1. Introduction
Polyurethane (PU) is widely used in diverse applications, such as coatings, foams, adhesives, sealants and elastomers. A key factor to the success of PU coatings is its versatility and durability. PU outperforms many other coating polymers. Abrasion resistance, flexibility, durability to washing and strength are by far higher in PU compared to other coating polymers. Therefore, PU is used in a lot of coating applications. For textile coating, polyester, polyether and polycarbonate PU are available. Their use is related to the final properties desired in an application. In general, the following properties can be related to the type of chemistry:
Polyester: good weather stability, poor resistance to hydrolysis;
Polyether: poor weather stability, good resistance to hydrolysis;
Polycarbonate: good weather stability + good resistance to hydrolysis.
Companies working with solvent-based PU coatings or diisocyanates, will eventually be forced to change to non-solvent or water-based technologies to comply with all the regulations. REACH regulation wants to guarantee the safe use of chemical substances and preparations throughout the entire industrial production chain. REACH imposes not only obligations concerning the gathering and distribution of information about the characteristics of the substance on the producers and importers of chemicals, but also on the downstream users, such as the textile coating companies processing PU since they use chemicals (e.g., DMF (dimethylformamide)). DMF is a substance of very high concern (SVHC), an obligation of communication if a coated textile contains more than 0.1% DMF. The next step is the prioritization for authorization followed by the authorization [
1]. Based on this information, alternative PU needs to be found to enable companies to continue their activities.
Currently, the biopolymers and synthetic polymers made from renewable raw materials are experiencing a renaissance, related to the trend to switch to CO
2-neutral renewable products. There is also a tendency towards the “bio, eco, natural” consciousness-awakening of the end consumer and the market-driven question to implement renewable materials. These developments are also applicable to polyols used for the synthesis of PU. Years ago, polyols were produced mainly from vegetable oils applied for the synthesis of strongly cross-linked PU, e.g., rigid foams and composite materials [
2,
3,
4,
5]. Natural oil polyols (NOPs) are used for PU synthesis, e.g., produced from soybean and castor oil, but polysaccharides and lignin are also frequently used raw materials to synthesize bio-based PU [
6]. According to the industry estimates, manufacturing polyester and polyether diols from NOPs (compared to similar polyols from oil) produces 36% less global warming emissions, 61% less non-renewable energy use and 23% less total energy demand [
7]. Fridrihsone et al. performed a life cycle analysis (LCA) study of rapeseed oil-based polyol for PU synthesis. Compared to petrochemical polyols, bio-based rapeseed oil polyols have a better environmental performance in 8 out of the 18 ReCiPe midpoint impact categories, and lower a cumulative energy demand [
8].
Two-component (2K) bio-based PU coatings were made based on rapeseed oil and applied via spray. The coatings showed good physical and mechanical properties and have the potential to be used as the inner coating for potable water tanks [
9]. Soybean oil phosphate ester polyols with a varying hydroxyl content were synthesized as polyols for 2K PU coating. These polyols can be used as the sole polyol or as the reactive diluent in 2K PU coating [
10]. The bio-based polyester polyols from eugenol were solvent free prepared and then applied to transparent and anticorrosive PU coatings. The developed coatings revealed excellent physico-mechanical properties, such as transparency, gloss, flexibility and cross-cut adhesion [
11]. Water-based polyols from epoxy cardanol modified with tartaric, citric and adipic acid were prepared [
12]. The effects of the different hardeners on the properties of castor oil-based 2K waterborne PU wood coatings were examined. Castor oil was modified using glycerol. The coating with hexamethylene diisocyanate had the best hardness, highest tensile strength and superior water resistance among all the tested coatings, and is suitable for wood coatings [
13]. The 2K PU adhesives, also based on castor oil, were synthesized for wood [
14]. Different bio-based PUs with a variable amount of cashew nutshell liquid were synthesized. The synthesized PU showed self-healing behavior, corrosion resistance and degraded when exposed to microbes [
15]. Isosorbide is one of the most applied sugar-based polyols in PU synthesis, owing to the fused ether rings providing rigidity [
16,
17,
18]. A series of polyester polyols was also prepared based on succinic acid, adipic acid, suberic acid and sebacic acid. All of them were bio-based except for the adipic acid. The polyester polyols were further reacted with 4,4′-methylenebis(phenylisocyanate) to prepare the 2K PU coatings. The sebacic acid-based formulation was considered to be the most suitable among all, for the coating performance (anticorrosive property, gloss and pencil hardness) [
19].
In some cases, particles were added to the 2K coating to improve the desired properties. Bio-based 2K PU coatings based on the sebacic acid and polyethylene glycol were made. Hydroxyapatite nanoparticles, synthesized from waste eggshells, were added in amounts of 1%, 3% and 5% to improve the physical properties of the coating [
20]. The 2K PU coating based on sorbitol was made, to which nano ZnO was added to improve the scratch resistance and anticorrosive properties. The addition of nano ZnO resulted in matt finishes [
21].
Since PU is the result of a reaction between hydroxyl compounds and isocyanates, bio-based PU can also be obtained by implementing bio-based isocyanates. PU dispersions were made from dimer fatty acid diisocyanates and castor oil. The addition of alkoxysilane modified castor oil increased the tensile strength and corrosion resistance [
22]. The use of ethyl ester L-lysine diisocyanate and ethyl ester
l-lysine triisocyanate in the PU films were reported. Compared to the isophorone diisocyanate-based PU, the bio-based PU tended to swell in the organic solvents [
23]. The bio-based PU adhesives for wood applications were synthesized by first reacting the cellulose acetate with 1,6-hexamethylene diisocyanate, followed by mixing with a variable amount of castor oil [
24]. Bio-based pentamethylene diisocyanate (PDI) has a significant bio-based content of 68% and is the first example of bio-based diisocyanate, which has been commercialized. The trimeric PDI is commercialized under the trade name DESMODUR
® eco N 7300 by Covestro [
25].
However, the majority of the reported applications of (bio-based) 2K PU coatings were on hard substrates, such as wood or metal and not on flexible substrates. This report describes the solvent-free synthesis and application of bio-based 2K PU on textiles for waterproof fabrics. A 2K PU coating formulation was made and directly applied to a polyester fabric prior to thermal curing in an oven. Bio-based polyol, bio-based isocyanate and non-toxic catalyst were used as the raw materials. The resulting PU was characterized via FT-IR, TGA and DSC, and the water barrier properties were assessed.
2. Materials and Methods
2.1. Materials
Bismuth neodecanoate (catalyst) was purchased from Sigma-Aldrich (Darmstadt, Germany). Desmodur Eco N7300 (bio-based polyisocyanate) was sampled by Covestro (Leverkusen, Germany). The Tego Airex 900 (organo modified polysiloxane) and Dynasylan 1189 (N-(n-butyl)-3-aminopropyltrimethoxysilane) were supplied by Evonik (Essen, Germany). Dynasylan 1189 was used as the adhesion promoter and Tego Airex 900 as the deaerator to remove air bubbles in the coating formulation. Tanacoat AWP (fluorocarbon emulsion) was sampled by Tanatex Chemicals (Ede, The Netherlands). The woven polyester fabric (105 g/m2) was purchased form Concordia Textiles (Waregem, Belgium). A cardanol-based polyester polyol (Polyol 1, Mw: 2000 g/mol; OH value: 291 mg/g KOH) and vegetable oil-based polyester polyol (Polyol 2, Mw: 3000 g/mol; OH value: 39 mg/g KOH) were used as the polyols.
2.2. Pre-Treatment of the Fabric
The fabric was pre-treated with Tanacoat AWP to prevent the penetration of the 2K coating into the fabric. Therefore, the fabric was immersed in a solution of 20% Tanacoat AWP in water. The fabrics were padded using a padding mangle pressure of 2 bar. After the padding process, the samples were dried for 1 min at 100 °C and 1 min at 155 °C, according the technical data sheet of the product.
2.3. Bio-Based 2K PU Coating
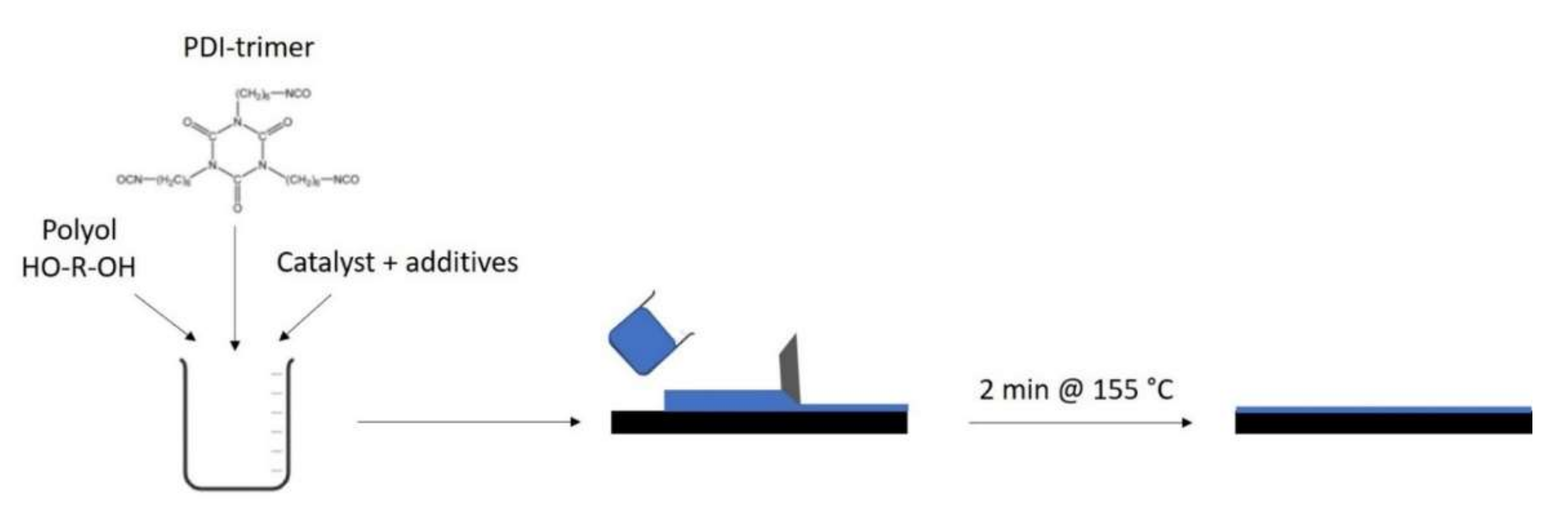
Figure 1 represents the flow chart of the coating process. The PU coatings were prepared by reacting renewable polyols with the Desmodur Eco N7300 in a molar equivalent ratio 1:1 (NCO:OH) using bismuth neodecanoate as a catalyst. A total of 9 g of cardanol-based polyester polyol (Mw: 2000 g/mol; OH value: 291 mg/g KOH) and 18 g of vegetable oil-based polyester polyol (Mw: 3000 g/mol; OH value: 39 mg/g KOH) were molten at 50 °C and mixed with 0.02 g of catalyst (bismuth neodecanoate). A total of 0.3 g of Dynasylan 1189 and 0.3 g of Tego Airex 900 were added. Tego Airex 900 was added to remove the air inclusion from the formulation, which would result in coating defects and a loss of water barrier properties. Subsequently, 11.68 g of Desmodur Eco N7300 (bio-based polyisocyanate) was added to the polyol mixture, which was kept at 40 °C. The 2K formulation was applied to a polyester fabric via the knife-over-roll coating method. The applied coating thickness was 50 µm. A total of 2 layers was applied and, after applying each layer, the coating formulation was cured for 2 min at 155 °C. Other bio-based 2K PU coatings were similarly produced (
Table 1).
2.4. Characterization
Fourier transform infrared spectra (in µ-ATR mode) were recorded using a Nicolet 6700 spectrometer from Thermofisher Scientific (Waltham, MA, USA). A spectral range from 500 to 4000 cm−1 with a resolution of 4 cm−1 was used. The infrared analysis was used to characterize the 2K PU. The surface morphology and the penetration of the coating into the fabric was visualized using field emission gun scanning electron microscopy (FEG-SEM) (JSM 7600 F from Jeol Europe, Zaventem, Belgium). To prevent charging, the specimens were sputtered with a palladium coating.
The thermogravimetric analysis (TGA) of the PU was performed to examine the thermal decomposition and char formation using a Q500 thermogravimetric analyzer (TA Instruments, Asse, Belgium). All the samples were conditioned at 23 °C and 50% relative humidity. Analyses were performed in air with a ramp rate of 10 °C/min from 30 to 600 °C. The thermograms were analyzed using Universal Analysis Software.
Differential scanning calorimetry (DSC) analysis of the PU was performed to measure the glass transition temperature (Tg) using TA Instruments Discovery DSC2500 (TA Instruments, Asse, Belgium). All the samples were conditioned at 23 °C and 50% relative humidity. All the samples were heated from 0 to 250 °C, cooled down from 250 to 0 °C and heated back from 0 to 250 °C. The analyses were performed with a heating and cooling rate of 10 °C/min.
The air permeability was assessed according to ISO 9237 using Textest FX 3300 apparatus (Textest AG, Schwerzenbach, Switzerland ). The rate of air flow passing perpendicularly through the coated fabric was measured at a pressure drop of 100 Pa across the fabric test area (20 cm2). A total of 10 measurements were performed.
The flexibility was assessed according to EN ISO 7854-C using a crumple flex tester (VVC, Linselles, France). The crumple flex tester simulates the flexing of the textile during use by twisting the coated fabric. Subsequently, an evaluation of the coating was conducted by assessing the presence of cracks or aspect loss.
Swelling experiments in ethyl acetate were performed to calculate the crosslinking density. The PU films were placed for 48 h in solvent. After removal, the residual amount of solvent on the PU films was wiped off, before being weighed. From the weight of the swollen polymer (w
s), the volume fraction of swollen polymer (V
p) can be calculated as follows:
where w
d is the dry weight of the polymer, and d
p and d
s are the densities of the polymer and solvent, respectively. The crosslink density (n) values were obtained from V
p with the Flory–Rehner equation:
where vs. is the molar volume of the solvent and χ is the polymer–solvent interaction parameter, which can be found from equation:
where R is a gas constant and T is the temperature (expressed in Kelvin), whereas δ
1 and δ
2 are the solubility parameters of the solvent and polymer.
The abrasion resistance was evaluated according to EN 530-2 using a Martindale wear and abrasion tester. The instrument was used in an inverted mode, i.e., the specimen was placed on the abradant table instead of in the test piece holder, and the abradant was mounted on the test piece holder. This provides an abraded area, which allows a measurement of resistance to hydrostatic pressure afterwards. The test was carried out for 500 cycles with an F2 abradant (sandpaper), and an applied pressure of 9 kPa on the sample. Besides determining the resistance to hydrostatic pressure, the mass loss was determined after 500 abrasion cycles.
Elongation at break and the break at stress were determined using an Instron electronic fabric tension tester (Instron, Norwood, MA, USA) according to ISO 13934-1 on 50 µm of 2K PU films. The tension loading speed was 100 mm/min. The resistance to the hydrostatic pressure was measured according to ISO 811 using a Textest FX 800 apparatus (Textest AG, Schwerzenbach, Switzerland). The coated fabric was subjected to a steadily increasing pressure of water on one face, under standard conditions, until penetration occurred in three places. The same test was repeated after washing and the exposure to UV or a high temperature and humidity to examine, respectively, the wash fastness, Q-panel Laboratory UltraViolet (QUV) and hydrolysis resistance. The wash fastness was evaluated according to ISO 6330. A total of 20 washing cycles at 60 °C were performed in a Wascator FOM 71 type A (James Heal, Halifax, UK). The detergent was the ECE detergent type 3 (Christeyns, Ghent, Belgium). The samples were dried at room temperature after washing. The hydrolysis resistance was measured according to ISO 1419-C (tropical test), by exposing the coated fabric for 3 weeks to a temperature of 70 °C and a relative humidity of 95%, and examining the resistance to hydrostatic pressure afterwards. The resistance towards aging by means of heat, light and humidity (QUV test) was evaluated according to ISO 4892. The details are presented in
Table 2. Generally, the total duration of the test was set at 500 h.