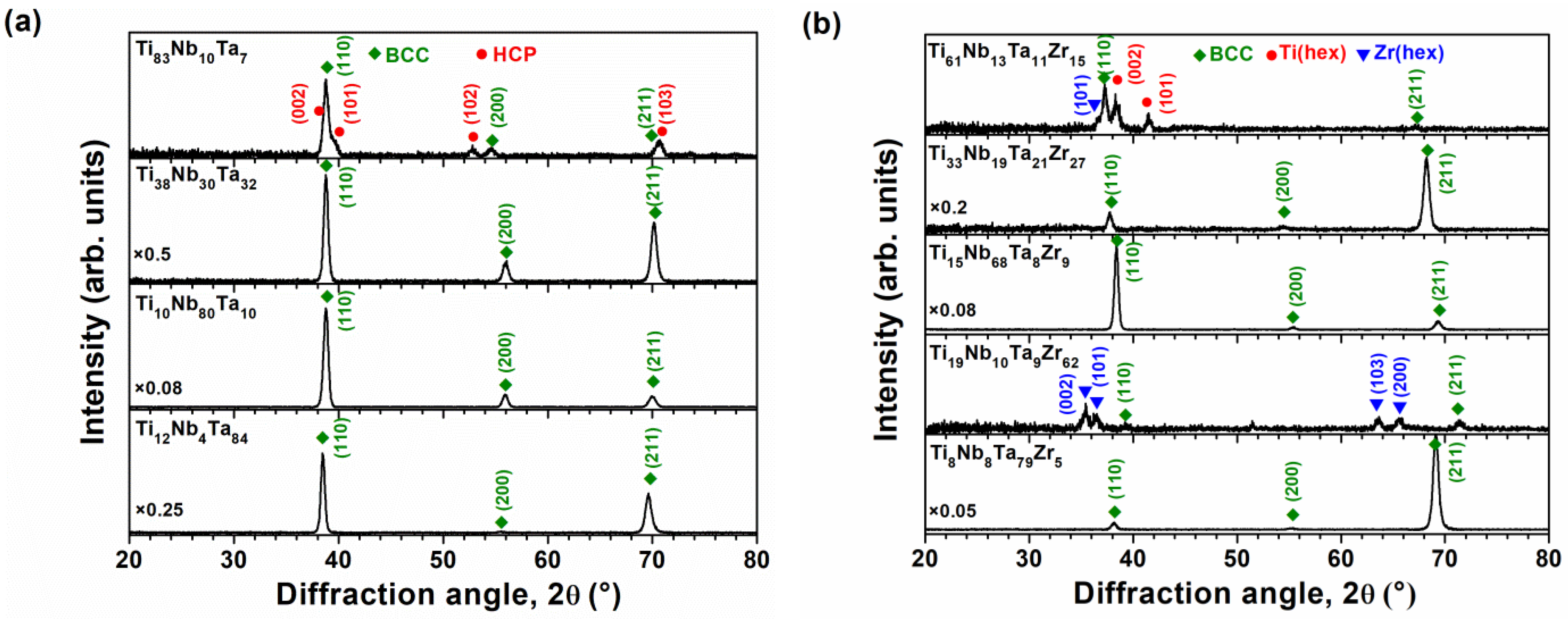
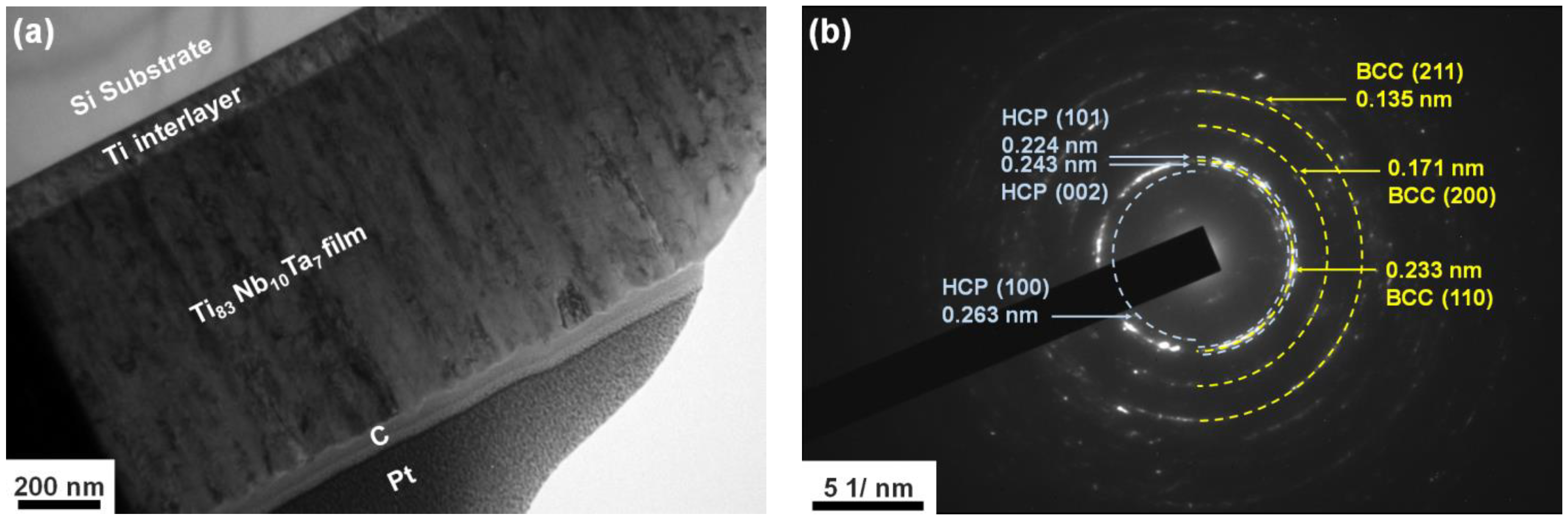
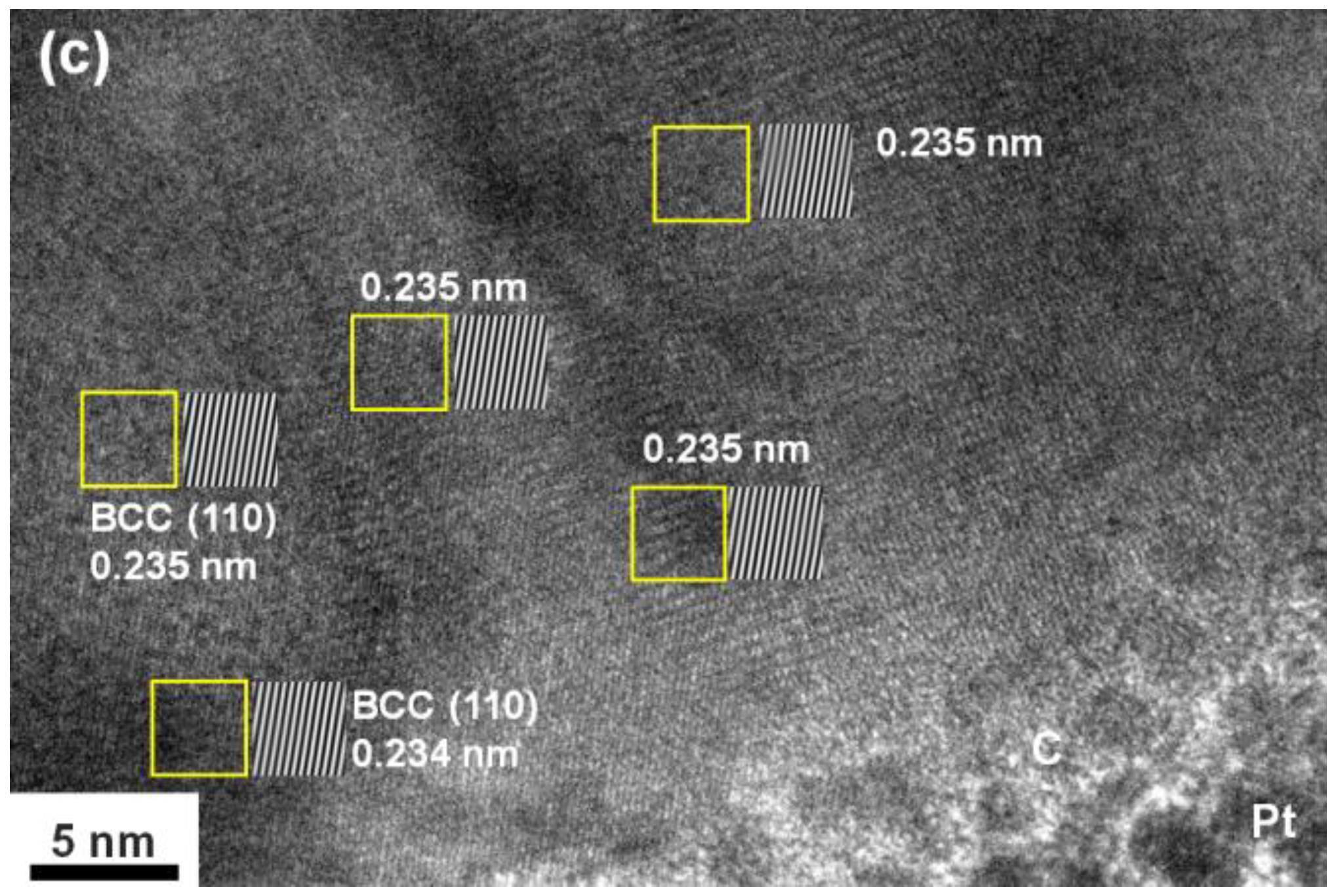
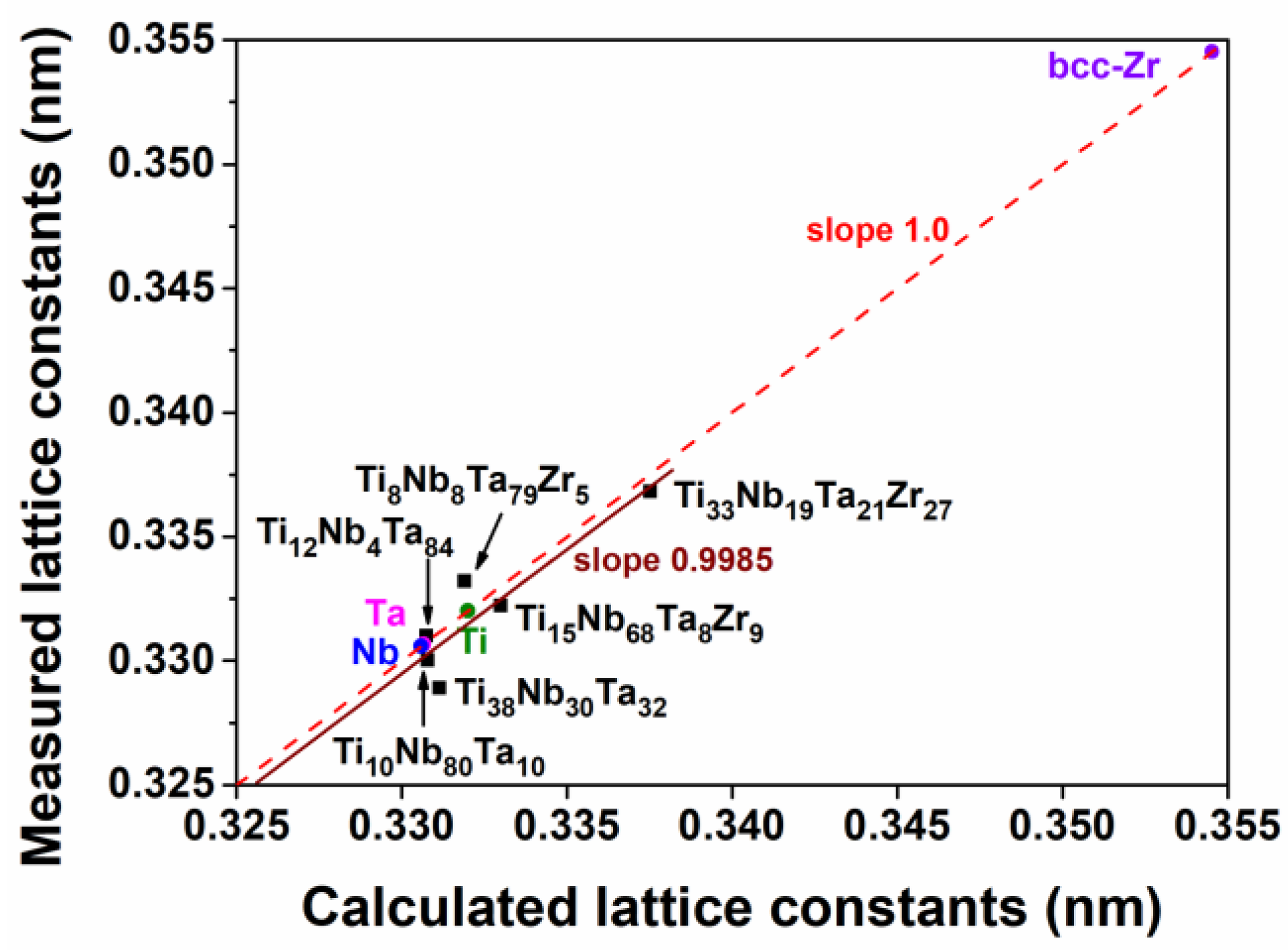
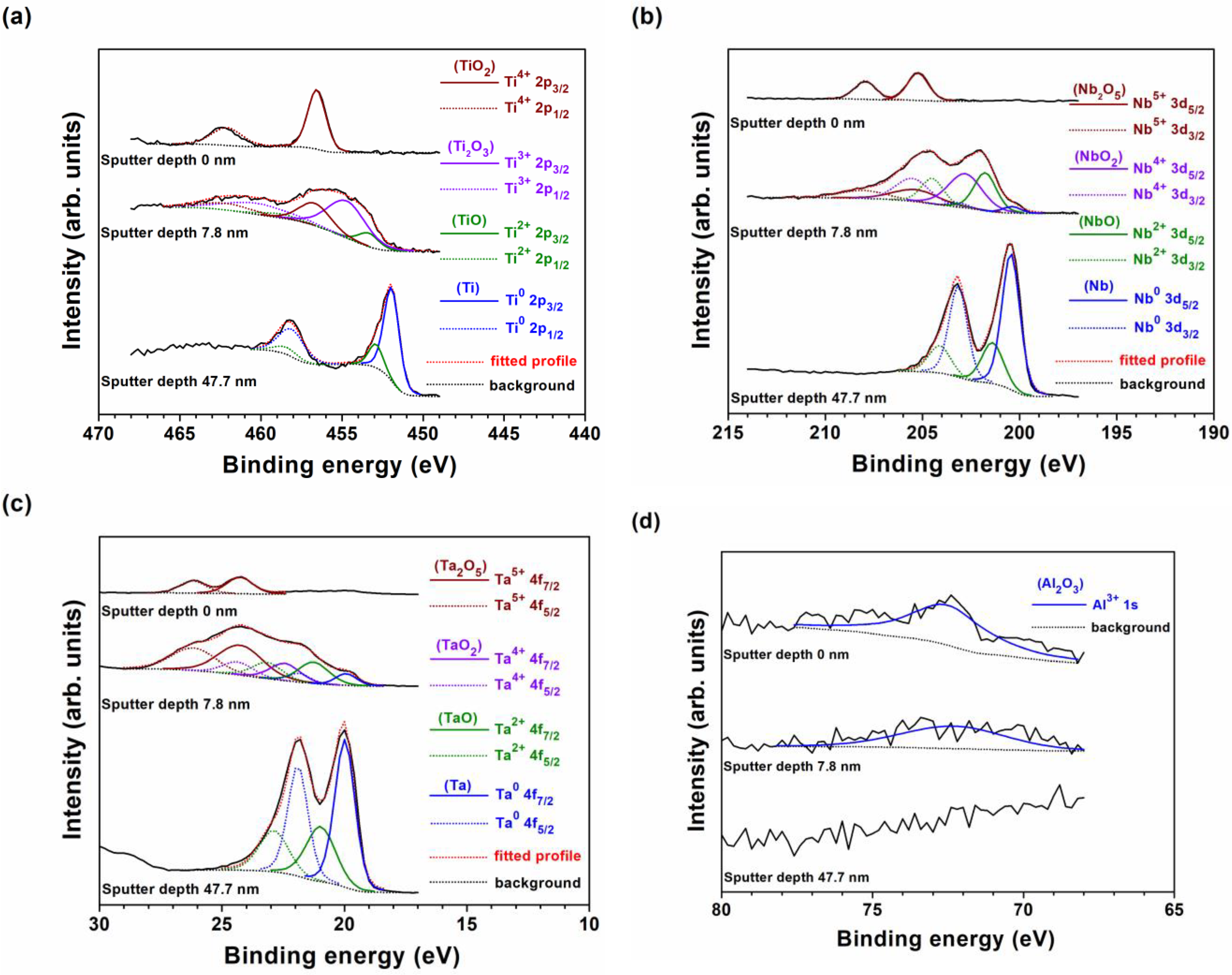
3.1. Chemical Compositions and Phase Structures
3.2. Mechanical Properties
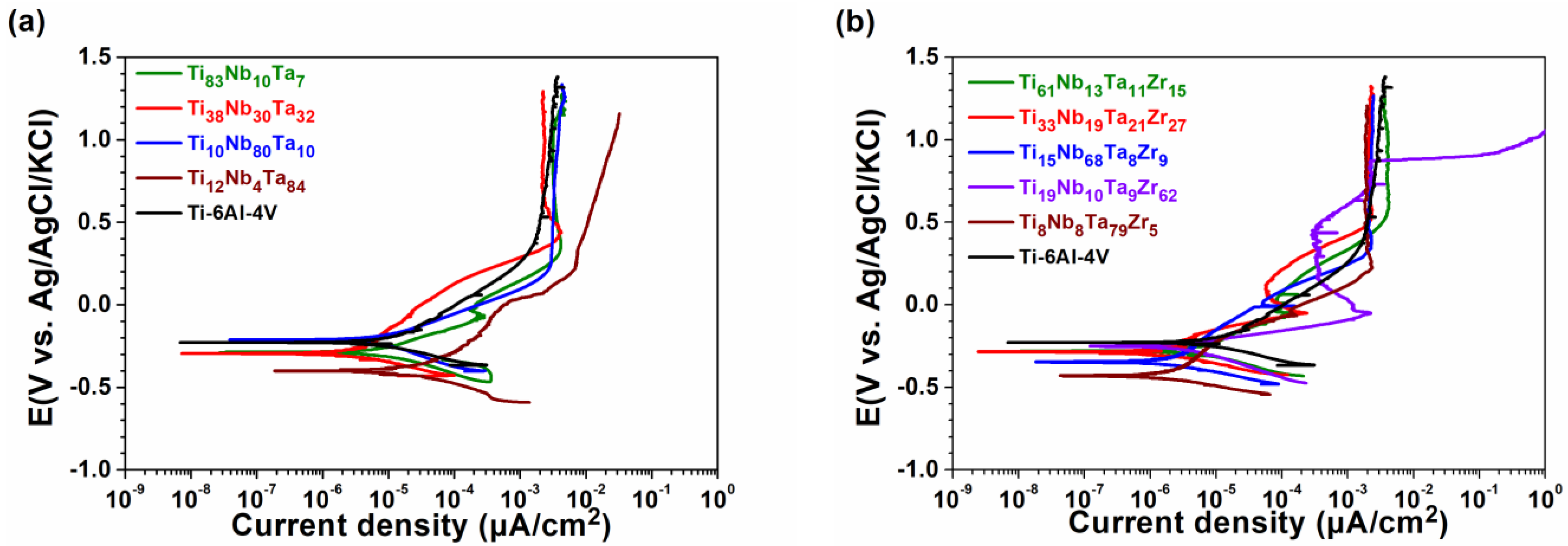
3.3. Corrosion Resistance
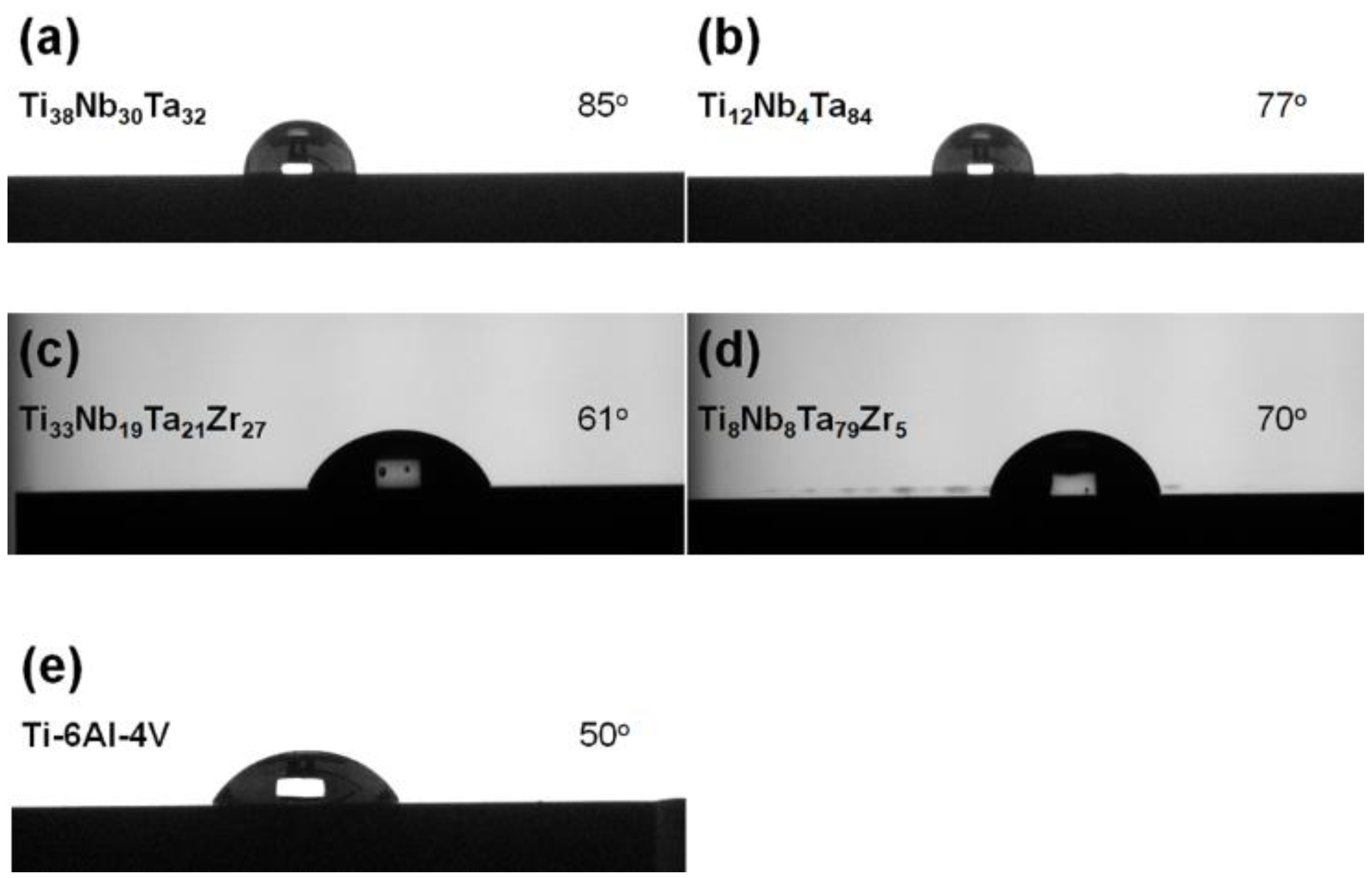
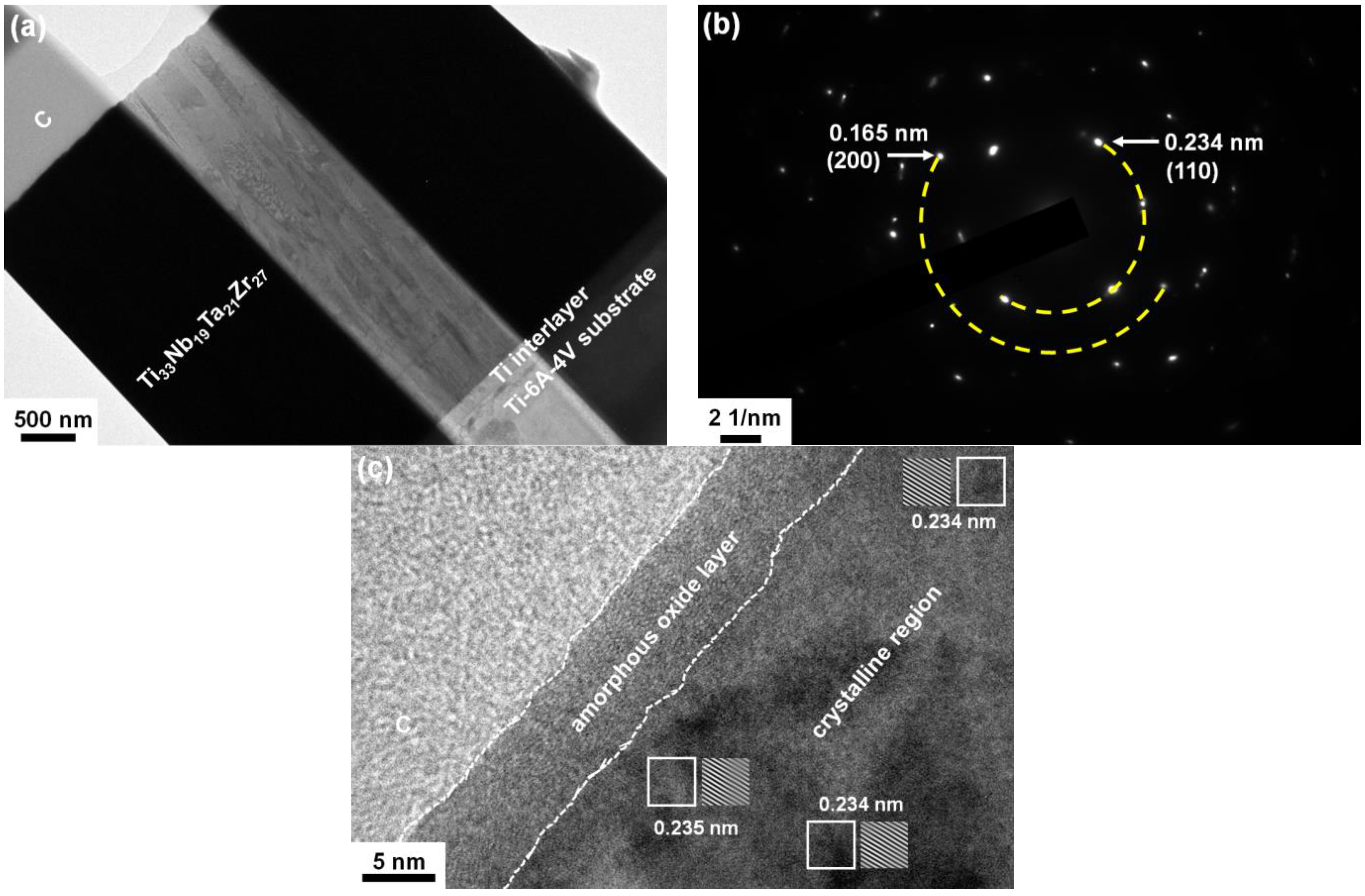
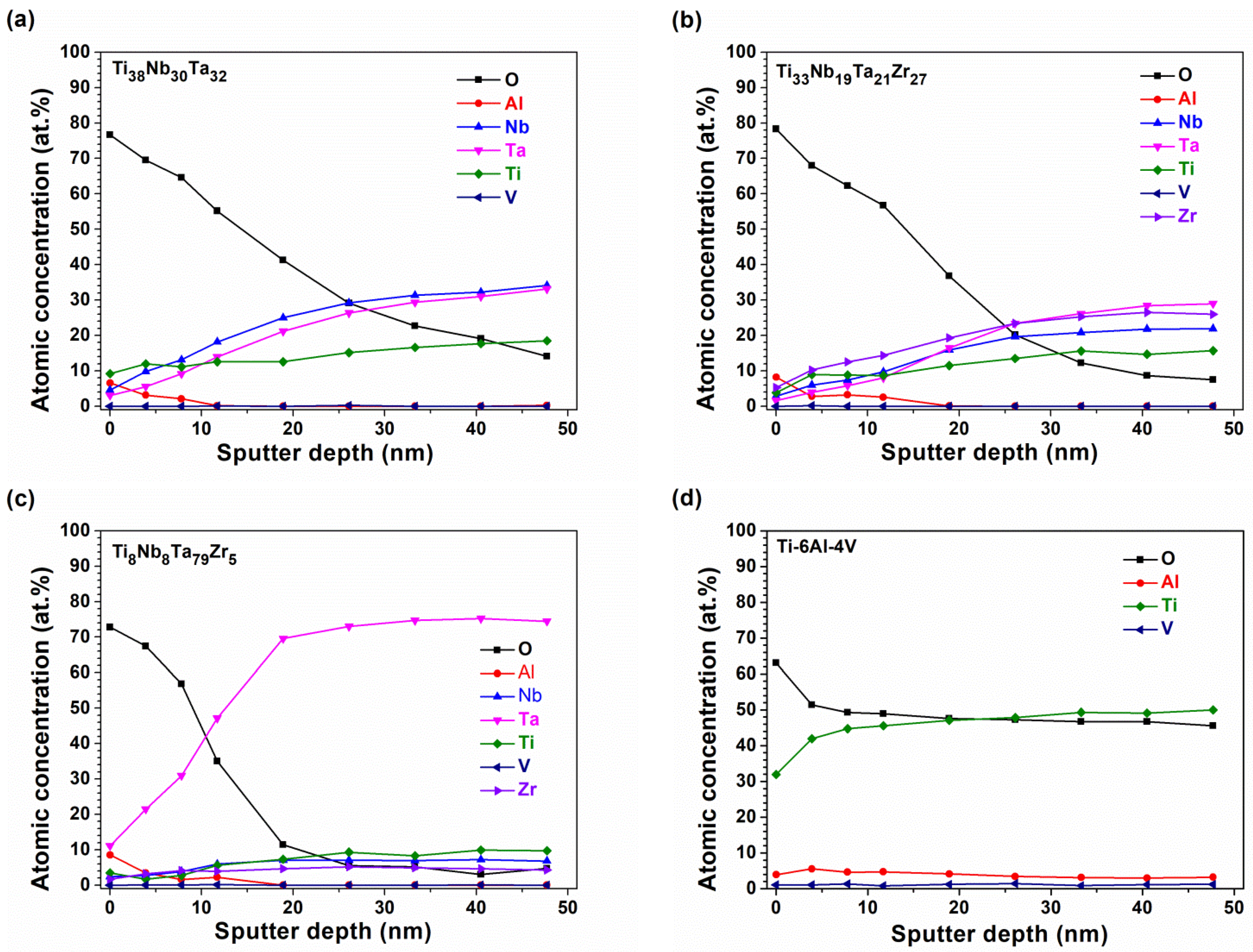
3.4. Elution Test and Formation of Surficial Oxide Layers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodil, S.E.; Olivares, R.; Arzate, H.; Muhl, S. Properties of carbon films and their biocompatibility using in-vitro tests. Diam. Relat. Mater. 2003, 12, 931–937. [Google Scholar] [CrossRef]
- Sharma, A.; Oh, M.C.; Kim, J.-T.; Srivastava, A.K.; Ahn, B. Investigation of electrochemical corrosion behavior of additive manufactured Ti–6Al–4V alloy for medical implants in different electrolytes. J. Alloys Compd. 2020, 830, 154620. [Google Scholar] [CrossRef]
- Hussein, A.H.; Gepreel, M.A.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti–Nb–Ta base alloys. Mater. Sci. Eng. C 2016, 61, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Van Lenthe, G.H.; de Waal Malefijt, M.C.; Huiskes, R. Stress shielding after total knee replacement may cause bone resorption in the distal femur. J. Bone Joint Surg. 1997, 79, 117–122. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huiskes, R.; Weinans, H.; Rietbergen, B.V. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Glassman, A.H.; Bobyn, J.D.; Tanzer, M. New femoral designs: Do they influence stress shielding? Clin. Orthop. Relat. Res. 2006, 453, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elbow Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef]
- Vilhena, L.M.; Shumayal, A.; Ramalho, A.; Ferreira, J.A.M. Tribocorrosion behaviour of Ti6Al4V produced by selective laser melting for dental implants. Lubricants 2020, 8, 22. [Google Scholar] [CrossRef]
- Fellah, M.; Labaïz, M.; Assala, O.; Dekhil, L.; Taleb, A.; Rezag, H.; Iost, A. Tribological behavior of Ti-6Al-4V and Ti-6Al-7Nb alloys for total hip prosthesis. Adv. Tribol. 2014, 2014, 451387. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Accumulation of aluminum in lamellar bone after implantation of titanium plates, Ti–6Al–4V screws, hydroxyapatite granules. Biomaterials 2004, 25, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sander, B.; Friák, M.; Ma, D.; Neugebauer, J. Theory-guided bottom-up design of β-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Niinomi, M.; Kuroda, D.; Fukunaga, K.; Morinaga, M.; Kato, Y.; Yashiro, T.; Suzuki, A. Corrosion wear fracture of new β type biomedical titanium alloys. Mater. Sci. Eng. A 1999, 263, 193–199. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chuang, W.S.; Huang, J.C.; Wang, X.; Chou, H.S.; Lai, Y.J.; Lin, P.H. On the bio-corrosion and biocompatibility of TiTaNb medium entropy alloy films. Appl. Surf. Sci. 2020, 508, 145307. [Google Scholar] [CrossRef]
- Lai, B.-W.; Chang, Y.-Y.; Shieh, T.-M.; Huang, H.-L. Biocompatibility and microstructure-based stress analyses of TiNbZrTa composite films. Materials 2022, 15, 29. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti–6Al–4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Alamdari, A.A.; Unal, U.; Motallebzadeh, A. In vitro biocompatibility evaluation of Ti1.5ZrTa0.5Nb0.5Hf0.5 refractory high-entropy alloy film for orthopedic implants: Microstructural, mechanical properties and corrosion behavior. J. Alloys Compd. 2021, 883, 160786. [Google Scholar] [CrossRef]
- Chen, Y.I.; Chen, C.Y.; Chang, L.C.; Kai, W. Characterization of cosputtered NbTaMoW films. J. Mater. Res. Technol. 2021, 15, 1090–1099. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible TiZrHfNbTa high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Lu, X.; Xia, Q.; Lei, B.; Yan, Y.; Shang, C.J. Face-centered-cubic titanium - A new crystal structure of Ti in a Ti- 8Mo-6Fe alloy. J. Alloys Compd. 2018, 748, 943–952. [Google Scholar] [CrossRef]
- Hua, K.; Zhang, Y.; Kou, H.; Li, J.; Gan, W.; Fundenberger, J.J.; Esling, C. Composite structure of α phase in metastable β Ti alloys induced by lattice strain during β to α phase transformation. Acta Mater. 2017, 132, 307–326. [Google Scholar] [CrossRef]
- Miura, K.; Yamada, N.; Hanada, S.; Jung, T.-K.; Itoi, E. The bone tissue compatibility of a new Ti–Nb–Sn alloy with a low Young’s modulus. Acta Biomater. 2011, 7, 2320–2326. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Cong, Z.; Li, Y.; Song, Z.; Chen, J. Effects of deposition temperature on the nanomechanical properties of refractory high entropy TaNbHfZr films. J. Alloys Compd. 2019, 797, 1025–1030. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M.; Bondar, O.V.; Postolnyi, B.O.; Zaleski, K.; Coy, E.; Jurga, S.; Lisovenko, M.O.; Konarski, P.; Rebouta, L.; et al. Superhard CrN/MoN coatings with multilayer architecture. Mater. Des. 2018, 153, 47–59. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Stern, M. A method for determining corrosion rates from linear polarization data. Corrosion 1958, 14, 60–64. [Google Scholar] [CrossRef]
- Ji, P.F.; Li, B.; Chen, B.H.; Wang, F.; Ma, W.; Zhang, X.Y.; Ma, M.Z.; Liu, R.P. Effect of Nb addition on the stability and biological corrosion resistance of Ti-Zr alloy passivation films. Corros. Sci. 2020, 170, 108696. [Google Scholar] [CrossRef]
- Alves, V.A.; Reis, R.Q.; Santos, I.C.B.; Souza, D.G.; de F. Gonçalves, T.; Pereira-da-Silva, M.A.; Rossi, A.; da Silva, L.A. In situ impedance spectroscopy study of the electrochemical corrosion of Ti and Ti–6Al–4V in simulated body fluid at 25 °C and 37 °C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Norby, T.; Middleton, H. Investigation of pitting resistance of titanium based on a modified point defect model. Corros. Sci. 2011, 53, 815–821. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Crouse, P.L.; Schmidth, M.J.J.; Li, L.; Garrod, D. Laser surface micro-texturing of Ti–6Al–4V substrates for improved cell integration. Appl. Surf. Sci. 2007, 253, 7738–7743. [Google Scholar] [CrossRef]
- Razazzadeh, A.; Atapour, M.; Enayati, M.H. Corrosion characteristics of TiNbMoMnFe high entropy thin film deposited on AISI316L for biomedical applications. Met. Mater. Int. 2021, 27, 2341–2352. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Eds.; Physical Electronics: Chanhassen, MN, USA, 1995. [Google Scholar]
- Krishna, D.N.G.; George, R.P.; Philip, J. Determination of nanoscale titanium oxide thin film phase composition using X-ray photoelectron spectroscopy valence band analysis. Thin Solid Films 2019, 681, 58–68. [Google Scholar] [CrossRef]
- Chi, M.; Sun, X.; Lozano-Blanco, G.; Tatarchuk, B.J. XPS and FTIR investigations of the transient photocatalytic decomposition of surface carbon contaminants from anatase TiO2 in UHV starved water/oxygen environments. Appl. Surf. Sci. 2021, 570, 151147. [Google Scholar] [CrossRef]

| Sample | Sputter Power (W) | Chemical Composition (at.%) | ΔSmix 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTi | PNb | PTa | PZr | Ti | Nb | Ta | Zr | O | (R) | |
| Ti83Nb10Ta7 | 200 | 20 | 10 | — | 76.3 ± 0.5 | 9.3 ± 0.1 | 6.9 ± 0.0 | — | 7.5 ± 0.4 | 0.58 |
| Ti38Nb30Ta32 | 180 | 80 | 70 | — | 36.4 ± 0.1 | 28.5 ± 0.1 | 30.1 ± 0.1 | — | 5.0 ± 0.1 | 1.09 |
| Ti10Nb80Ta10 | 30 | 200 | 20 | — | 9.2 ± 0.1 | 76.2 ± 0.3 | 10.0 ± 0.0 | — | 4.6 ± 0.4 | 0.64 |
| Ti12Nb4Ta84 | 70 | 20 | 200 | — | 10.9 ± 0.2 | 4.2 ± 0.6 | 78.2 ± 1.3 | — | 6.7 ± 2.0 | 0.54 |
| Ti61Nb13Ta11Zr15 | 200 | 30 | 20 | 30 | 61.3 ± 0.5 | 12.6 ± 0.2 | 11.3 ± 0.3 | 14.8 ± 0.0 | — | 1.09 |
| Ti33Nb19Ta21Zr27 | 180 | 80 | 70 | 140 | 32.8 ± 0.2 | 18.9 ± 0.1 | 21.0 ± 0.3 | 27.3 ± 0.1 | — | 1.36 |
| Ti15Nb68Ta8Zr9 | 70 | 200 | 20 | 30 | 14.5 ± 0.3 | 68.2 ± 0.3 | 8.1 ± 0.4 | 9.2 ± 0.2 | — | 0.96 |
| Ti19Nb10Ta9Zr62 | 70 | 30 | 200 | 30 | 19.1 ± 0.2 | 10.5 ± 0.1 | 8.5 ± 0.0 | 61.9 ± 0.1 | — | 1.06 |
| Ti8Nb8Ta79Zr5 | 70 | 30 | 20 | 200 | 7.6 ± 0.2 | 8.1 ± 0.1 | 78.9 ± 0.6 | 5.4 ± 0.6 | — | 0.74 |
| Sample | H 1 (GPa) | E 2 (GPa) | H/E | H3/E2 (GPa) |
|---|---|---|---|---|
| Ti-6Al-4V | 4.9 ± 0.4 | 124 ± 8 | 0.040 | 0.008 |
| Ti83Nb10Ta7 | 2.7 ± 0.3 | 110 ± 7 | 0.025 | 0.002 |
| Ti38Nb30Ta32 | 5.0 ± 0.2 | 150 ± 10 | 0.033 | 0.006 |
| Ti10Nb80Ta10 | 4.2 ± 0.5 | 142 ± 8 | 0.030 | 0.004 |
| Ti12Nb4Ta84 | 7.7 ± 0.3 | 216 ± 6 | 0.036 | 0.010 |
| Ti61Nb13Ta11Zr15 | 3.9 ± 1.0 | 108 ± 17 | 0.036 | 0.005 |
| Ti33Nb19Ta21Zr27 | 5.9 ± 0.9 | 163 ± 12 | 0.036 | 0.008 |
| Ti15Nb68Ta8Zr9 | 9.6 ± 1.1 | 165 ± 14 | 0.058 | 0.032 |
| Ti19Nb10Ta9Zr62 | 7.0 ± 0.3 | 115 ± 4 | 0.061 | 0.026 |
| Ti8Nb8Ta79Zr5 | 12.1 ± 1.0 | 226 ± 9 | 0.054 | 0.035 |
| Sample | Ecorr (mV) | Icorr (μA/cm2) | Rp (KΩcm2) | Rp Ratio |
|---|---|---|---|---|
| Ti-6Al-4V | −250 | 0.004 | 4.5 × 103 | 1.0 |
| Ti83Nb10Ta7 | −268 | 0.006 | 4.1 × 103 | 0.9 |
| Ti38Nb30Ta32 | −315 | 0.002 | 8.8 × 103 | 2.0 |
| Ti10Nb80Ta10 | −214 | 0.003 | 7.6 × 103 | 1.7 |
| Ti12Nb4Ta84 | −375 | 0.001 | 1.5 × 104 | 3.3 |
| Ti61Nb13Ta11Zr15 | −308 | 0.005 | 5.3 × 103 | 1.2 |
| Ti33Nb19Ta21Zr27 | −281 | 0.001 | 2.9 × 104 | 6.4 |
| Ti15Nb68Ta8Zr9 | −365 | 0.001 | 2.1 × 104 | 4.7 |
| Ti19Nb10Ta9Zr62 | −247 | 0.002 | 7.7 × 103 | 1.7 |
| Ti8Nb8Ta79Zr5 | −441 | 0.001 | 1.9 × 104 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-I.; Chen, Y.-J.; Lai, C.-Y.; Chang, L.-C. Mechanical and Anticorrosive Properties of TiNbTa and TiNbTaZr Films on Ti-6Al-4V Alloy. Coatings 2022, 12, 1985. https://doi.org/10.3390/coatings12121985
Chen Y-I, Chen Y-J, Lai C-Y, Chang L-C. Mechanical and Anticorrosive Properties of TiNbTa and TiNbTaZr Films on Ti-6Al-4V Alloy. Coatings. 2022; 12(12):1985. https://doi.org/10.3390/coatings12121985
Chicago/Turabian StyleChen, Yung-I, Yi-Jyun Chen, Cheng-Yi Lai, and Li-Chun Chang. 2022. "Mechanical and Anticorrosive Properties of TiNbTa and TiNbTaZr Films on Ti-6Al-4V Alloy" Coatings 12, no. 12: 1985. https://doi.org/10.3390/coatings12121985





