Effect of Oxidation and Crystallization on Pitting Initiation Behavior of Fe-Based Amorphous Coatings
Abstract
:1. Introduction
2. Methods
3. Results
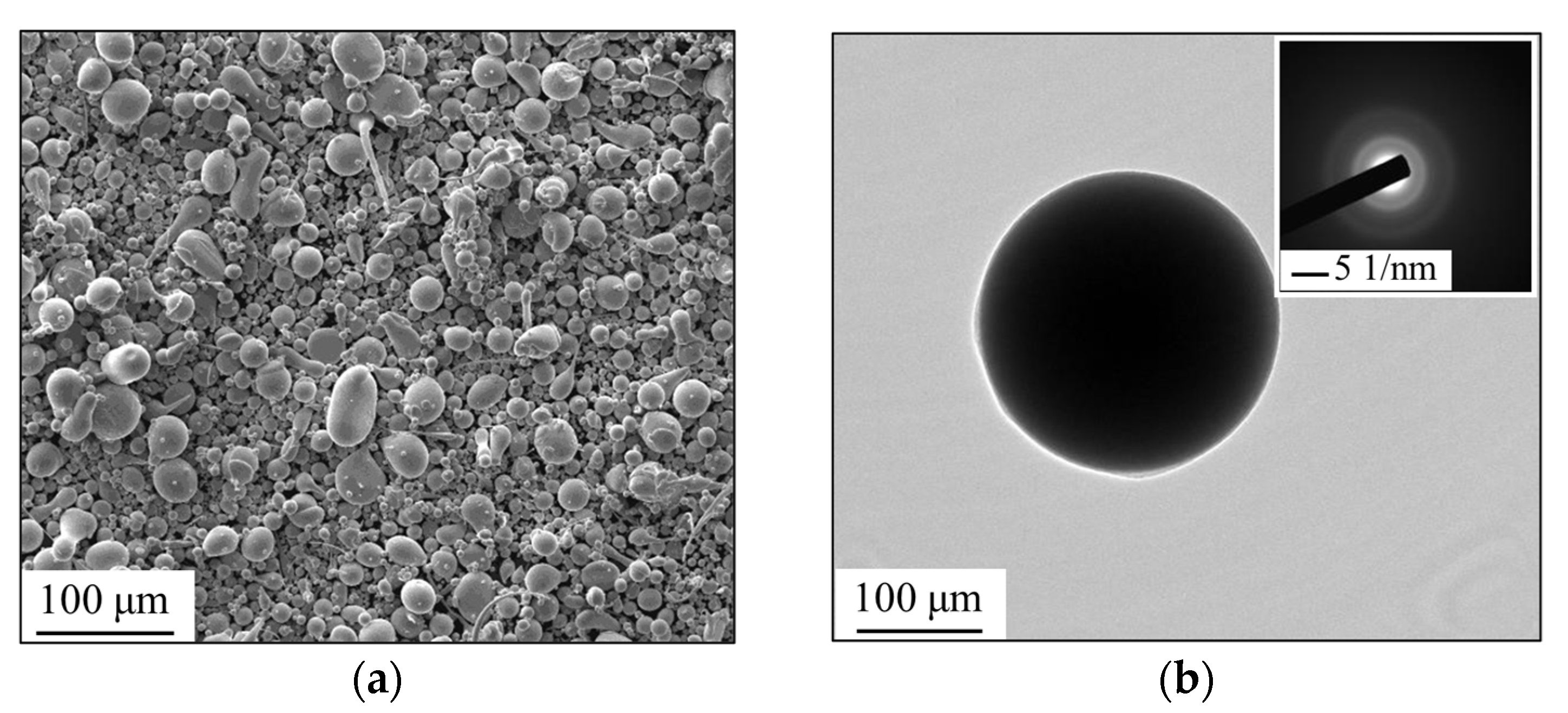
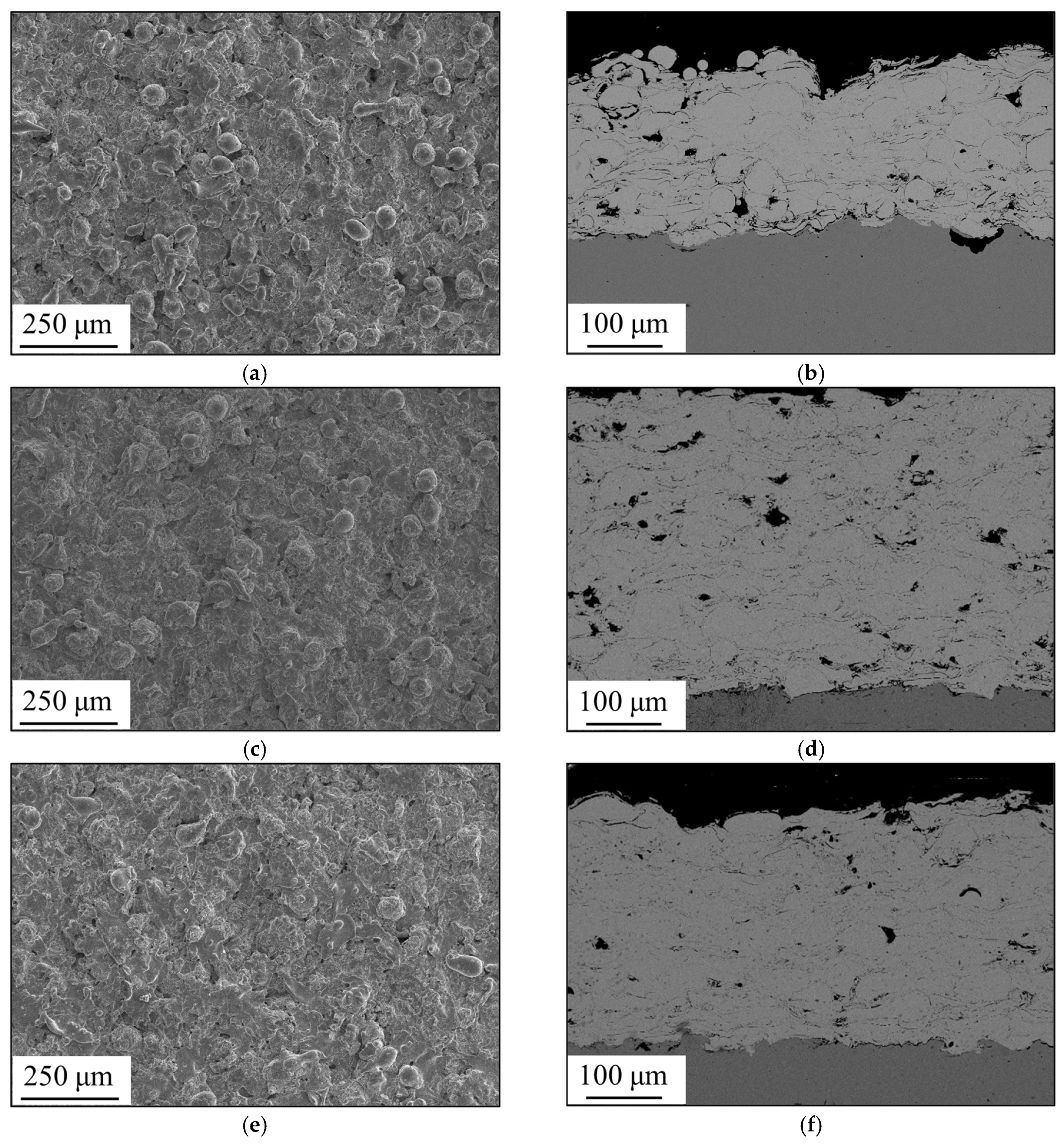
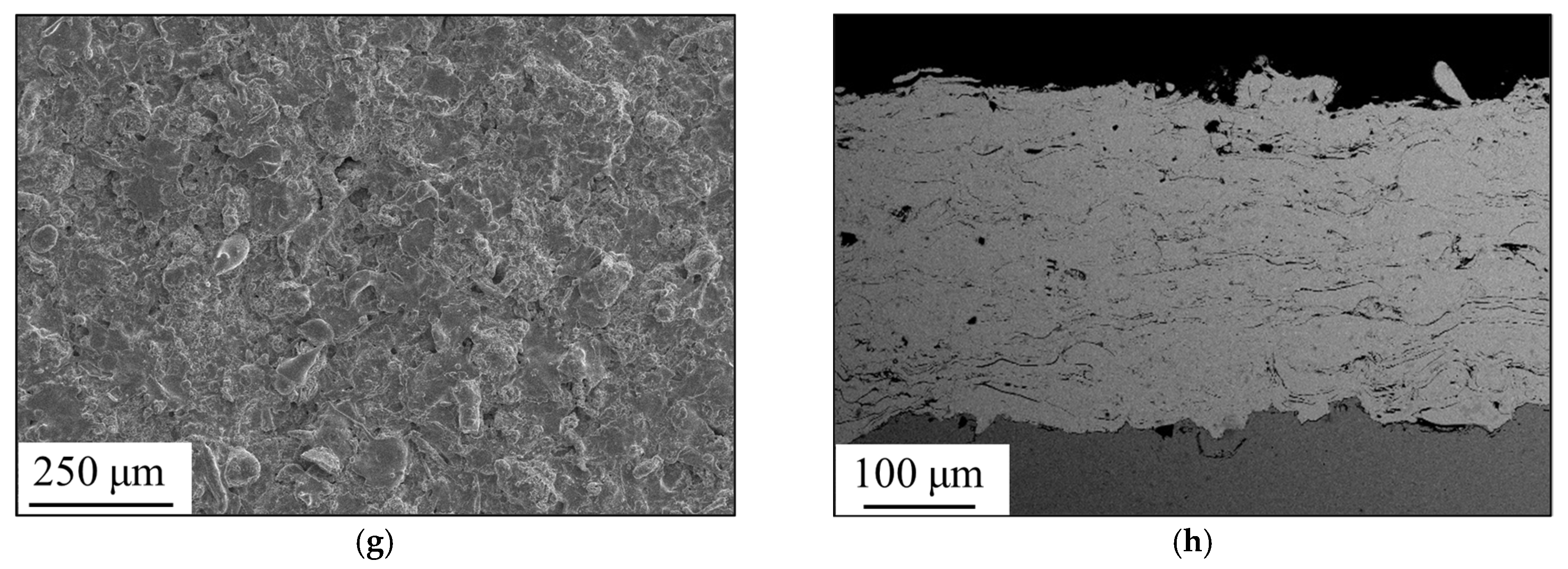
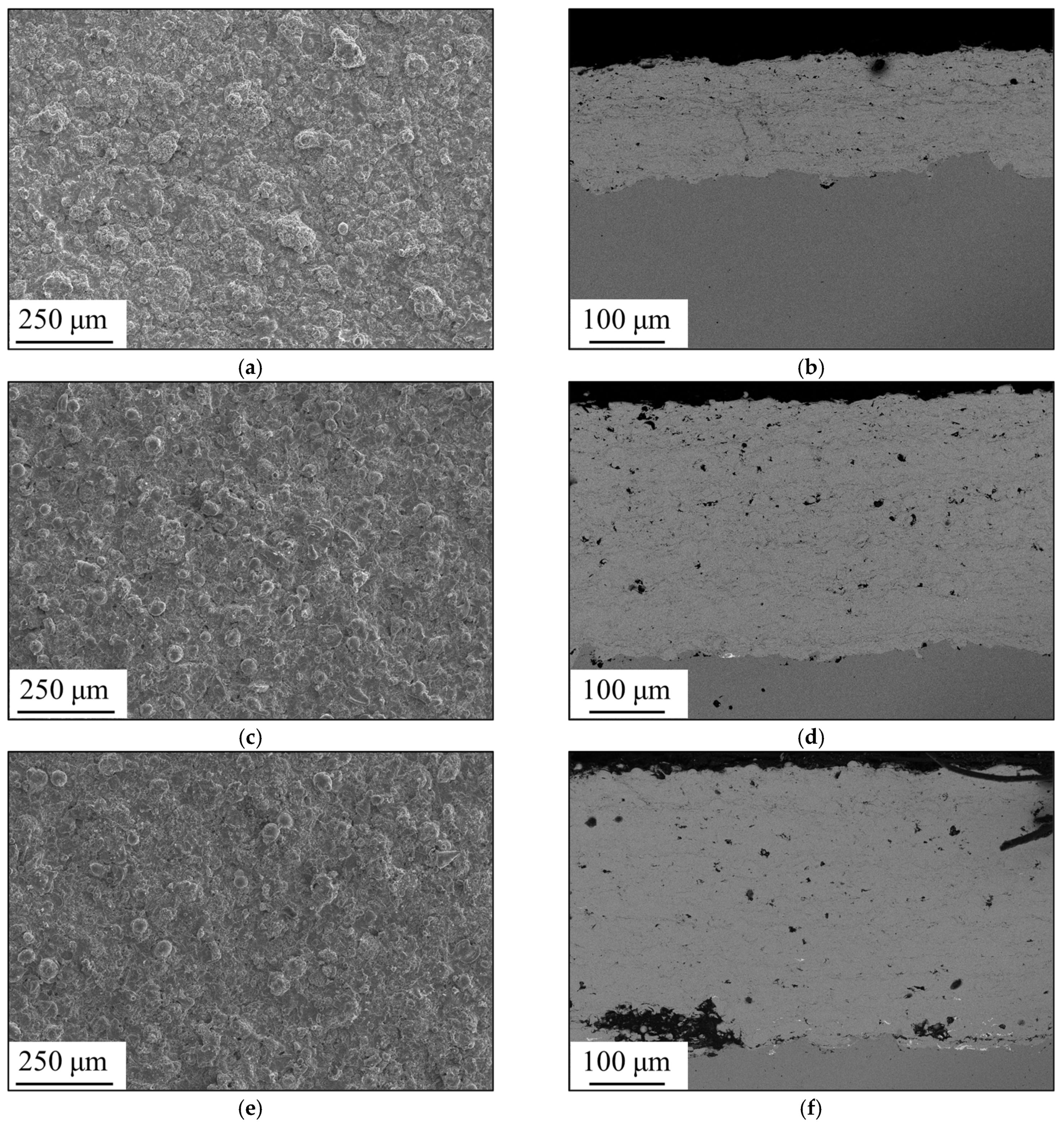
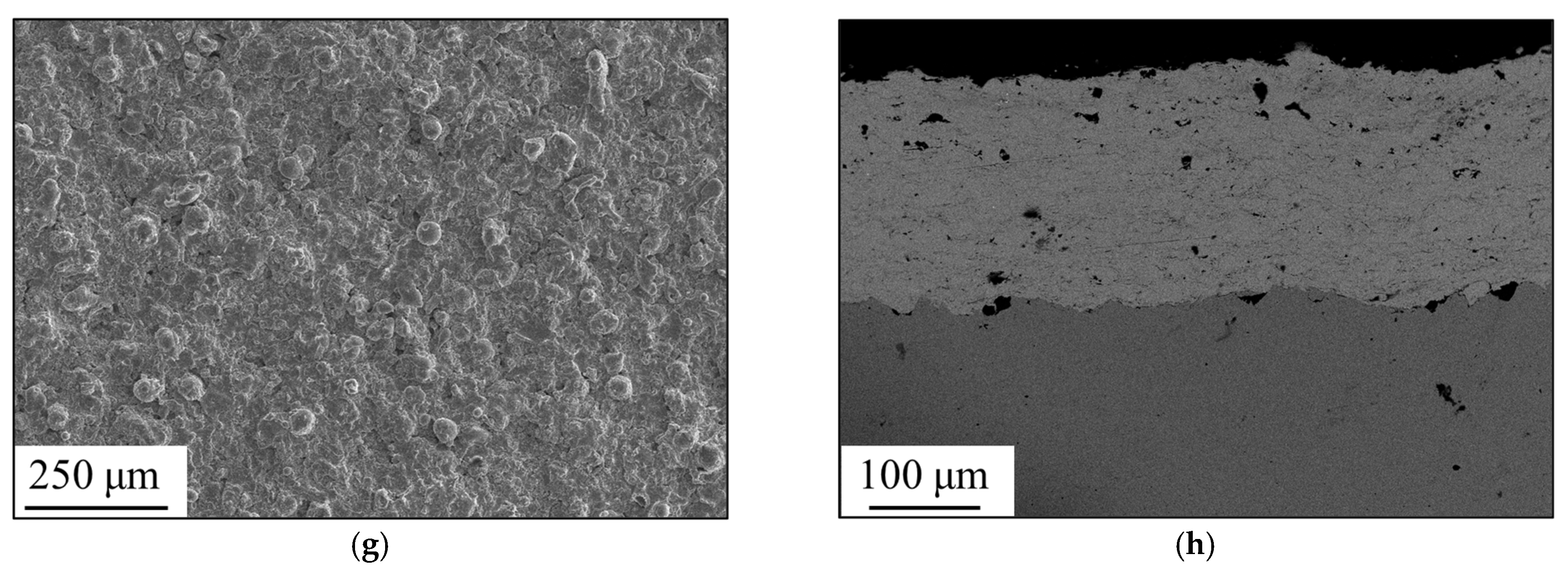
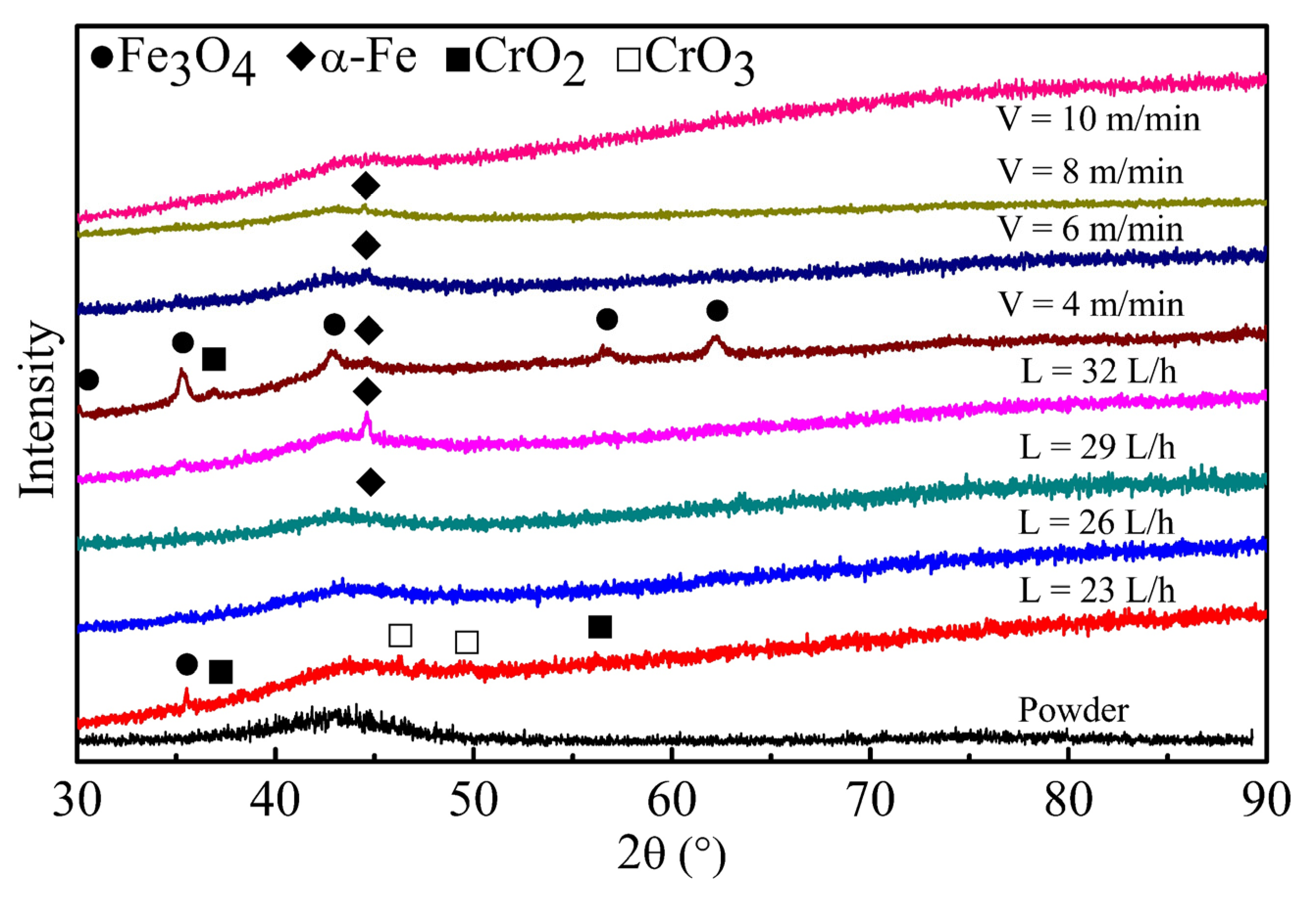
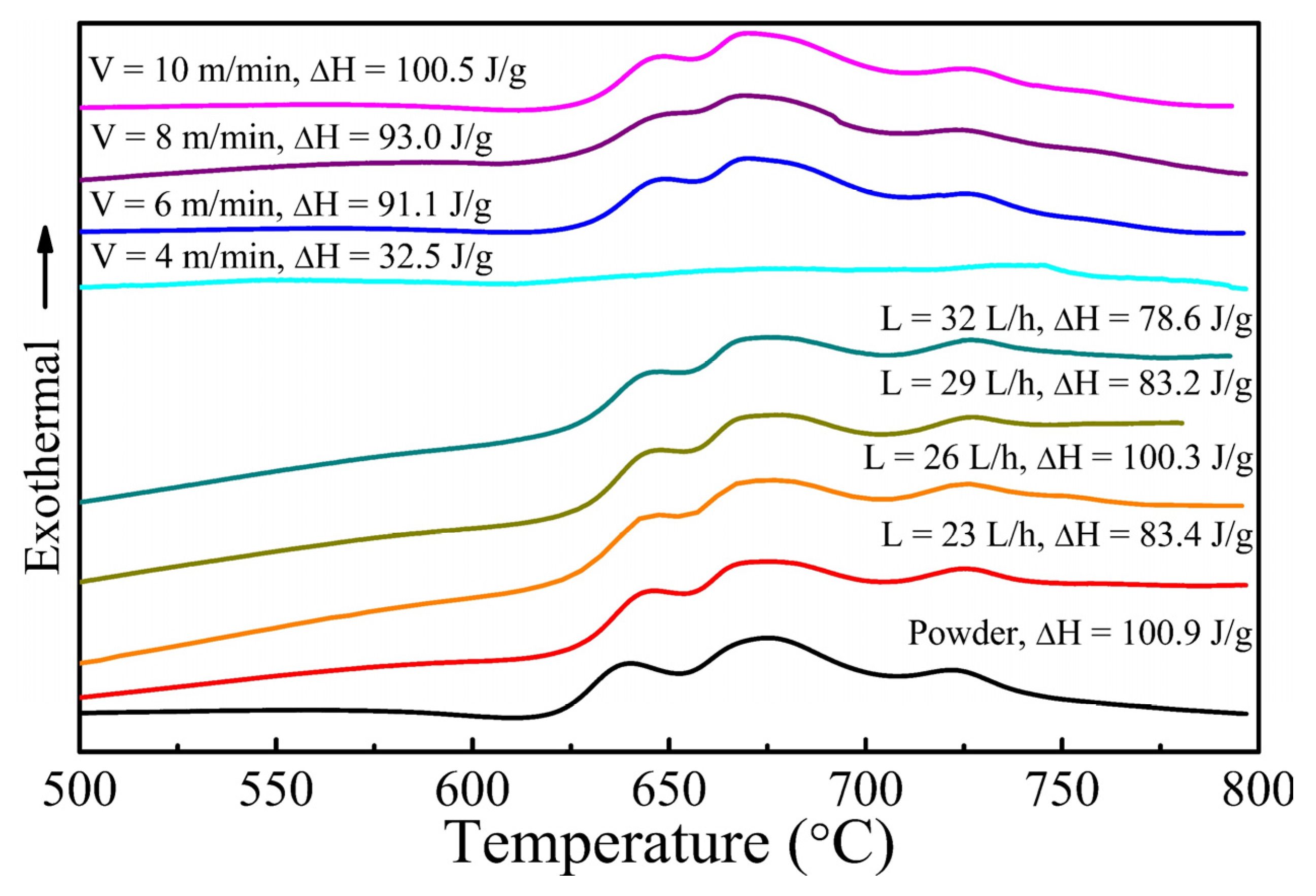
3.1. Formation of Amorphous Coatings
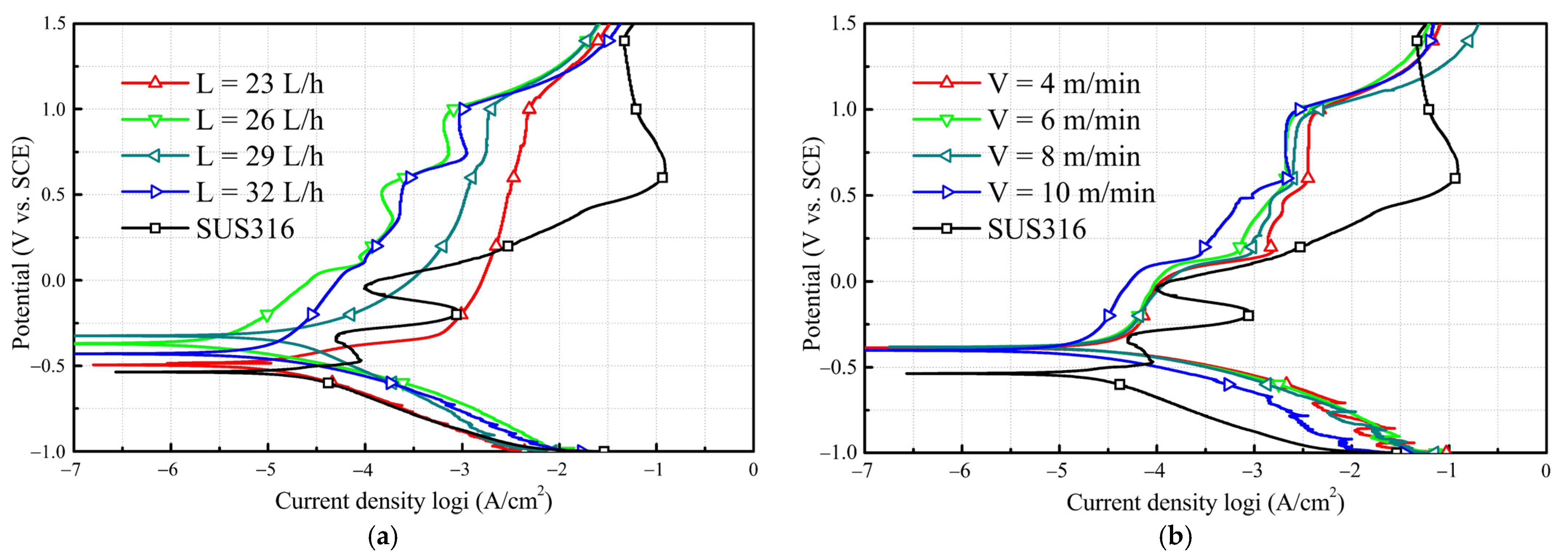
3.2. Corrosion Resistance of Amorphous Coatings
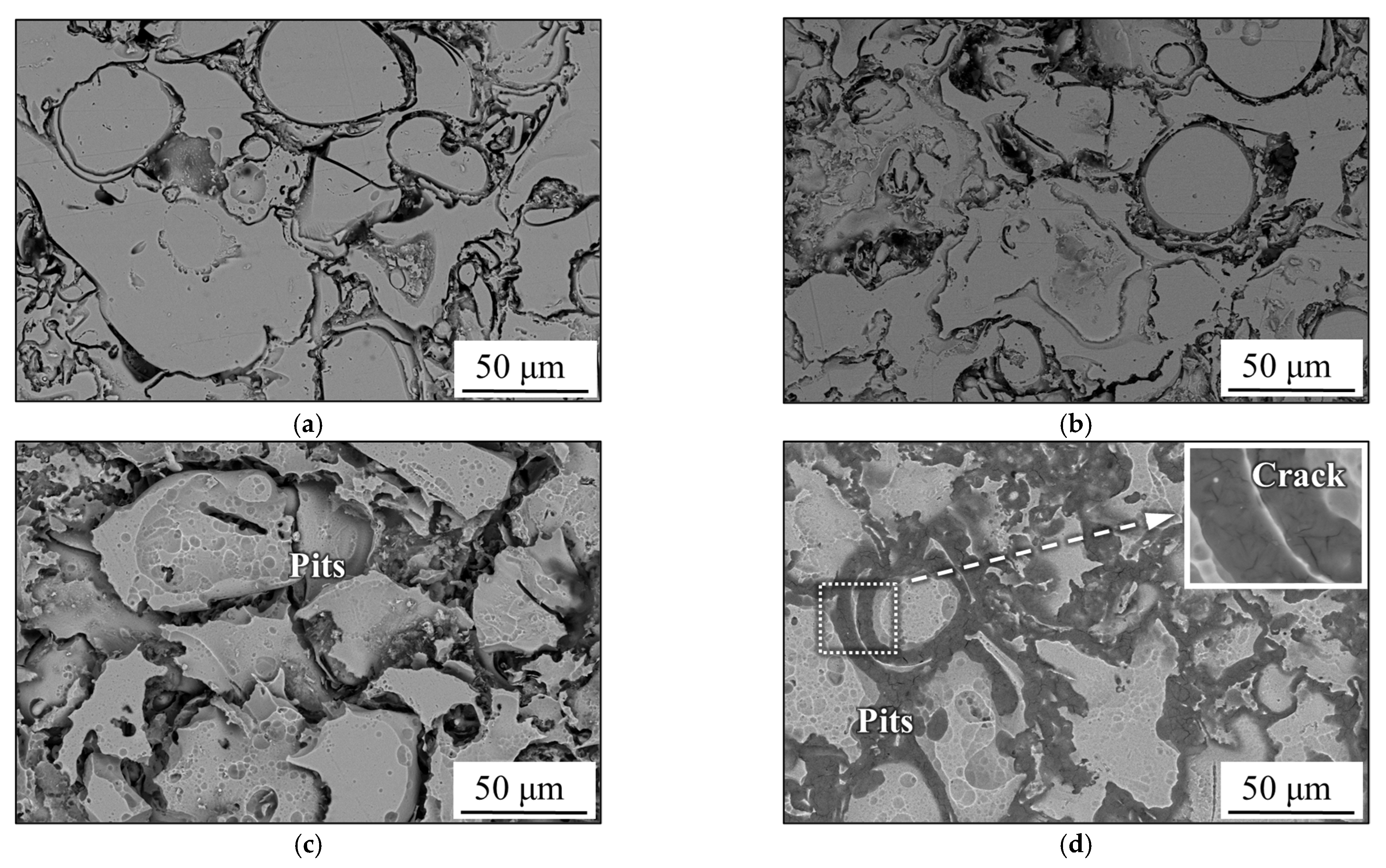
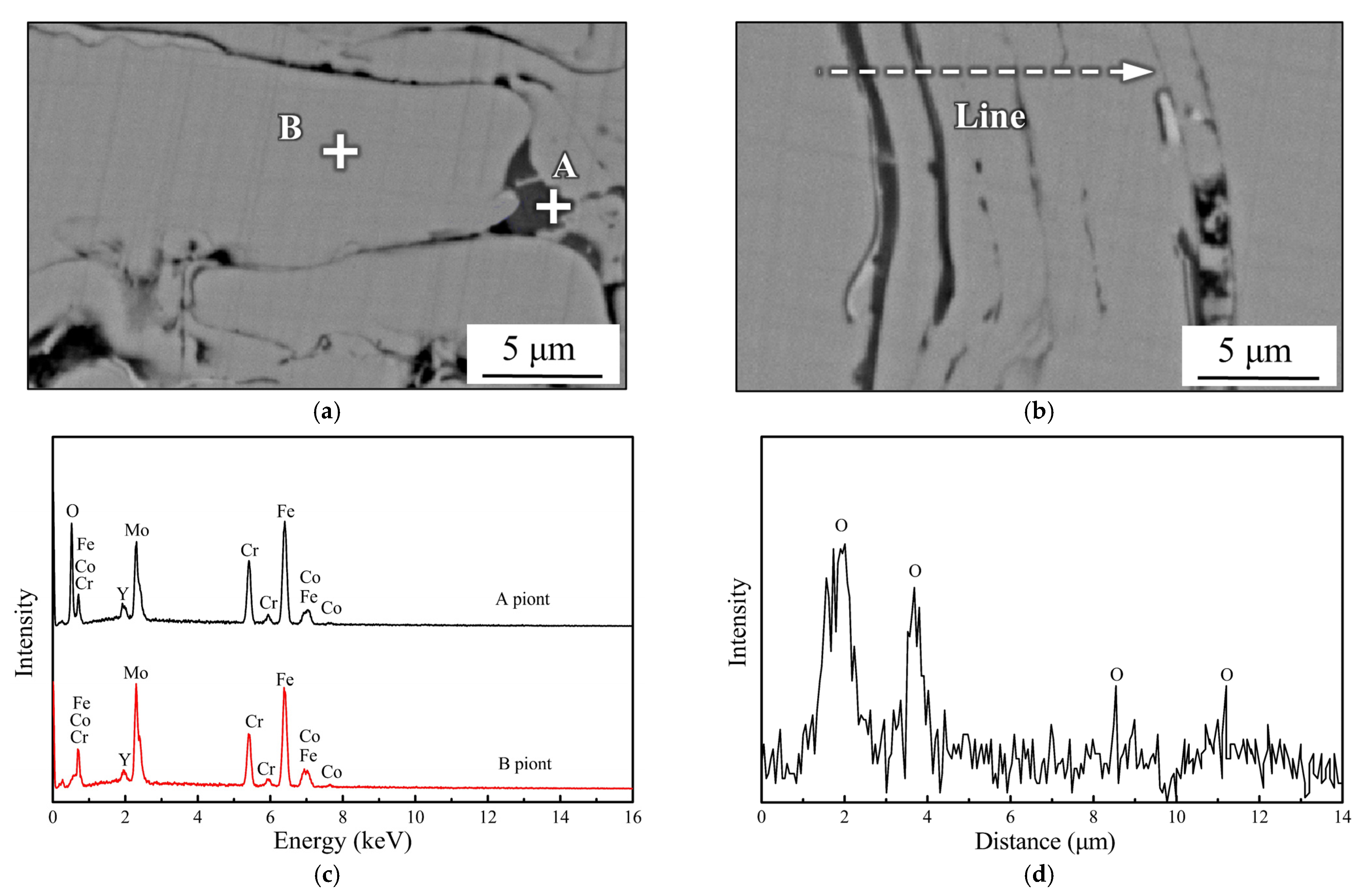
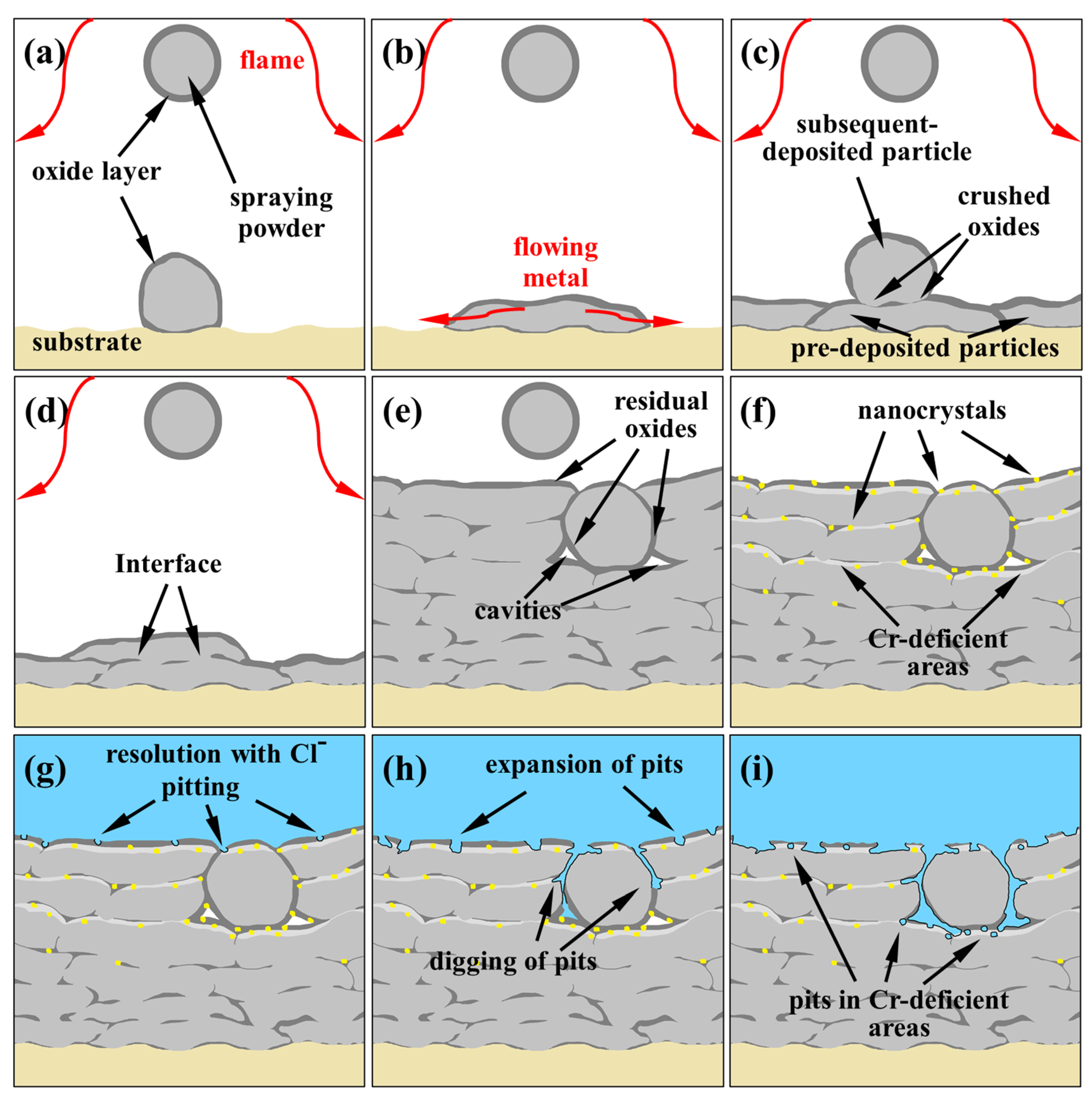
3.3. Pitting Corrosion at the Interface
4. Discussion
5. Conclusions
- Fe-based amorphous coating with high amorphous content and low porosity is obtained by high-velocity oxygen-fuel spraying (HVOF) using industrial raw materials; the amorphous volume fraction exceeds 99% and the porosity is lower than 1.0%.
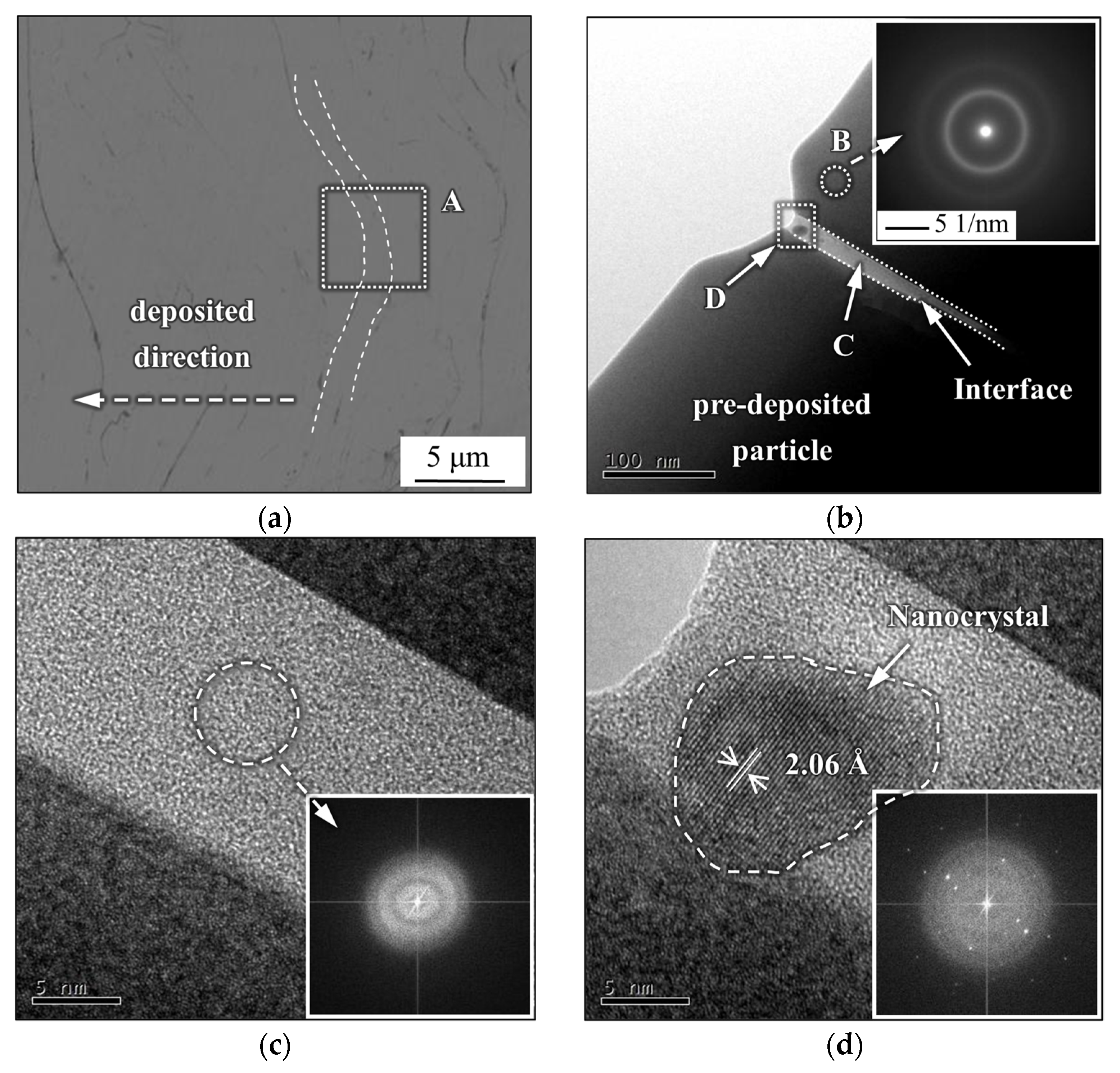
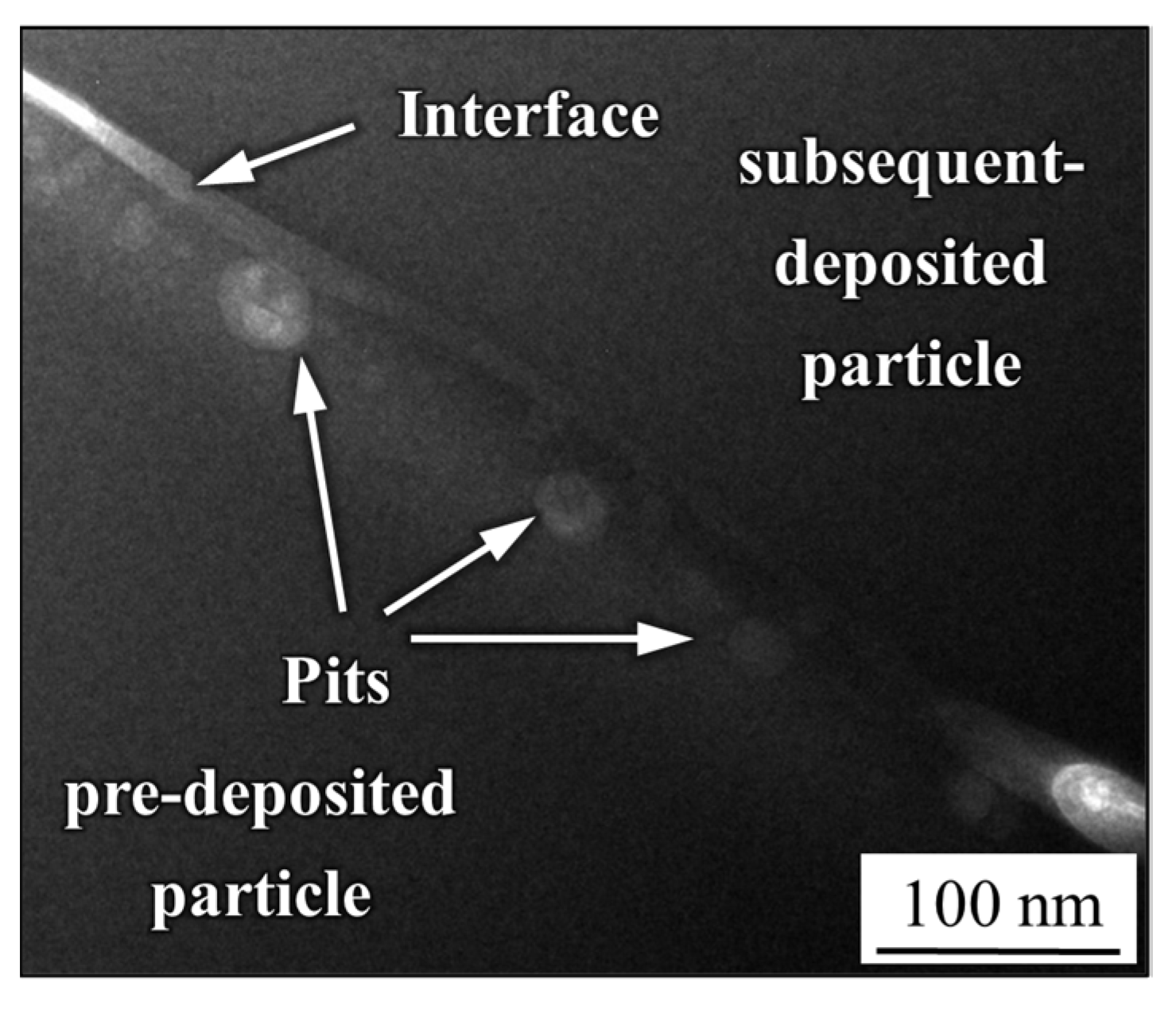
- The mechanical interlocking effect and metallurgical bonding act synergistically on the interface between the spray particles, and a model is proposed for the crystallization around the interface.
- Fe-based amorphous coatings exhibit superior corrosion resistance than SUS316 in the chloride solution.
- Pitting corrosion emerges along the pre-deposited side near the interface owing to the heterogeneity of the microstructure and chemical composition in certain areas, resulting from the nanocrystal precipitation and considerable Cr depletion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farmer, J.C.; Haslam, J.; Day, S.; Lian, T.; Saw, C.; Hailey, P.; Choi, J.; Rebak, R.; Payer, J.; Blue, C.; et al. The corrosion resistance of Fe-based amorphous metals: Fe49.7Cr17.7Mn1.9Mo7.4W1.6B15.2C3.8Si2.4 and other compositions. In Proceedings of the Materials Science & Technology, Conference and Exhibition, Detroit, MI, USA, 16–20 September 2007; pp. 318–392. [Google Scholar]
- Blink, J.; Farmer, J.C.; Choi, J.S.; Saw, C. Applications in the nuclear industry for thermal spray amorphous metal and ceramic coatings. Met. Mater. Tran. A 2009, 40, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.R.; Mei, X.X.; Zhang, X.N.; Li, X.; Wang, Y.; Sun, J.; Wang, Y. H+-induced irradiation damage resistance in Fe- and Ni-based metallic glass. Nucl. Instrum. Methods Phys. Res. B 2016, 375, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Hou, W.L.; Chang, X.C.; Wang, J.Q. Slurry erosion-corrosion behavior of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings for marine pump in sand-containing NaCl solutions. Corros. Sci. 2011, 53, 3177–3185. [Google Scholar] [CrossRef]
- Lu, J.; Liu, C.; Han, Y.; Xu, G. Analysis of utilizing anti-corrosion Fe-based amorphous coating material with the flue gas waste heat in the power plant boiler. Elect. Power Sci. Eng. 2014, 30, 13–18. [Google Scholar] [CrossRef]
- Kishitake, K.; Era, H.; Otsubo, F. Thermal-sprayed Fe-based Fe-10Cr-13P-7C amorphous coatings possessing excellent corrosion resistance. J. Therm. Spay. Technol. 1996, 5, 476–482. [Google Scholar] [CrossRef]
- Lu, W.Y.; Wang, D.B.; Wang, Q.; Yang, F.; Li, T.R.; Shi, Y.T.; Zhang, S.; Yang, B. Sensitivity of Corrosion Behavior for Fe-Based Amorphous Coating to Temperature and Chloride Concentration. Coatings 2021, 11, 331. [Google Scholar] [CrossRef]
- Otsubo, F.; Kishitake, K. Corrosion resistance of Fe-10%Cr-30%Mo-(C,B,P) amorphous coatings sprayed by HVOF and APS processes. Mater. Tran. 2005, 46, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.F.; Deng, C.M.; Song, J.B.; Deng, C.G.; Liu, M.; Dai, M.J. Electrochemical Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed. Coatings 2019, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, C.; Peng, Y.; Yu, Y.; Liu, L. Effects of crystallization on the corrosion resistance of Fe-based amorphous coatings. Corros. Sci. 2012, 59, 10–19. [Google Scholar] [CrossRef]
- Gavrilovic, A.; Rafailovic, L.D.; Artner, W.; Wosik, J.; Whitehead, A.H. The corrosion behavior of amorphous and nanocrystallince Fe73.5Cu1Nb3Si15.5B7 alloy. Corros. Sci. 2011, 53, 2400–2405. [Google Scholar] [CrossRef]
- Baron, A.; Szewieczek, D.; Nawrat, G. Corrosion of amorphous and nanocrystalline Fe-based alloys and its influence on their magnetic behavior. Electr. Acta 2007, 52, 5690–5695. [Google Scholar] [CrossRef]
- Liaw, M.M.P. (Ed.) Bulk Metallic Glasses an Overview; Springer: New York, NY, USA, 2008; p. 10. [Google Scholar]
- Scully, J.R.; Gebert, A.; Payer, J.H. Corrosion and related mechanical properties of bulk metallic glasses. J. Mater. Res. 2007, 22, 302–309. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.Y.; Zhu, F.; Dong, W.T.; Sun, L.L. Pitting corrosion mechanism of Cl- and S2- induced by oxide inclusion in Fe-based amorphous metallic coatings. Surf. Coat. Technol. 2020, 385, 125449. [Google Scholar] [CrossRef]
- Zhang, C.; Chan, K.C.; Wu, Y.; Liu, L. Pitting initiation in Fe-based amorphous coatings. Acta Mater. 2012, 60, 4152–4159. [Google Scholar] [CrossRef]
- Liu, S.L.; Zhu, Y.S.; Lai, X.Y.; Zheng, X.P.; Jia, R.N.; Yuan, X.S. Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings. Coatings 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Gostin, P.F.; Oswald, S.; Schultz, L.; Gebert, A. Acid corrosion process of Fe-based bulk metallic glass. Corros. Sci. 2012, 62, 112–121. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, C.; Wang, W.; Liu, L. Pitting mechanism in a stainless steel-reinforced Fe-based amorphous coating. Electrochim. Acta 2016, 206, 61–69. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.D.; Sun, W.H.; Gao, Y.; Wang, J.Q. Enhanced corrosion resistance in Fe-based amorphous coatings through eliminating Cr-depleted zones. Corros. Sci. 2018, 136, 161–173. [Google Scholar] [CrossRef]
- Liu, G.; An, Y.L.; Guo, Z.H.; Chen, J.M.; Hou, G.L.; Chen, J. Structure and corrosion behavior of iron-based metallic glass coatings prepared by LPPS. Appl. Surf. Sci. 2015, 258, 5380–5386. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coating by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Wang, S.L.; Chen, J.C.; Yi, S.H.; Ke, L.M. Corrosion resistance of Fe-based amorphous metallic matrix coating fabricated by HVOF thermal spraying. Trans. Nonferrous Met. Soc. China 2014, 24, 146–151. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, H.X.; Zhang, X.F.; Yi, S. Effects of Cr contents in Fe-based bulk metallic glasses on the glass forming ability and the corrosion resistance. Mat. Chem. Phys. 2009, 113, 878–883. [Google Scholar] [CrossRef]
- Li, L.Y.; Yan, J.; Xiao, J.; Sun, L.; Wang, J. A comparative study of corrosion behavior of S-phase with AISI 304 austenitic stainless steel in H2S/CO2/Cl- media. Corros. Sci. 2021, 187, 109472. [Google Scholar] [CrossRef]
- Wang, S.G.; Sun, M.; Xu, Y.H.; Long, K.; Zhang, Z.D. Enhanced localized and uniform corrosion resistances of bulk nanocrystalline 304 stainless steel in high-concentration hydrochloric acid solutions at room temperature. J. Mater. Sci. Technol. 2018, 34, 2498–2506. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Hou, G.L.; An, Y.L.; Liu, G. The effect of high-velocity oxy-fuel spraying parameters on microstructure, corrosion and wear resistance of Fe-based metallic glass coatings. J. Non-Cryst. Solid 2014, 406, 37–44. [Google Scholar] [CrossRef]
- An, Y.L.; Hou, G.L.; Chen, J.; Zhao, X.Q.; Liu, G.; Zhou, H.D.; Chen, J. Microstructure and tribological properties of iron-based metallic glass coatings prepared by atmospheric plasma spraying. Vacuum 2014, 107, 132–140. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.T.; Huang, L.P.; Huang, S.S.; Zheng, X.B. Effect of feedstock particle size on wear resistance of plasma sprayed Fe-based amorphous coatings. Surf. Coat. Technol. 2014, 258, 495–502. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, C.; Zhou, H.; Liu, L. On the bonding strength in thermally sprayed Fe-based amorphous coatings. Surf. Coat. Technol. 2013, 218, 17–22. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C. Fe-based amorphous coatings: Structures and properties. Thin Solid Film 2014, 561, 70–86. [Google Scholar] [CrossRef]
- Bijalwan, P.; Singh, C.; Kumar, A.; Sarkar, K.; Rani, N.; Laha, T.; Banerjee, A.; Mondal, K. Corrosion behavior of plasma sprayed Fe-based metallic glass (Fe73Cr2Si11B11C3 at%) coatings in 3.5% Nacl solution. J. Non-Cryst. Solid 2021, 567, 120913. [Google Scholar] [CrossRef]
- Su, J.; Kang, J.J.; Yue, W.; Ma, G.Z.; Wang, C.B. Comparison of tribological behavior of Fe-based metallic glass coatings fabricated by cold spraying and high velocity air fuel spraying. J. Non-Cryst. Solid 2019, 522, 119582. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wang, S.L.; Yang, X.L.; Hao, S.L.; Chen, Y.H.; Li, H.X.; Pan, D. Interfacial characteristic and microstructure of Fe-based amorphous coating on magnesium alloy. Surf. Coat. Technol. 2021, 425, 127659. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wu, H.Y.; Wang, S.L.; Chen, Y.H.; Huang, Y.D.; Li, H.X. Reinforcing mechanism of WC particles in Fe-based amorphous matrix coating on magnesium alloy surface. Materials 2021, 14, 6571. [Google Scholar] [CrossRef] [PubMed]
- Si, C.R.; Duan, B.B.; Zhang, Q.; Cai, J.; Wu, W.C. Microstructure, corrosion-resistance, and wear-resistance properties of subsonic flame sprayed amorphous Fe–Mo–Cr–Co coating with extremely high amorphous rate. J. Mater. Res. Technol. 2020, 9, 3292–3303. [Google Scholar] [CrossRef]
- Li, J.W.; Yang, L.J.; Ma, H.R.; Jiang, K.M.; Chang, C.T.; Wang, J.Q.; Song, Z.; Wang, X.; Li, R.-W. Improved corrosion resistance of novel Fe-based amorphous alloys. Mater. Des. 2016, 95, 225–230. [Google Scholar] [CrossRef]
- Wang, S.L.; Yi, S. The corrosion behaviors of Fe-based bulk metallic glasses in a sulfuric solution at 70 °C. Intermetallic 2010, 18, 1950–1953. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, H.M.; Li, W.S.; Fan, X.J.; Li, X.Q.; Ning, W.C.; Xiong, D. Microstructure and corrosion resistance of Fe-based amorphous coating prepared by detonation spray. Surf. Coat. Technol. 2020, 399, 126096. [Google Scholar] [CrossRef]
- Kang, Y.H.; Chen, Y.M.; Wen, Y.X.; Wu, B.; Song, M.B. Effects of structural relaxation and crystallization on the corrosion resistance of an Fe-based amorphous coating. J. Non-Cryst. Solid 2020, 550, 120378. [Google Scholar] [CrossRef]
- Liu, M.M.; Hu, H.X.; Zheng, Y.G.; Wang, J.Q.; Qiu, S. Effect of sol-gel sealing treatment loaded with different cerium salts on the corrosion resistance of Fe-based amorphous coating. Surf. Coat. Technol. 2019, 367, 311–326. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, R.Q.; Yang, Y.; Wu, Y.; Liu, L. Influence of the size of spraying powders on the microstructure and corrosion resistance of Fe-based amorphous coatings. Electrochim. Acta 2011, 56, 6380–6388. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Wang, J.Q. Corrosion of high-velocity oxy-fuel sprayed iron-based amorphous metallic coatings for marine pump in sodium chloride solutions. Mater. Corros. 2012, 63, 685–694. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylen, P. Microstructural characteristics and corrosion behavior of HVAF- and HVOF- sprayed Fe-based coatings. Surf. Coat. Technol. 2017, 318, 365–373. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behavior of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Lott, S.E.; Alkire, R.C. The role of inclusions on initiation of crevice corrosion of stainless steel. J. Electrochem. Soc. 1989, 136, 973–979. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.D.; Sun, W.H.; Wang, J.Q. Influence of oxidation related structural defeats on localized corrosion in HVAF-sprayed Fe-based metallic coatings. Surf. Coat. Technol. 2018, 335, 205–218. [Google Scholar] [CrossRef]
- Gou, R.Q.; Zhang, C.; Chen, Q.; Yang, Y.; Li, N.; Liu, L. Study of structure and corrosion resistance of Fe-based amorphous coatings prepared by HVAF and HVOF. Corros. Sci. 2011, 53, 2351–2356. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, H.X.; Jeong, Y.U.; Yi, S. Effects of electrolyte pH on the electrochemical behavior of Fe-based bulk metallic glass. Met. Mater. Int. 2012, 18, 791–797. [Google Scholar] [CrossRef]


| No. | Oxygen Flow Rate (m3/h) | Kerosene Flow Rate (L/h) | Feed Rate (g/min) | Spraying Distance (mm) | Speed of Gun (m/min) |
|---|---|---|---|---|---|
| 1 | 50 | 32 | 72 | 380 | 10 |
| 2 | 50 | 29 | 72 | 380 | 10 |
| 3 | 50 | 26 | 72 | 380 | 10 |
| 4 | 50 | 23 | 72 | 380 | 10 |
| 5 | 42 | 15 | 50 | 300 | 10 |
| 6 | 42 | 15 | 50 | 300 | 8 |
| 7 | 42 | 15 | 50 | 300 | 6 |
| 8 | 42 | 15 | 50 | 300 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, S.; Li, H.; Wang, S.; Chen, Y. Effect of Oxidation and Crystallization on Pitting Initiation Behavior of Fe-Based Amorphous Coatings. Coatings 2022, 12, 176. https://doi.org/10.3390/coatings12020176
Zhang H, Wang S, Li H, Wang S, Chen Y. Effect of Oxidation and Crystallization on Pitting Initiation Behavior of Fe-Based Amorphous Coatings. Coatings. 2022; 12(2):176. https://doi.org/10.3390/coatings12020176
Chicago/Turabian StyleZhang, Haoran, Shanlin Wang, Hongxiang Li, Shuaixing Wang, and Yuhua Chen. 2022. "Effect of Oxidation and Crystallization on Pitting Initiation Behavior of Fe-Based Amorphous Coatings" Coatings 12, no. 2: 176. https://doi.org/10.3390/coatings12020176
APA StyleZhang, H., Wang, S., Li, H., Wang, S., & Chen, Y. (2022). Effect of Oxidation and Crystallization on Pitting Initiation Behavior of Fe-Based Amorphous Coatings. Coatings, 12(2), 176. https://doi.org/10.3390/coatings12020176







