Influence of Annealing on the Microstructure and Mechanical Properties of Ni-W/Boron Composite Coatings
Abstract
:1. Introduction
2. Materials and Methods
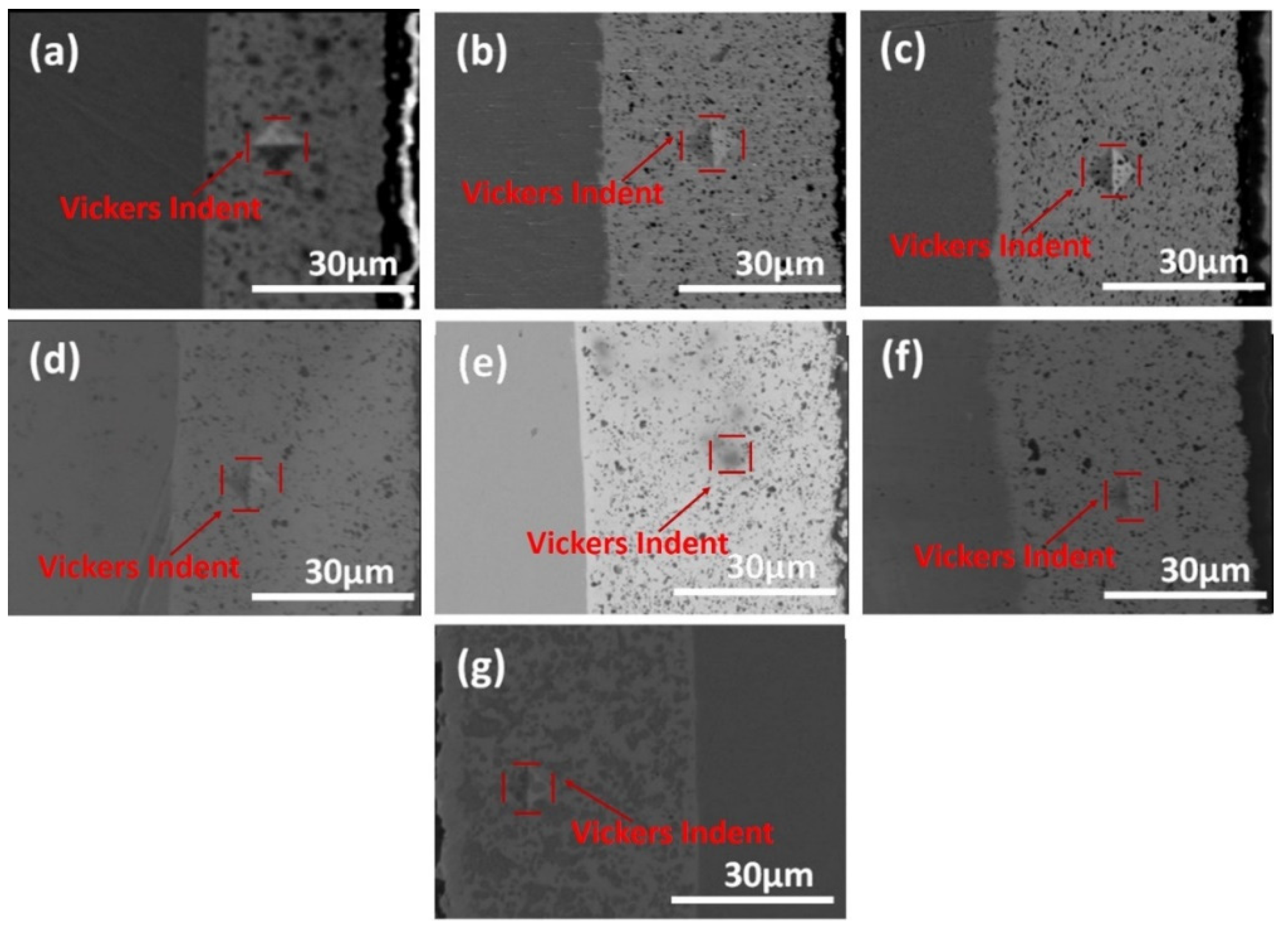
3. Results
4. Conclusions
- Ni-W/B composite coatings can be deposited by adding the boron particles into the Ni-W electrodeposition electrolyte. The SEM results demonstrate that the boron particles can be uniformly co-deposited in the Ni-W matrix;
- With the heat treatment, new phases can be formed, especially at heat treatment temperatures beyond 400 °C, which can be determined by the XRD results;
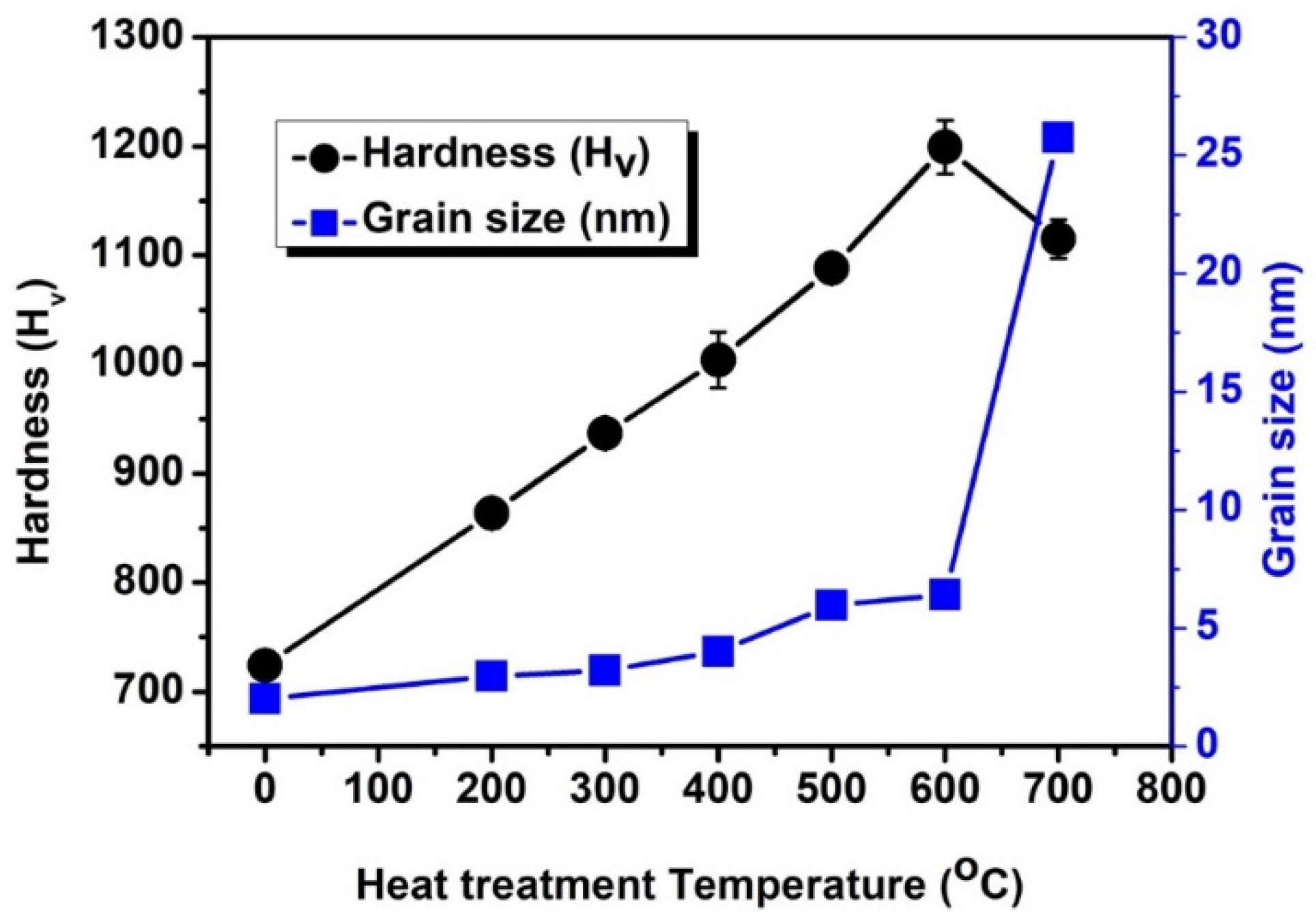
- The grain size of the deposits is smaller than 10 nm with the heat treatment temperatures lower than 600 °C, which increases with the heat treatment temperature increasing. While the sample treated at the temperature of 700 °C, the grain size increases up to 25.8 nm;
- When the heat treatment temperature is lower than 600 °C, the hardness increases because of the formation of the Ni4W phase and the inverse Hall–Petch relationship. While the grain coarsening causes the hardness of the deposits to decrease at the temperature of 700 °C;
- The heat treatment of the Ni-W/B samples also exhibit a superior wear performance. The wear rate is significantly lower for the Ni-W/B samples heat treated at 600 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boonyongmaneerat, Y.; Saengkiettiyut, K.; Saenapitak, S.; Sangsuk, S. Pulse co-electrodeposition and characterization of NiW–WC composite coatings. J. Alloy. Compd. 2010, 506, 151–154. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Aliofkhazraei, M.; Lotfi, N. Review of Nickel-Based Electrodeposited Tribo-Coatings. Trans. Indian Inst. Met. 2018, 71, 257–295. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rouhaghdam, A.R.S.; Torabinejad, V. Electrodeposition of Ni–W–Al2O3 nanocomposite coating with functionally graded microstructure. J. Alloy. Compd. 2016, 666, 217–226. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Torabinejad, V. Electrodeposition of multilayer Ni–W and Ni–W–alumina nanocomposite coatings. Surf. Eng. 2017, 33, 327–336. [Google Scholar] [CrossRef]
- Schuh, C.A.; Nieh, T.G.; Iwasaki, H. The effect of solid solution W additions on the mechanical properties of nanocrystalline Ni. Acta Mater. 2003, 51, 431–443. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Ganesh Sundara Raman, S.; Seshadri, S.K. Synthesis and evaluation of hardness and sliding wear resistance of electrodeposited nanocrystalline Ni–W alloys. Mater. Sci. Eng. A 2006, 418, 303–311. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Zhang, W.; Huan, Y. Preparation of Ni-W/SiC nanocomposite coatings by electrochemical deposition. J. Alloy. Compd. 2017, 702, 38–50. [Google Scholar] [CrossRef]
- Hou, K.-H.; Han, T.; Sheu, H.-H.; Ger, M.-D. Preparation and wear resistance of electrodeposited Ni–W/diamond composite coatings. Appl. Surf. Sci. 2014, 308, 372–379. [Google Scholar] [CrossRef]
- Boonyongmaneerat, Y.; Saengkiettiyut, K.; Saenapitak, S.; Sangsuk, S. Effects of WC addition on structure and hardness of electrodeposited Ni–W. Surf. Coat. Technol. 2009, 203, 3590–3594. [Google Scholar] [CrossRef]
- Sangeetha, S.; Kalaignan, G.P. Tribological and electrochemical corrosion behavior of Ni–W/BN (hexagonal) nano-composite coatings. Ceram. Int. 2015, 41, 10415–10424. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Umporntheep, K.; Auejitthavorn, V.; Li, R.; Wangyao, P.; Boonyongmaneerat, Y.; Limpanart, S.; Ma, M.; Liu, R. Electrodeposition and Mechanical Properties of Ni-W Matrix Composite Coatings with Embedded Amorphous Boron Particles. Int. J. Electrochem. Sci. 2016, 11, 9529–9541. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Das, M.K.; Hao, R.; Zhong, H.; Thueploy, A.; Limpanart, S.; Boonyongmaneerat, Y.; Ma, M.; Liu, R. Co-electrodeposition of hard Ni-W/diamond nanocomposite coatings. Sci. Rep. 2016, 6, 22285. [Google Scholar] [CrossRef] [Green Version]
- Das, M.K.; Li, R.; Qin, J.; Zhang, X.; Das, K.; Thueploy, A.; Limpanart, S.; Boonyongmaneerat, Y.; Ma, M.; Liu, R. Effect of electrodeposition conditions on structure and mechanical properties of Ni-W/diamond composite coatings. Surf. Coat. Technol. 2017, 309, 337–343. [Google Scholar] [CrossRef]
- Su, C.; Sa, Z.; Liu, Y.; Zhao, L.; Wu, F.; Bai, W. Excellent Properties of Ni-15 wt.% W Alloy Electrodeposited from a Low-Temperature Pyrophosphate System. Coatings 2021, 11, 1262. [Google Scholar] [CrossRef]
- Wang, H.-T.; Sheu, H.-H.; Ger, M.-D.; Hou, K.-H. The effect of heat treatment on the microstructure and mechanical properties of electrodeposited nanocrystalline Ni–W/diamond composite coatings. Surf. Coat. Technol. 2014, 259 Part B, 268–273. [Google Scholar] [CrossRef]
- Hou, K.-H.; Jeng, M.-C.; Ger, M.-D. The heat treatment effects on the structure and wear behavior of pulse electroforming Ni–P alloy coatings. J. Alloy. Compd. 2007, 437, 289–297. [Google Scholar] [CrossRef]
- Zhang, C.; Si, W.; Wang, Y.; Dai, S.; Shu, D. Investigations on the Influence of Annealing on Microstructure and Mechanical Properties of Electrodeposited Ni-Mo and Ni-Mo-W Alloy Coatings. Coatings 2021, 11, 1428. [Google Scholar] [CrossRef]
- Martín, A.; Rodríguez, J.; Llorca, J. Temperature effects on the wear behavior of particulate reinforced Al-based composites. Wear 1999, 225–229, 615–620. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- D’Agostino, A.T. Determination of thin metal film thickness by X-ray diffractometry using the Scherrer equation, atomic absorption analysis and transmission/reflection visible spectroscopy. Anal. Chim. Acta 1992, 262, 269–275. [Google Scholar] [CrossRef]
- Dyck, T.; Bund, A. An adaption of the Archard equation for electrical contacts with thin coatings. Tribol. Int. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Oriňáková, R.; Oriňák, A.; Kupková, M.; Sabalová, M.; Fedorková, A.S.; Kabátová, M.; Kaľavský, F.; Sedlaříková, M. Effect of heat treatment on the corrosion and mechanical properties of electrolytical composite Ni-B coatings. Int. J. Electrochem. Sci. 2014, 9, 4268–4286. [Google Scholar]
- Benea, L.; Bonora, P.L.; Borello, A.; Martelli, S.; Wenger, F.; Ponthiaux, P.; Galland, J. Preparation and investigation of nanostructured SiC–nickel layers by electrodeposition. Solid State Ion. 2002, 151, 89–95. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Ashrafi, A.; Golgoon, A.; Roozbehani, B. Effect of pulse plating parameters on the structure and properties of electrodeposited NiMo films. Mater. Chem. Phys. 2016, 175, 215–222. [Google Scholar] [CrossRef]
- Bund, A.; Thiemig, D. Influence of bath composition and pH on the electrocodeposition of alumina nanoparticles and nickel. Surf. Coat. Technol. 2007, 201, 7092–7099. [Google Scholar] [CrossRef]
- Abdel Aal, A. Hard and corrosion resistant nanocomposite coating for Al alloy. Mater. Sci. Eng. A 2008, 474, 181–187. [Google Scholar] [CrossRef]
- Kovacı, H.; Yetim, A.F.; Baran, Ö.; Çelik, A. Tribological behavior of DLC films and duplex ceramic coatings under different sliding conditions. Ceram Int. 2018, 44, 7151–7158. [Google Scholar] [CrossRef]





| Plating Bath Composition | Electrodeposition Parameters | ||
|---|---|---|---|
| NiSO4.6H2O | 18 g/L | Temperature | 75 °C |
| Na2WO4 | 53 g/L | Current density | 0.1 A/cm2 |
| Na3C6H5O7 | 168 g/L | Stirring rate | 100 rpm |
| NH4Cl | 31 g/L | pH | 8.9 |
| NaBr | 18 g/L | ||
| Boron powder | 5 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, M.K.; Pinitpuwadol, W.; Wonlopsiri, K.; Wangyao, P.; Qin, J. Influence of Annealing on the Microstructure and Mechanical Properties of Ni-W/Boron Composite Coatings. Coatings 2022, 12, 1992. https://doi.org/10.3390/coatings12121992
Das MK, Pinitpuwadol W, Wonlopsiri K, Wangyao P, Qin J. Influence of Annealing on the Microstructure and Mechanical Properties of Ni-W/Boron Composite Coatings. Coatings. 2022; 12(12):1992. https://doi.org/10.3390/coatings12121992
Chicago/Turabian StyleDas, Malay Kumar, Waralee Pinitpuwadol, Kohpong Wonlopsiri, Panyawat Wangyao, and Jiaqian Qin. 2022. "Influence of Annealing on the Microstructure and Mechanical Properties of Ni-W/Boron Composite Coatings" Coatings 12, no. 12: 1992. https://doi.org/10.3390/coatings12121992




