The Influence of Pickling Treatment Parameters on the Surface State and Pre-Passivation Behavior of Super 13Cr Martensitic Stainless Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. The Pre-Treatment in Acid Solution and Pre-Passivation
2.3. Electrochemical Test
2.4. Observation of the Microstructure
3. Results
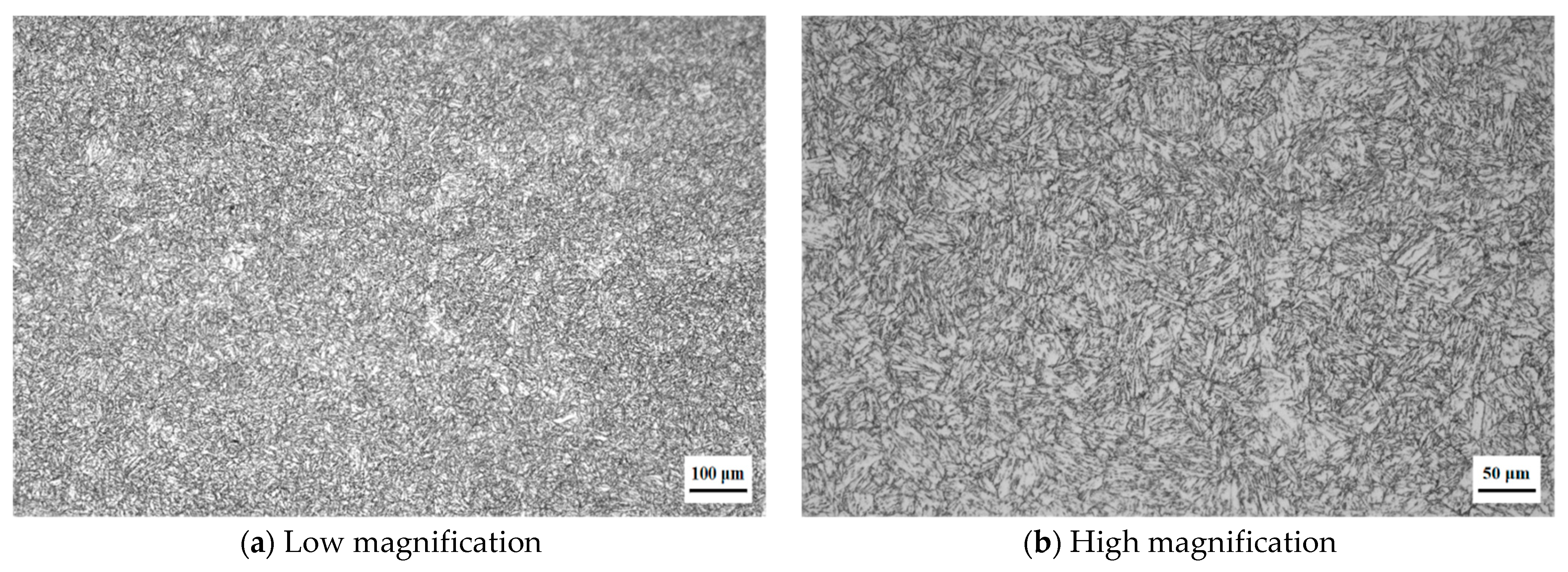
3.1. Microstructure of Super 13Cr
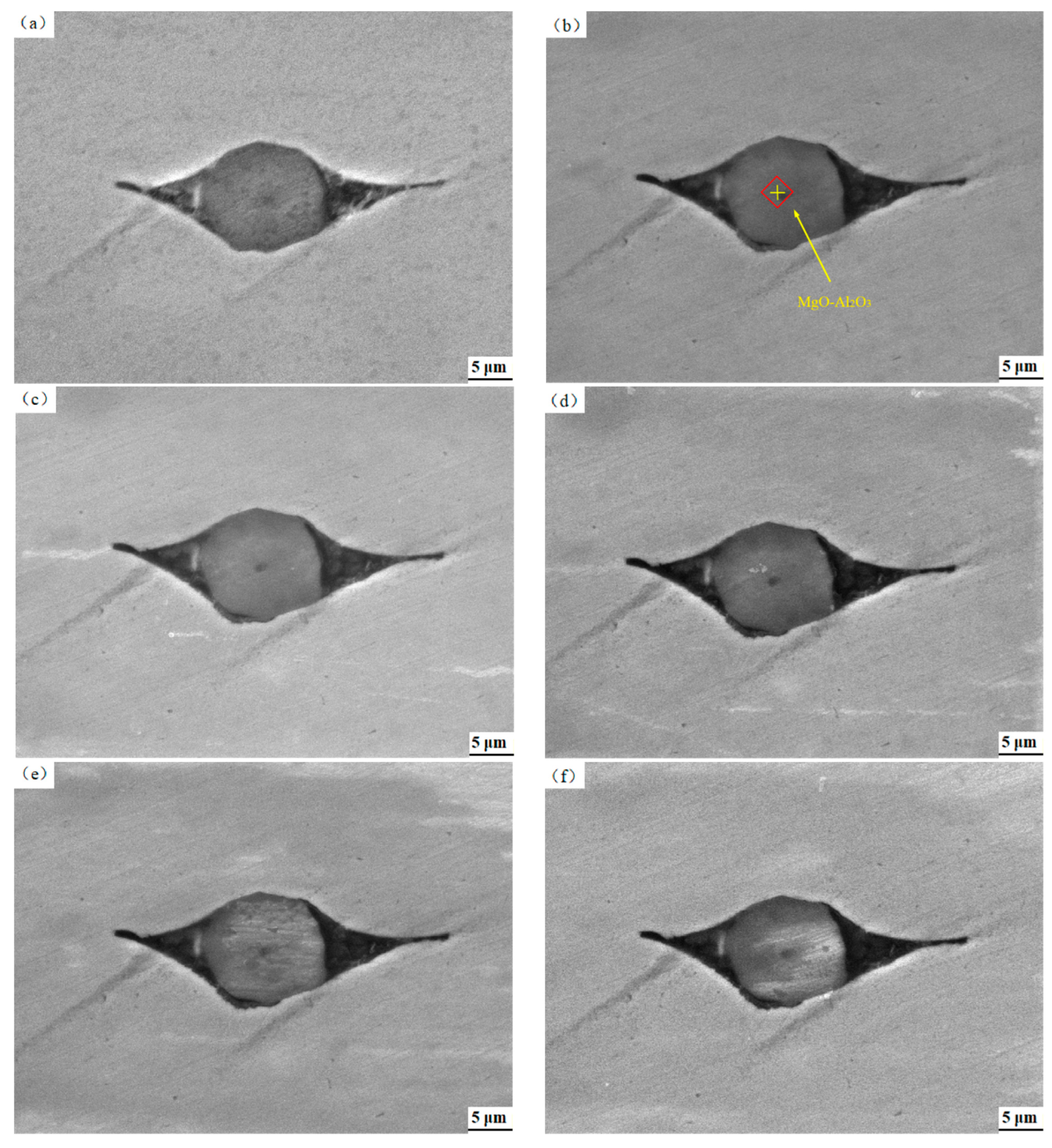
3.2. Surface Characteristic Depended on the Acidification Duration
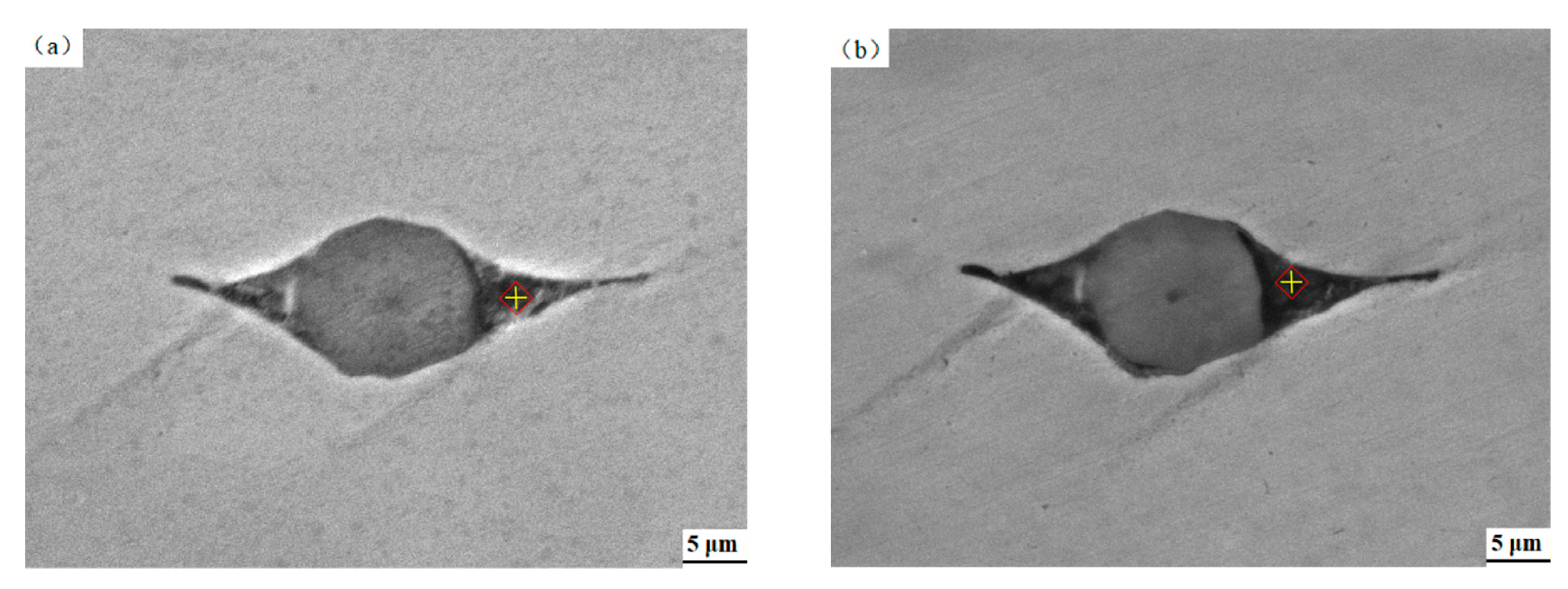
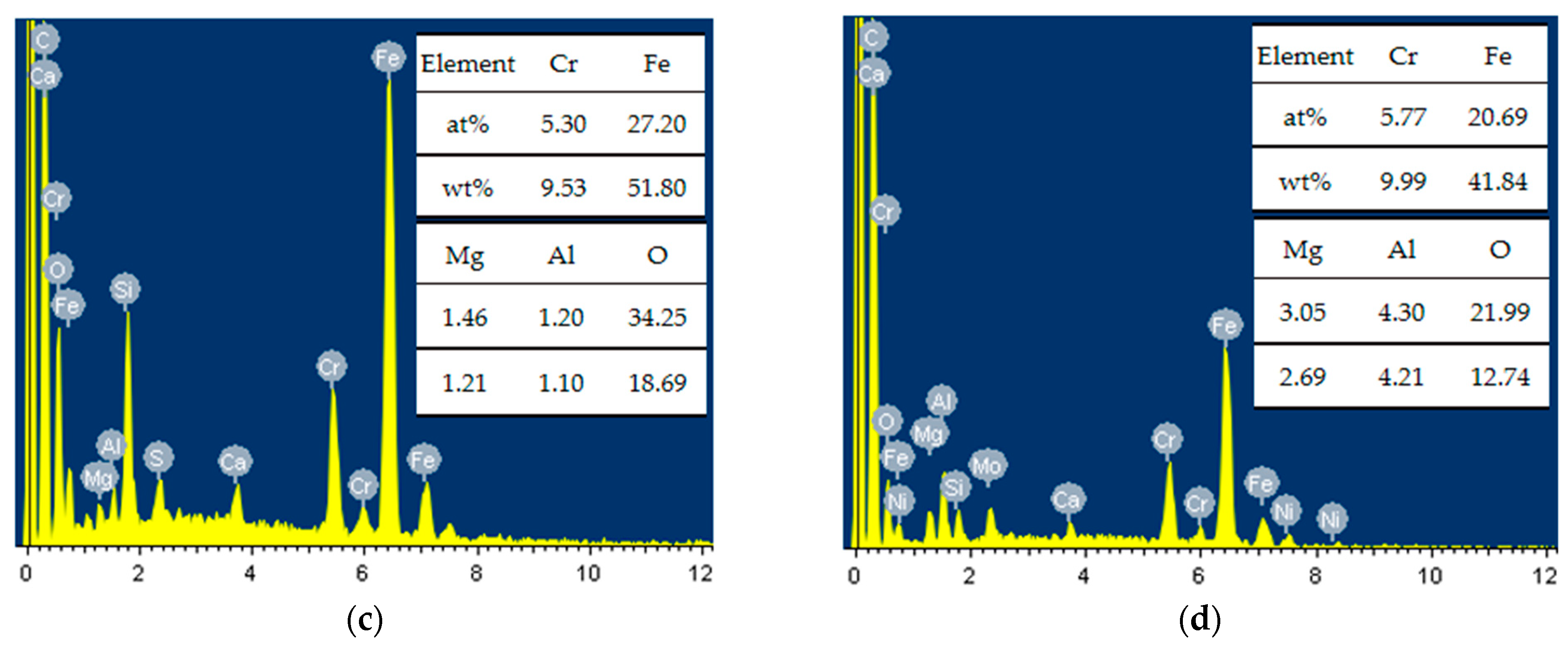
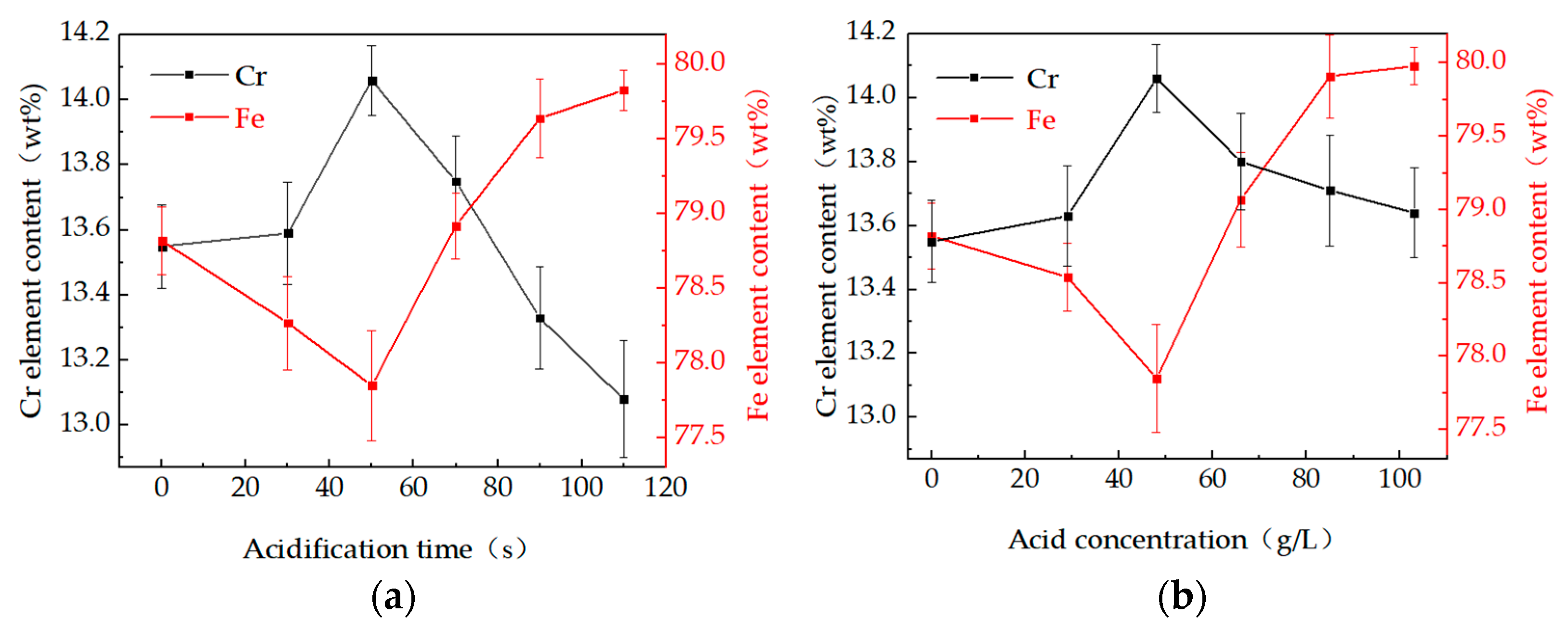
3.3. Effect of Pickling Treatment on Super 13Cr Surface Chemical Composition
3.4. Electrochemical Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, G.Y.; Li, Y.Y.; Hou, B.S.; Zhang, Q.H.; Zhang, G.A. Corrosion behavior of 13Cr stainless steel under stress and crevice in high pressure CO2/O2 environment. J. Mater. Sci. Technol. 2021, 88, 79–89. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Tan, S.Z.; Yu, Z.M.; Dong, B.J.; Yi, Y.G.; Tian, G.; Yu, H.Y.; Shi, S.Z. Study on CO2 corrosion behavior of super 13Cr steel in high temperature steam environment. Petroleum 2020, 6, 106–113. [Google Scholar] [CrossRef]
- Li, K.Y.; Zeng, Y.M.; Luo, J.L. Influence of H2S on the general corrosion and sulfide stress cracking of pipelines steels for supercritical CO2 transportation. Corros. Sci. 2021, 190, 109639. [Google Scholar] [CrossRef]
- Akpanyung, K.V.; Loto, R.T. Pitting corrosion evaluation: A review. J. Phys. Conf. Ser. 2019, 1378, 022088. [Google Scholar] [CrossRef]
- Williams, D.E.; Zhu, Y.Y. Explanation for Initiation of Pitting Corrosion of Stainless Steels at Sulfide Inclusions. J. Electrochem. Soc. 2000, 147, 1763–1766. [Google Scholar] [CrossRef]
- Pickering, H.W.; Frankenthal, R.P. On the Mechanism of Localized Corrosion of Iron and Stainless Steel: I. Electrochemical Studies. J. Electrochem. Soc. 1972, 119, 1297–1304. [Google Scholar] [CrossRef]
- Li, X.P.; Zhao, Y.; Qi, W.L.; Xie, J.X.; Wang, J.L.; Liu, B.; Zeng, G.X.; Zhang, T.; Wang, F.H. Effect of extremely aggressive environment on the nature of corrosion scales of HP-13Cr stainless steel. Appl. Surf. Sci. 2019, 469, 146–161. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.S.; Li, J.; Liu, W.Y.; Liu, M.Q.; Gao, W.X.; Shi, T.H. Stress corrosion crack evaluation of super 13Cr tubing in high-temperature and high-pressure gas wells. Eng. Fail. Anal. 2019, 95, 263–272. [Google Scholar] [CrossRef]
- Lei, X.W.; Feng, Y.R.; Zhang, J.X.; Fu, A.Q.; Yin, C.X.; Digby, D.M. Impact of reversed austenite on the pitting corrosion behavior of super 13Cr martensitic stainless steel. Electrochimica Acta 2016, 191, 640–650. [Google Scholar] [CrossRef]
- Lei, X.W.; Wang, H.Y.; Wang, N.; Ren, D.Z.; Fu, A.Q.; Yin, C.X.; Zhang, J.P.; Feng, Y.R. Passivity of martensitic stainless steel in borate buffer solution: Influence of sulfide ion. Appl. Surf. Sci. 2019, 478, 255–265. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, H.B.; Yuan, R.; Zhang, P.C.; Zhang, Z.H.; Song, S. Effect of Temperature and Composition on Corrosion Behavior of Medium Cr Steel in a Salt Solution Containing CO2. Corrosion 2021, 77, 655–667. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, W.; Fan, Y.M.; Zhang, T.Y.; Dong, B.J.; Chen, L.J.; Wang, Y.B. Influence of microstructure on the corrosion behavior of super 13Cr martensitic stainless steel under heat treatment. Mater. Charact. 2021, 175, 111066. [Google Scholar] [CrossRef]
- Kultamaa, M.; Mönkkönen, K.; Saarinen, J.J.; Suvanto, M. Corrosion Protection of Injection Molded Porous 440C Stainless Steel by Electroplated Zinc Coating. Coatings 2021, 11, 949. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, W.; Dong, B.J.; Wang, Y.T.; Fan, Y.M.; Zhang, T.Y.; Banthukul, W.T. Effects of Microstructure and Material Composition on the Formation Kinetics of Passive Film and Pitting Behavior of Super 13Cr Stainless Steel. Metall. Mater. Trans. A 2021, 52, 1985–1998. [Google Scholar] [CrossRef]
- Sun, Y.T.; Tan, X.; Lei, L.L.; Li, J. Revisiting the effect of molybdenum on pitting resistance of stainless steels. Tungsten 2021, 3, 329–333. [Google Scholar] [CrossRef]
- Jang, H.J.; Kwon, H.S. In situ study on the effects of Ni and Mo on the passive film formed on Fe–20Cr alloys by photoelectrochemical and Mott–Schottky techniques. J. Electroanal. Chem. 2006, 590, 120–125. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Du, Z.X.; Hu, Z.F. Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding. Coatings 2021, 11, 1139. [Google Scholar] [CrossRef]
- Loable, C.; Vicosa, I.N.; Mesquita, T.J.; Mantel, M.; Nogueira, R.P.; Berthomé, G.; Chauveau, E.; Roche, V. Synergy between molybdenum and nitrogen on the pitting corrosion and passive film resistance of austenitic stainless steels as a PH-dependent effect. Mater. Chem. Phys. 2017, 186, 237–245. [Google Scholar] [CrossRef]
- Moon, J.; Ha, H.Y.; Park, S.J.; Lee, T.H.; Jang, J.H.; Lee, C.H.; Han, H.N.; Hong, H.U. Effect of Mo and Cr additions on the microstructure, mechanical properties and pitting corrosion resistance of austenitic Fe-30Mn-10.5Al-1.1C lightweight steels. J. Alloys Compd. 2019, 775, 1136–1146. [Google Scholar] [CrossRef]
- Qi, X.; Mao, H.H.; Yang, Y.T. Corrosion behavior of nitrogen alloyed martensitic stainless steel in chloride containing solutions. Corros. Sci. 2017, 120, 90–98. [Google Scholar] [CrossRef]
- Xu, L.N.; Xu, X.Q.; Yin, C.X.; Qiao, L.J. CO2 corrosion behavior of 1% Cr–13% Cr steel in relation to Cr content changes. Mater. Res. Express 2019, 6, 096512. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, Z.L.; Hu, J.D.; Hou, Z.G.; Tian, Q.C.; Wang, H.Z. Influence of Cr Content on Corrosion Behaviour of Tube Pile Steel in Half-Immersion Environment. Trans. Indian Inst. Met. 2018, 71, 209–218. [Google Scholar] [CrossRef]
- JFE Steel Corporation. Martensitic Stainless Steel Seamless Pipe For Oil Country Tubular Goods, And Method For Manufacturing Same. In Patent Application Approval Process; USPTO 20200283866; Energy Weekly News; JFE Steel Corporation: Tokyo, Japan, 2020. [Google Scholar]
- Ćurković, L.; Otmačić, Ć.H.; Žmak, I.; Mustafa, M.K.; Gabelica, I. Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel. Coatings 2021, 11, 988. [Google Scholar] [CrossRef]
- Tadahiro, O.; Atsushi, O.; Masakazu, N.; Kawada, K.; Watanabe, T.; Nakagawa, Y.; Miyoshi, S.; Takahashi, S.; Chen, M.S. The Technology of Chromium Oxide Passivation on Stainless Steel Surface. J. Electrochem. Soc. 2019, 140, 1691–1699. [Google Scholar] [CrossRef]
- Wegrelius, L.; Falkenberg, F.; Olefjord, I. Passivation of Stainless Steels in Hydrochloric Acid. J. Electrochem. Soc. 2019, 146, 1397–1406. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Cao, T.S.; Zhang, L.; Meng, X.M.; Zhang, Z.P.; Cheng, C.Q. Improvement on the pitting corrosion resistance of 304 stainless steel via duplex passivation treatment. Mater. Corros. 2019, 70, 1764–1775. [Google Scholar] [CrossRef]
- Li, M.; Pascalidou, E.M.; Wiame, F.; Zanna, S.; Maurice, V.; Marcus, P. Passivation mechanisms and pre-oxidation effects on model surfaces of FeCrNi austenitic stainless steel. Corros. Sci. 2020, 167, 108483. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Wei, Y.H.; Yang, J.; Li, J.; Emori, W. Effects of nitric acid passivation on the corrosion behavior of ZG06Cr13Ni4Mo stainless steel in simulated marine atmosphere. Mater. Corros. 2020, 71, 1576–1590. [Google Scholar] [CrossRef]
- Li, J.D.; Chen, C.; Zhang, S.J.; Lin, B.; Wng, Y.Y.; Zhu, Y.Q.; Tang, J.L. Research progress on localized corrosion behavior of stainless steel under different stress conditions. Surf. Technol. 2021, 50, 101–115. [Google Scholar] [CrossRef]
- Chen, X.R.; Cheng, G.G.; Li, J.Y.; Hou, Y.Y.; Pan, J.X.; Ruan, Q. Characteristics and Formation Mechanism of Inclusions in 304L Stainless Steel during the VOD Refining Process. Metals 2018, 8, 1024. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Todoroki., H. Control of MgO·Al2O3 Spinel Inclusions in Stainless Steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Kent, P.; Yang, S.F.; Li, J.S.; Zhang, L.F.; Wang, Z.F. Behavior of MgO·Al2O3 Based Inclusions in Alloy Steel During Refining Process. J. Iron Steel Res. Int. 2010, 17, 1–6. [Google Scholar] [CrossRef]
- Cheng, R.J.; Li, R.C.; Cheng, D.; Liu, J.S.; Fang, Q.; Zhou, J.A.; Dong, W.L.; Zhang, H.; Ni, H.W. Revolution and Control of Fe-Al-(Mg, Ti)-O Oxide Inclusions in IF Steel during 260t BOF-RH-CC Process. Metals 2020, 10, 528. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.G.; Ma, R.Y.; Zhou, Y.T.; Liu, Y.; Daniel, E.F.; Li, X.F.; Wang, P.; Dong, J.H.; Ke, W. Effects of rare earth modifying inclusions on the pitting corrosion of 13Cr4Ni martensitic stainless steel. J. Mater. Sci. Technol. 2021, 93, 232–243. [Google Scholar] [CrossRef]
- Sun, B.Z.; Zuo, X.M.; Cheng, X.J.; Li, X.G. The role of chromium content in the long-term atmospheric corrosion process. NPJ Mater. Degrad. 2020, 4. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Xu, X.X.; Li, Y.; Lv, X.W. The function of Cr on the rust formed on weathering steel performed in a simulated tropical marine atmosphere environment. Constr. Build. Mater. 2021, 277, 122298. [Google Scholar] [CrossRef]
- Wang, Z.C.; Paschalidou, E.M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo Enrichments in the Passive Oxide Film on 316L Austenitic Stainless Steel. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.Y.; Sun, F.L.; Chu, F.Z.; Wang, L.W.; Wang, X.; Cui, Z.Y. Passivation behavior and surface chemistry of 316 SS in the environment containing Cl− and NH4+. J. Electroanal. Chem. 2021, 886, 115138. [Google Scholar] [CrossRef]
- Kim, E.T.; Muhammad, I.; Han, J.C.; Ko, K.K.; Bae, H.J.; Sung, H.; Kim, G.J.; Seol, J.B. Near atomic-scale comparison of passive film on a 17 wt% Cr-added 18 wt% Mn steel with those on typical austenitic stainless steels. Scr. Mater. 2021, 203, 114112. [Google Scholar] [CrossRef]




| C | Si | Mn | P | S | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.017 | 0.23 | 0.49 | 0.012 | 0.0043 | 13.3 | 5.01 | 1.85 | Bal. |
| Pickling Parameters | Native Film | 48 g/L—50 s | 103 g/L—110 s |
|---|---|---|---|
| Icorr/(μA/cm2) | 10.542 | 0.266 | 7.291 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Luo, Z.; Han, Y.; Liu, Y.-M.; Sun, L.; Zhai, W.-Y. The Influence of Pickling Treatment Parameters on the Surface State and Pre-Passivation Behavior of Super 13Cr Martensitic Stainless Steel. Coatings 2022, 12, 127. https://doi.org/10.3390/coatings12020127
Dong H, Luo Z, Han Y, Liu Y-M, Sun L, Zhai W-Y. The Influence of Pickling Treatment Parameters on the Surface State and Pre-Passivation Behavior of Super 13Cr Martensitic Stainless Steel. Coatings. 2022; 12(2):127. https://doi.org/10.3390/coatings12020127
Chicago/Turabian StyleDong, Hui, Zhuo Luo, Yan Han, Yan-Ming Liu, Liang Sun, and Wen-Yan Zhai. 2022. "The Influence of Pickling Treatment Parameters on the Surface State and Pre-Passivation Behavior of Super 13Cr Martensitic Stainless Steel" Coatings 12, no. 2: 127. https://doi.org/10.3390/coatings12020127





