Multiwalled-Carbon-Nanotubes (MWCNTs)–GPTMS/Tannic-Acid-Nanocomposite-Coated Cotton Fabric for Sustainable Antibacterial Properties and Electrical Conductivity
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Chemicals
2.3. Preparation of GPTMS Sol
2.4. Coating of Fabrics Using MWCNTs–GPTMS Nano Sol
2.5. Coating of Fabrics Using GPTMS/MWCNTs/Tannic Acid Nanocomposite
3. Characterization
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. Scanning Electron Micrograph SEM/EDX Analysis
3.3. Antibacterial Test
3.4. UV Protection Factor
3.5. Electrical Conductivity Properties
3.6. Tensile Strength
3.7. Roughness
3.8. Statistical Analysis
4. Results and Discussion
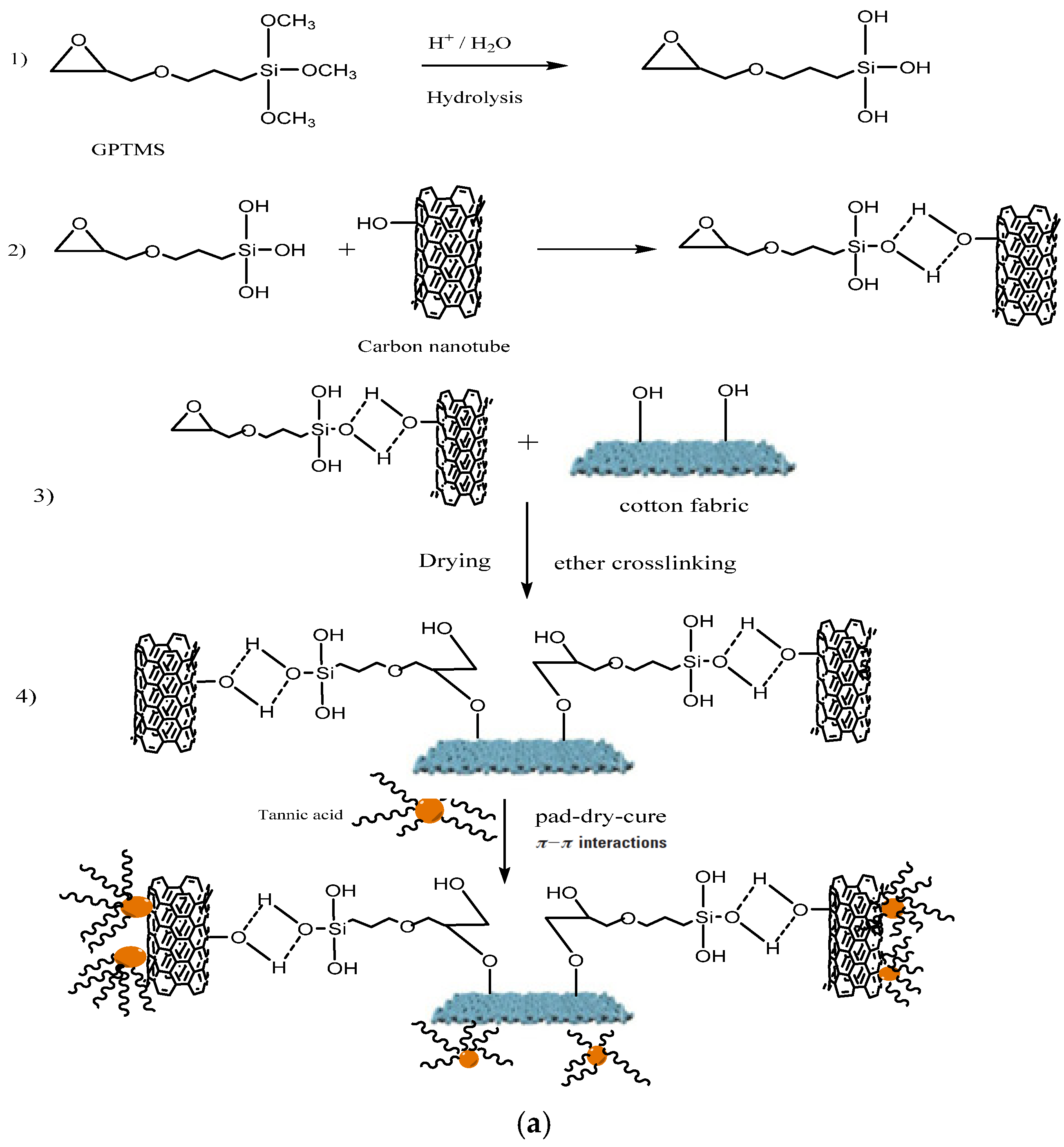
4.1. Mechanism of Deposition for MWCNTs–GPTMS-Tannic Nanocomposite on Cotton Fabric
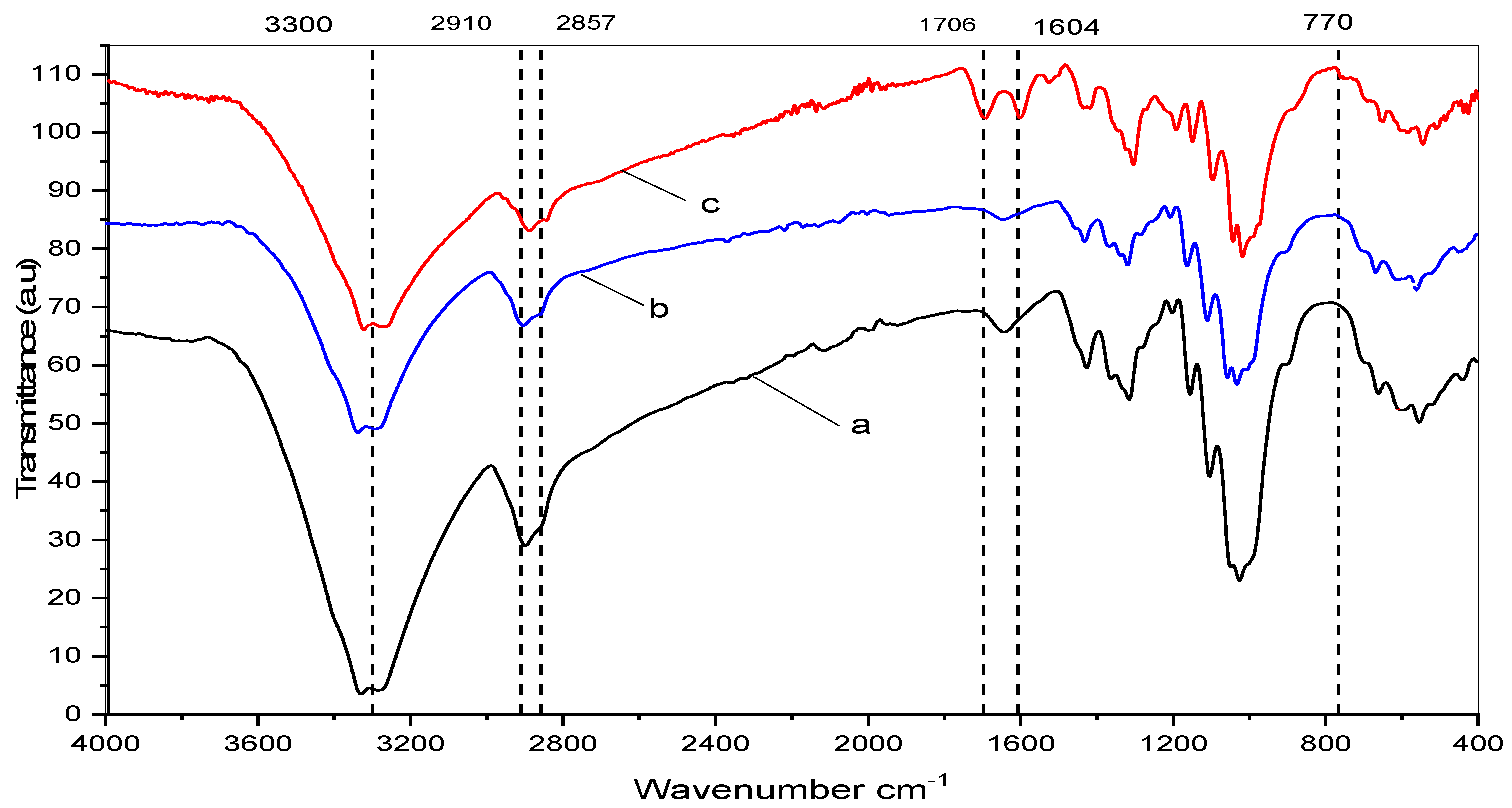
4.2. IR Analysis
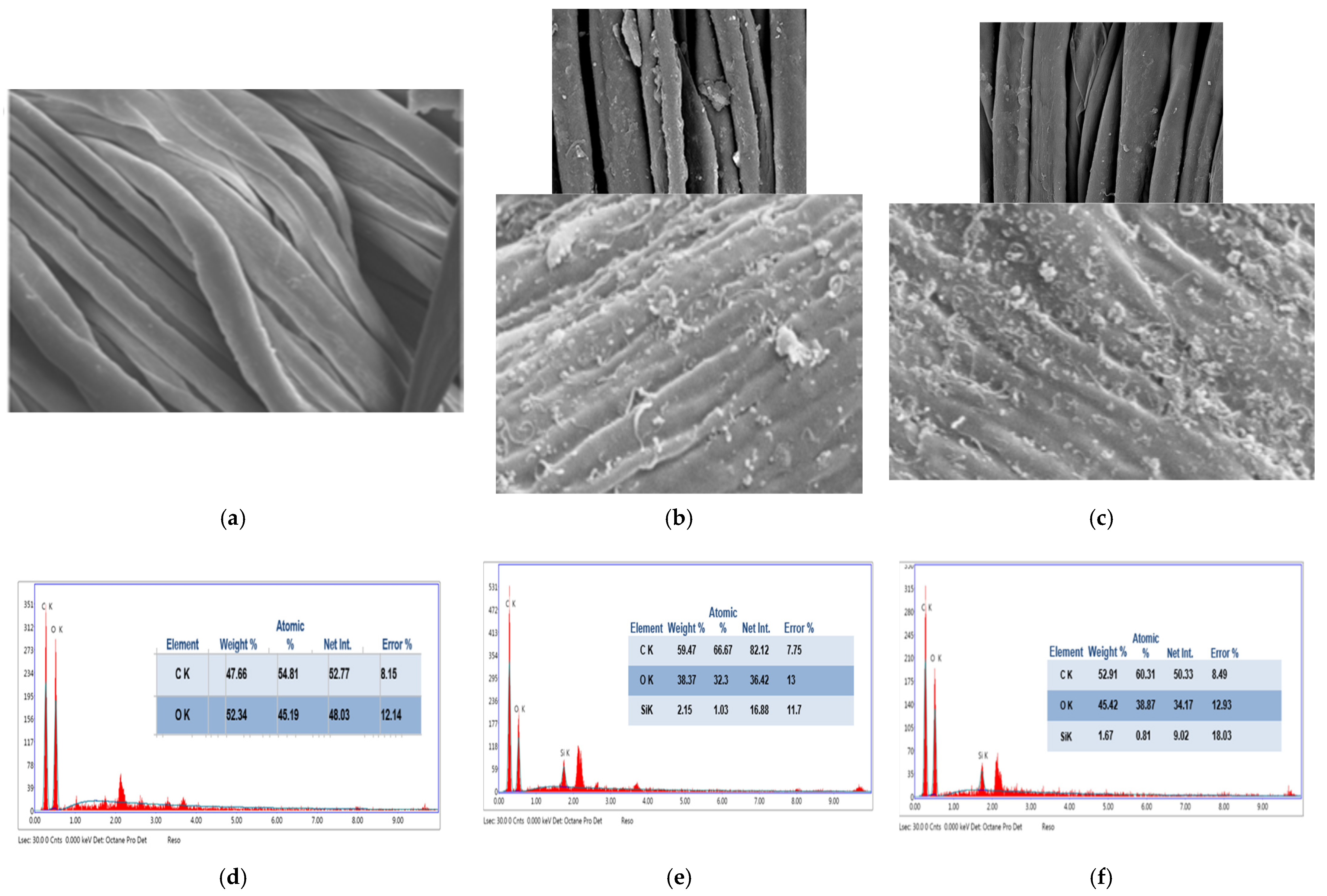
4.3. SEM Analysis
4.4. Antibacterial Activity
4.5. UV Protection Properties
4.6. Analysis of Conductivity
4.7. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bharath, S.P.; Manjanna, J.; Javeed, A.; Yallappa, S. Multi-walled carbon nanotube-coated cotton fabric for possible energy storage devices. Bull. Mater. Sci. 2015, 38, 169–172. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Naggar, M.E.; Tawfik, S.; Zaghloul, S.; Sharaf, S. Hyperbranched polymer–silver nanohybrid induce super antibacterial activity and high performance to cotton fabric. Cellulose 2019, 26, 3543–3555. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Higazy, A.; AbdelHady, M.; Sharaf, S. Novel route for antibacterial finishing of cotton fabric based on Ag loaded cyclodextrin—PAN copolymers. Egypt. J. Chem. 2016, 59, 887–910. [Google Scholar]
- Karst, D.; Yang, Y. Potential Advantages and Risks of Nanotechnology for Textiles. AATCC Rev. 2006, 6, 44–48. [Google Scholar]
- Abd El-Hady, M.; Farouk, A.; Sharaf, S. Flame retardancy and UV protection of cotton-based fabrics using nano ZnO and polycarboxylic acids. Carbohydr. Polym. 2013, 92, 400–406. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. High performance fabrics via innovative reinforcement route using cellulose nanoparticles. J. Text. Inst. 2018, 109, 186–194. [Google Scholar] [CrossRef]
- Sharaf, S.; Farouk, A.; El-Hady, M. Novel conductive textile fabric based on polyaniline and CuO nanoparticles. Int. J. PharmTech Res. 2016, 9, 461–472. [Google Scholar]
- Abd El-Hady, M.; Sharaf, S.; Farouk, A. Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 2020, 27, 1099–1110. [Google Scholar] [CrossRef]
- Farouk, A.; Saeed, S.E.S.; Sharaf, S.; Abd El-Hady, M.M. Photocatalytic activity and antibacterial properties of linen fabric using reduced graphene oxide/silver nanocomposite. RSC Adv. 2020, 10, 41600–41611. [Google Scholar] [CrossRef]
- El-Tahlawy, K.F.; El-Bendary, M.A.; Elhendawy, A.G.; Hudson, S.M. The antimicrobial activity of cotton fabrics treated with different crosslinking agents and chitosan. Carbohydr. Polym. 2005, 60, 421–430. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Huang, Z.K.; Huang, Z.Z.; Tseng, Z.S. Antimicrobial and physical properties of woolen fabrics cured with citric acid and chitosan. J. Appl. Polym. Sci. 2004, 94, 1999–2007. [Google Scholar] [CrossRef]
- Ghigo, G.; Berto, S.; Minella, M.; Vione, D.; Alladio, E.; Nurchi, V.M.; Lachowicz, J.; Daniele, P.G. New insights into the protogenic and spectroscopic properties of commercial tannic acid: The role of gallic acid impurities. New J. Chem. 2018, 42, 7703–7712. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Liu, R.; Luo, J.; Zhu, Y.; Wei, W.; Liu, X. Tannic acid functionalized UV-curable carbon nanotube: Effective reinforcement of acrylated epoxidized soybean oil coating. Prog. Org. Coat. 2019, 130, 214–220. [Google Scholar] [CrossRef]
- Montazer, M.; Shamei, A.; Alimohammadi, F. Stabilized nanosilver loaded nylon knitted fabric using BTCA without yellowing. Prog. Org. Coat. 2012, 74, 270–276. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Shamei, A. A novel method for coating of carbon nanotube on cellulose fiber using 1, 2, 3, 4-butanetetracarboxylic acid as a cross-linking agent. Prog. Org. Coat. 2012, 74, 470–478. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Shamei, A. Functional cellulose fibers via polycarboxylic acid/carbon nanotube composite coating. J. Coat. Technol. Res. 2013, 10, 123–132. [Google Scholar] [CrossRef]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- Xue, P.; Park, K.H.; Tao, X.M.; Chen, W.; Cheng, X.Y. Electrically conductive yarns based on PVA/carbon nanotubes. Compos. Struct. 2007, 78, 271–277. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Li, Z.; Liu, P.; Chen, D.; Zhang, Y. Mechanical strength improvement of polypropylene threads modified by PVA/CNT composite coatings. Mater. Lett. 2008, 62, 4380–4382. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Mozaffari, A. Polyvinylpyrrolidone/carbon nanotube/cotton functional nanocomposite: Preparation and characterization of properties. Fibers Polym. 2018, 19, 1940–1947. [Google Scholar] [CrossRef]
- Zheng, L.; Su, X.; Lai, X.; Chen, W.; Li, H.; Zeng, X. Conductive superhydrophobic cotton fabrics via layer-by-layer assembly of carbon nanotubes for oil-water separation and human motion detection. Mater. Lett. 2019, 253, 230–233. [Google Scholar] [CrossRef]
- Fugetsu, B.; Akiba, E.; Hachiya, M.; Endo, M. The production of soft, durable, and electrically conductive polyester multifilament yarns by dye-printing them with carbon nanotubes. Carbon 2009, 47, 527–530. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, H.; Zheng, H.; Bai, R. A facile approach for the fabrication of highly stable superhydrophobic cotton fabric with multi-walled carbon nanotubes−azide polymer composites. Langmuir 2010, 26, 7529–7534. [Google Scholar] [CrossRef] [PubMed]
- Karimi, L.; Zohoori, S.; Amini, A. Multi-wall carbon nanotubes and nano titanium dioxide coated on cotton fabric for superior self-cleaning and UV blocking. New Carbon Mater. 2014, 29, 380–385. [Google Scholar] [CrossRef]
- Baker, C.N.; Stocker, S.A.; Culver, D.H.; Thornsberry, C. Comparison of the E Test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J. Clin. Microbiol. 1991, 29, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkerfeldt, M.; Strååt, M.; Walkenström, P. Electrically conductive textile coating with a PEDOT-PSS dispersion and a polyurethane binder. Text. Res. J. 2013, 83, 618–627. [Google Scholar] [CrossRef]
- Kasaw, E.; Haile, A.; Getnet, M. Conductive Coatings of Cotton Fabric Consisting of Carbonized Charcoal for E-Textile. Coatings 2020, 10, 579. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L.; Roberson, E.D. Quantifying biomarkers of cognitive dysfunction and neuronal network hyperexcitability in mouse models of Alzheimer’s disease: Depletion of calcium-dependent proteins and inhibitory hippocampal remodeling. In Alzheimer’s Disease and Frontotemporal Dementia; Springer: Basel, Switzerland, 2010; pp. 245–262. [Google Scholar]
- Rosace, G.; Trovato, V.; Colleoni, C.; Caldara, M.; Re, V.; Brucale, M.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; De Luca, G.; et al. Structural and morphological characterizations of MWCNTs hybrid coating onto cotton fabric as potential humidity and temperature wearable sensor. Sens. Actuators B Chem. 2017, 252, 428–439. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption of phenolic compounds by carbon nanotubes: Role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, C.; Su, W.; Wangyan, L.V.; Zhu, S.; Wang, F.; Fu, Q. Water dispersed multi-walled carbon nanotubes modified by tannin acid. Mater. Lett. 2014, 123, 44–47. [Google Scholar] [CrossRef]
- Samanta, K.K.; Basak, S.; Chattopadhyay, S. Sustainable dyeing and finishing of textiles using natural ingredients and water-free technologies. In Textiles and Clothing Sustainability; Springer: Basel, Switzerland, 2017; pp. 99–131. [Google Scholar]
- Zhang, W.; Ji, X.; Yin, Y.; Wang, C. Temperature induced color changing cotton fabricated via grafting epoxy modified thermochromic capsules. Cellulose 2019, 26, 5745–5756. [Google Scholar] [CrossRef]
- Suarez Vega, A.; Agustín-Sáenz, C.; Brusciotti, F.; Somers, A.; Forsyth, M. Effect of lanthanum 4-hydroxy cinnamate on the polymerisation, condensation and thermal stability of hybrid sol–gel formulations. J. Sol-Gel Sci. Technol. 2020, 96, 91–107. [Google Scholar] [CrossRef]
- Sangili, A.; Annalakshmi, M.; Chen, S.M.; Balasubramanian, P.; Sundrarajan, M. Synthesis of silver nanoparticles decorated on core-shell structured tannic acid-coated iron oxide nanospheres for excellent electrochemical detection and efficient catalytic reduction of hazardous 4-nitrophenol. Compos. Part B Eng. 2019, 162, 33–42. [Google Scholar] [CrossRef]
- Shang, Q.; Hu, L.; Yang, X.; Hu, Y.; Bo, C.; Pan, Z.; Ren, X.; Liu, C.; Zhou, Y. Superhydrophobic cotton fabric coated with tannic acid/polyhedral oligomeric silsesquioxane for highly effective oil/water separation. Prog. Org. Coat. 2021, 154, 106191. [Google Scholar] [CrossRef]
- Gashti, M.P.; Alimohammadi, F.; Song, G.; Kiumarsi, A. Characterization of nanocomposite coatings on textiles: A brief review on microscopic technology. Curr. Microsc. Contrib. Adv. Sci. Technol. 2012, 2, 1424–1437. [Google Scholar]
- Matsuoka, M.; Akasaka, T.; Totsuka, Y.; Watari, F. Strong adhesion of Saos-2 cells to multi-walled carbon nanotubes. Mater. Sci. Eng. B 2010, 173, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Upadhyayula, V.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Tucci, M.; Benghuzzi, H. Evaluation of the antimicrobial efficacy of green tea extract (egcg) against streptococcus pyogenes in vitro-biomed 2011. Biomed. Sci. Instrum. 2011, 47, 177–182. [Google Scholar]
- Khatoon, U.T.; Nageswara Rao, G.V.S.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Comparative study of antifungal activity of silver and gold nanoparticles synthesized by facile chemical approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Yamout, S.Z.; Sidani, M.M. Tannins protect against skin tumor promotion induced by ultraviolet-B radiation in hairless mice. Nutr. Cancer 2000, 37, 73–77. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, Z.; Wang, P.; Zhu, Z.; Xu, J.; Chen, S.; Wang, H. The Use of Copper Ions and Tannic Acid to Enhance the UV Protection of Cotton Fabrics. J. Nat. Fibers 2020, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Qi, K.; Xin, J.H. Functionalization of cotton with carbon nanotubes. J. Mater. Chem. 2008, 18, 3454–3460. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Gou, J.; Leng, J.; Du, S. Electrical properties and shape-memory behavior of self-assembled carbon nanofiber nano paper incorporated with shape-memory polymer. Smart Mater. Struct. 2010, 19, 075021. [Google Scholar] [CrossRef]
- Peng, M.; Qi, J.; Zhou, Z.; Liao, Z.; Zhu, Z.; Guo, H. Carbon nanotubes noncovalently functionalized by an organic−inorganic hybrid: New building blocks for constructing superhydrophobic conductive coatings. Langmuir 2010, 26, 13062–13064. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Jarrais, B.; Pereira, C.; Morgado, J.; Freire, C.; Pereira, M.F.R. Functionalization of textiles with multi-walled carbon nanotubes by a novel dyeing-like process. J. Mater. Sci. 2012, 47, 5263–5275. [Google Scholar] [CrossRef]
- Gashti, M.P.; Almasian, A. UV radiation induced flame retardant cellulose fiber by using polyvinylphosphonic acid/carbon nanotube composite coating. Compos. Part B Eng. 2013, 45, 282–289. [Google Scholar] [CrossRef]
- Yazhini, K.B.; Prabu, H.G. Antibacterial activity of cotton coated with ZnO and ZnO-CNT composites. Appl. Biochem. Biotechnol. 2015, 175, 85–92. [Google Scholar] [CrossRef] [PubMed]

| Inhibition Zone (mm per 1 cm Sample) | ||||||||
|---|---|---|---|---|---|---|---|---|
| G+ | G− | |||||||
| Substrate/Treatment of Fabric | S. aureus | B. subtilis | E. coli | P. aeruginosa | ||||
| No. of Washing Cycle | 1 | 20 | 1 | 20 | 1 | 20 | 1 | 20 |
| cotton/GPTMS | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| cotton/tannic acid | 21 | 20 | 26 | 23 | 20 | 19 | 17 | 15 |
| cotton/GPTMS/MWCNTs | 16 | 15 | 20 | 17 | 19 | 18 | 17 | 15 |
| cotton/GPTMS/MWCNTs then tannic acid | 26 | 25 | 22 | 21 | 24 | 23 | 23 | 22 |
| tannic acid/cotton/GPTMS/MWCNTs | 18 | 16 | 17 | 16 | 16 | 15 | 18 | 17 |
| Treatment | UPF Value | UV Protection | |
|---|---|---|---|
| Blank | 7.1 | 5 | Non-ratable |
| Cotton/GPTMS | 6.8 | 5 | Non-ratable |
| Cotton/tannic acid | 96.2 | 50+ | Excellent |
| Cotton/GPTMS/MWCNTs | 73.6 | 50+ | Excellent |
| Cotton/GPTMS/MWCNTs then tannic acid | 152.1 | 50+ | Excellent |
| Tannic acid/cotton/GPTMS/MWCNTs | 75.3 | 50+ | Excellent |
| Treatment | Resistivity Ω/cm |
|---|---|
| Untreated | 245 |
| Cotton/GPTMS | 148 |
| Cotton/tannic acid | 150 |
| Tannic acid/cotton/GPTMS/MWCNTs | 115 |
| Cotton/GPTMS/MWCNTs | 120 |
| Cotton/GPTMS/MWCNTs then tannic acid | 145 |
| Treatment | Tensile Strength (Kg f) | Roughness (µm) |
|---|---|---|
| Untreated cotton fabric | 55 ± 0.86 | 14.5 ± 0.41 |
| Cotton-GPTMS | 46 ± 0.76 | 12.3 ± 0.37 |
| Cotton-tannic acid | 52 ± 1.50 | 12.7 ± 0.25 |
| Cotton-GPTMS-MWCNTs | 78 ± 0.36 | 15.4 ± 0.35 |
| Cotton-GPTMS–MWCNTs drying then tannic acid | 75 ± 0.5 | 14.9 ± 0.26 |
| Tannic acid-cotton-GPTMS–MWCNTs | 72 ± 0.65 | 15 ± 0.21 |
| Previous Work | Our Work | ||
|---|---|---|---|
| Treatment | Functionalities | Treatment | Functionalities |
| Cotton fabrics were treated with nano titanium dioxide and multiwalled carbon nanotubes (MWCNTs) using succinic acid as a crosslinking agent [26] | -The abrasion resistance and UV blocking capability are improved, - tensile strength decreases -significant influence on the self-cleaning properties of the treated cotton fabricsparticularly when exposed to sunlight | Treatment of cotton fabric in Presence of ecofriendly tannic acid as well as GPTMS is the mean key role in improvement of functions | Extra antibacterial, UV protection, improvement in tensile strength, electrical conductivity |
| A dip–dry–cure finishing process was used to coat cotton fabric with MWCNTs functionalized with poly(butylacrylate) [48]. | -Durable superhydrophobicity, -self-cleaning and flame retardancy -improved mechanical resistance and UV protection | ||
| Coating of MWCNTs onto cotton by ultrasonic irradiation followed by the dipping method [24] | hydrophobicity to the fabric, this property was not durable | ||
| MWCNTs were coated with tetraethylorthosilicate and a fluoroalkoxysilane after being noncovalently functionalized with an organic–inorganic hybrid consisting of silica and an amphiphilic copolymer of styrene and maleic anhydride [50]. | Hydrophobicity and improved conductivity | ||
| The exhaustion method was used to coat carbon nanotubes (CNTs) and fix them on a cotton surface utilising 1,2,3,4-butanetetracarboxylic acid (BTCA) as a crosslinking agent [17] | Increase the thermal stability of the substrate. | ||
| A dyeing-like method was used to successfully incorporate MWCNTs into cotton and polyester substrates [51]. | Superhydrophobic behavior, flame-retardant | ||
| MWCNTs were stabilized on a cotton surface using vinylphosphonic acid via UV irradiation [52] | High-efficient flame retardant finishing of cotton fabrics and improvement of its thermal properties. | ||
| (BTCA) and ZnO-BTCA-carbon nanotube (CNT) composites were fabricated and coated on cotton fabric by pad-dry-cure [53] | Very good antibacterial activity | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hady, M.M.; Farouk, A.; Sharaf, S. Multiwalled-Carbon-Nanotubes (MWCNTs)–GPTMS/Tannic-Acid-Nanocomposite-Coated Cotton Fabric for Sustainable Antibacterial Properties and Electrical Conductivity. Coatings 2022, 12, 178. https://doi.org/10.3390/coatings12020178
Abd El-Hady MM, Farouk A, Sharaf S. Multiwalled-Carbon-Nanotubes (MWCNTs)–GPTMS/Tannic-Acid-Nanocomposite-Coated Cotton Fabric for Sustainable Antibacterial Properties and Electrical Conductivity. Coatings. 2022; 12(2):178. https://doi.org/10.3390/coatings12020178
Chicago/Turabian StyleAbd El-Hady, Marwa M., Asmaa Farouk, and Samar Sharaf. 2022. "Multiwalled-Carbon-Nanotubes (MWCNTs)–GPTMS/Tannic-Acid-Nanocomposite-Coated Cotton Fabric for Sustainable Antibacterial Properties and Electrical Conductivity" Coatings 12, no. 2: 178. https://doi.org/10.3390/coatings12020178






