Surface Modification of 6xxx Series Aluminum Alloys
Abstract
:1. Introduction
1.1. 6xxx Grade Al Alloys
1.1.1. Alloy 6005A
1.1.2. Alloy 6061
1.1.3. Alloy 6063
1.1.4. Alloy 6082
2. Need for Surface Modification of 6xxx Series Al Alloys
- (i)
- pitting corrosion;
- (ii)
- stress corrosion cracking;
- (iii)
- intergranular corrosion.
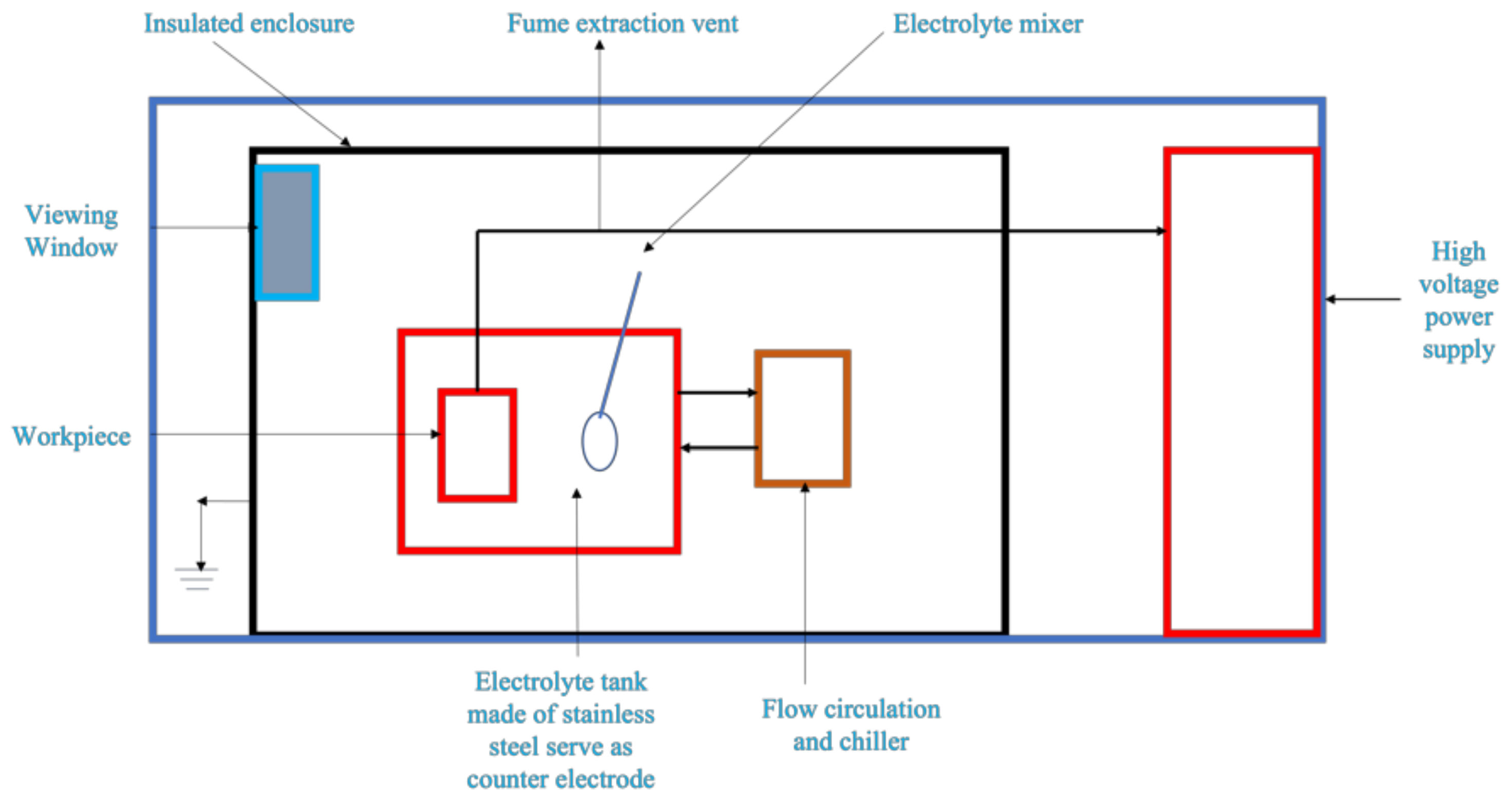
3. Anodizing
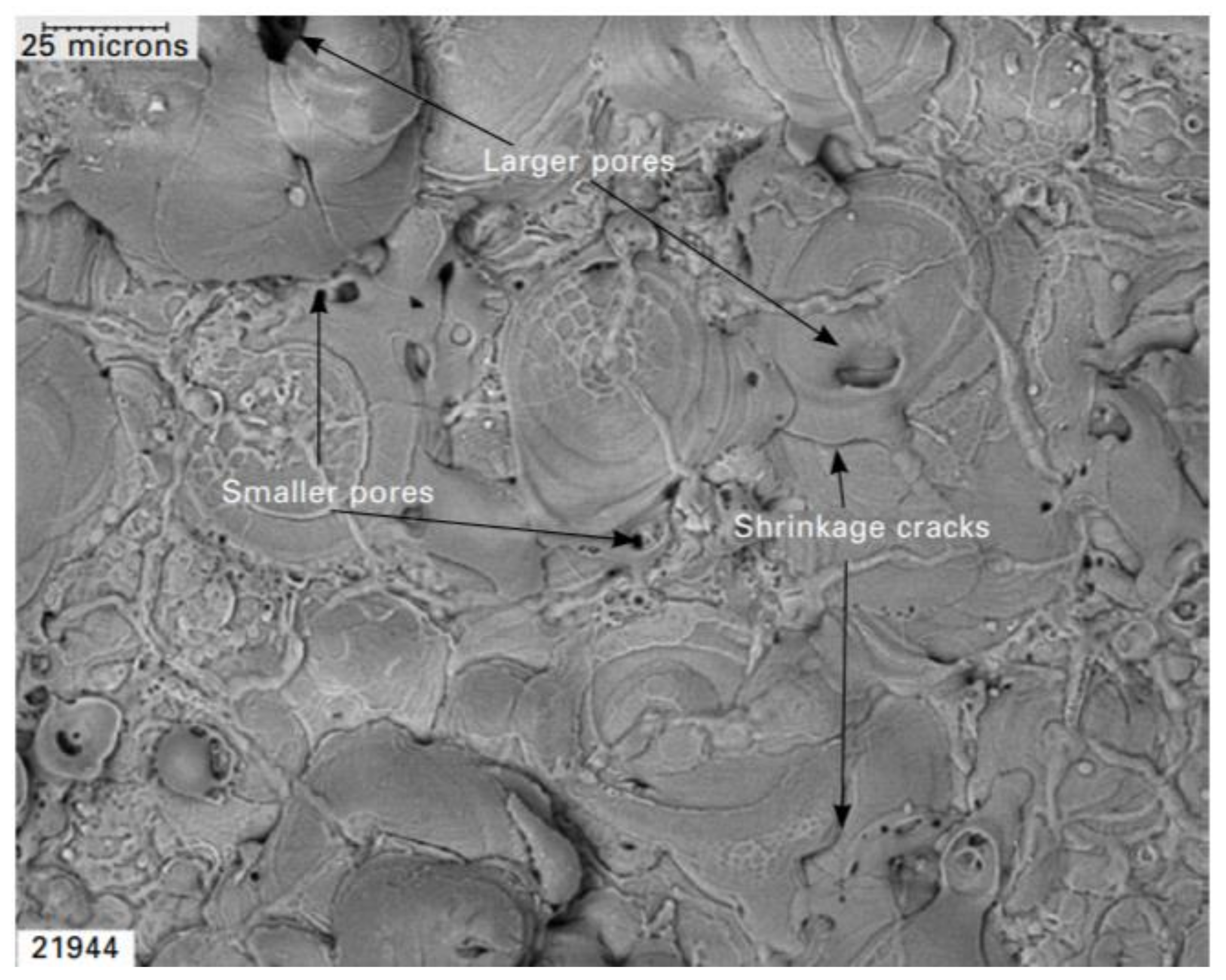
4. Plasma Electrolytic Oxidation (PEO)
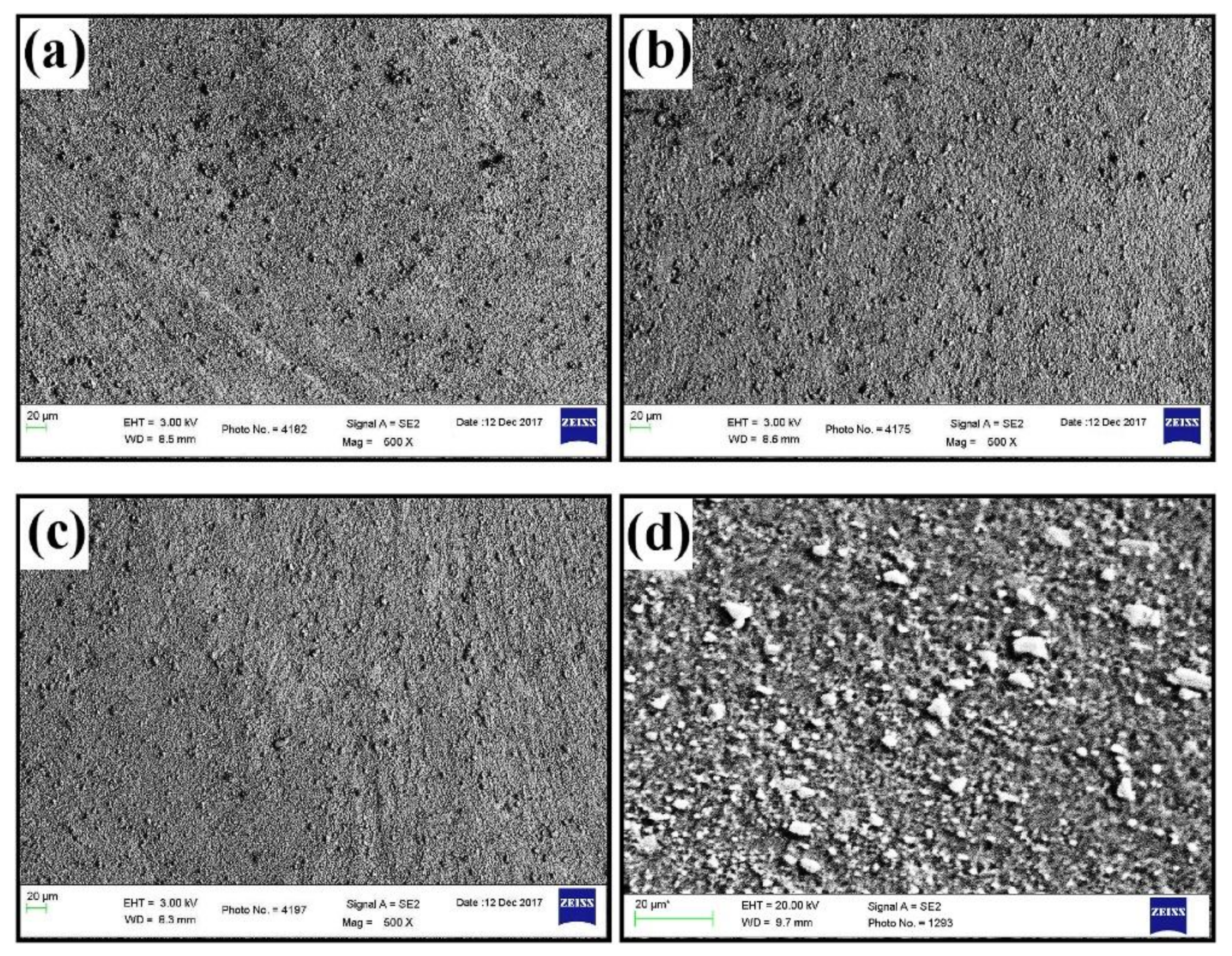
4.1. PEO of 6061 Al Alloys
4.2. PEO of 6063 Al Alloys
4.3. PEO of 6082 Al Alloys
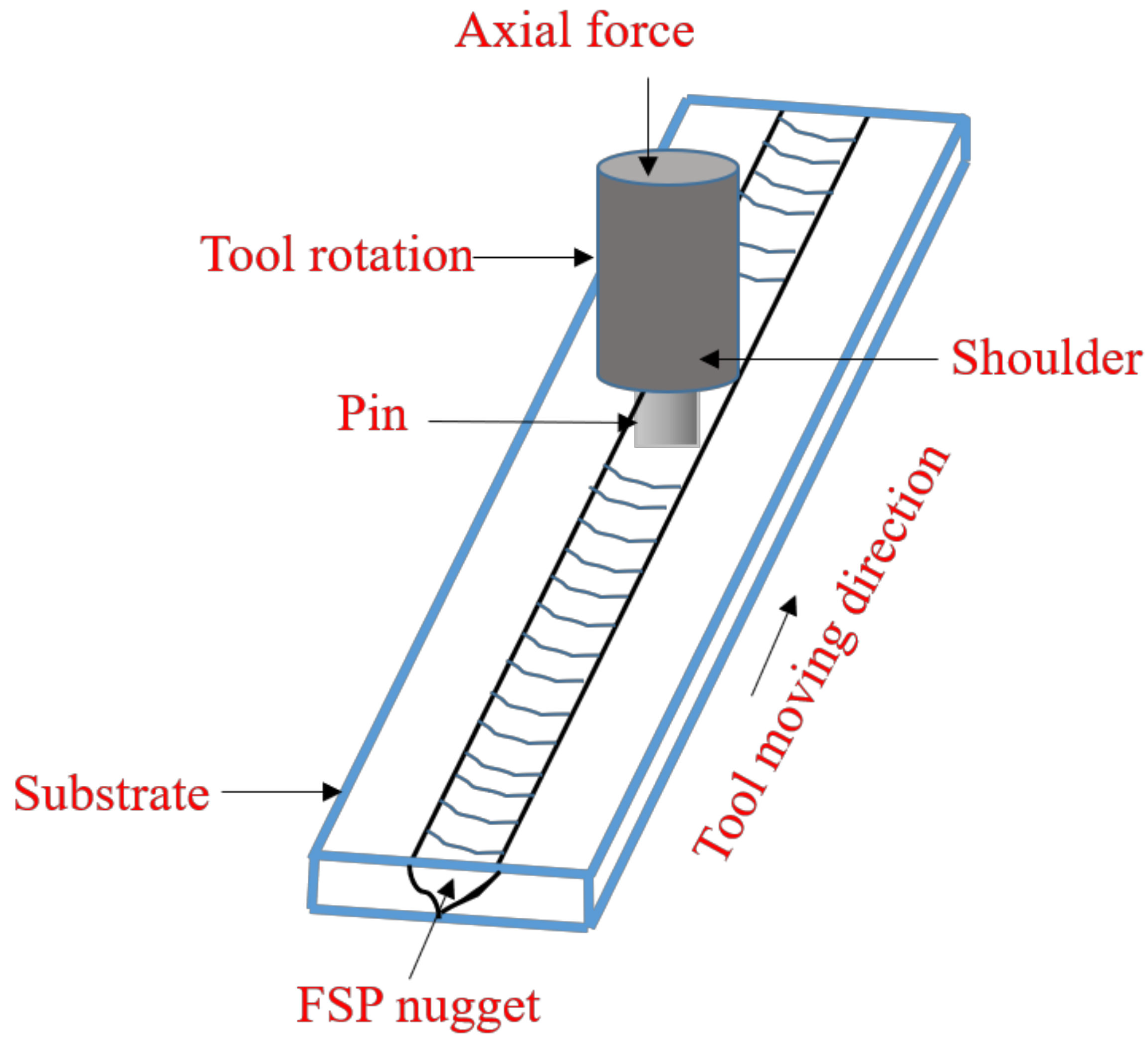
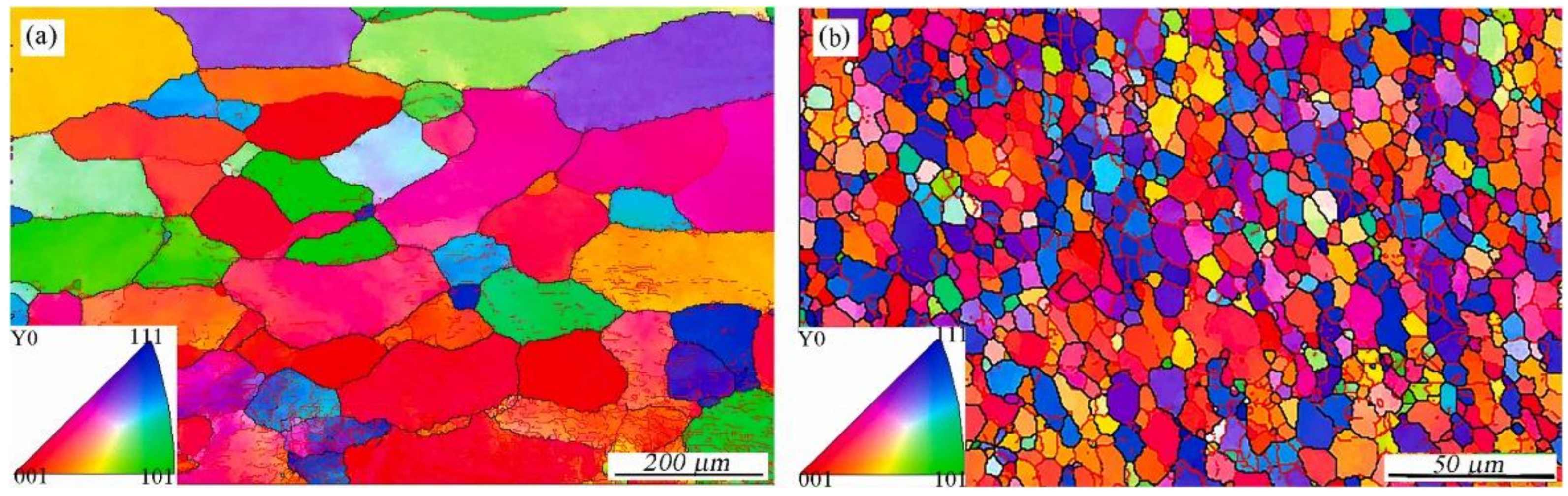
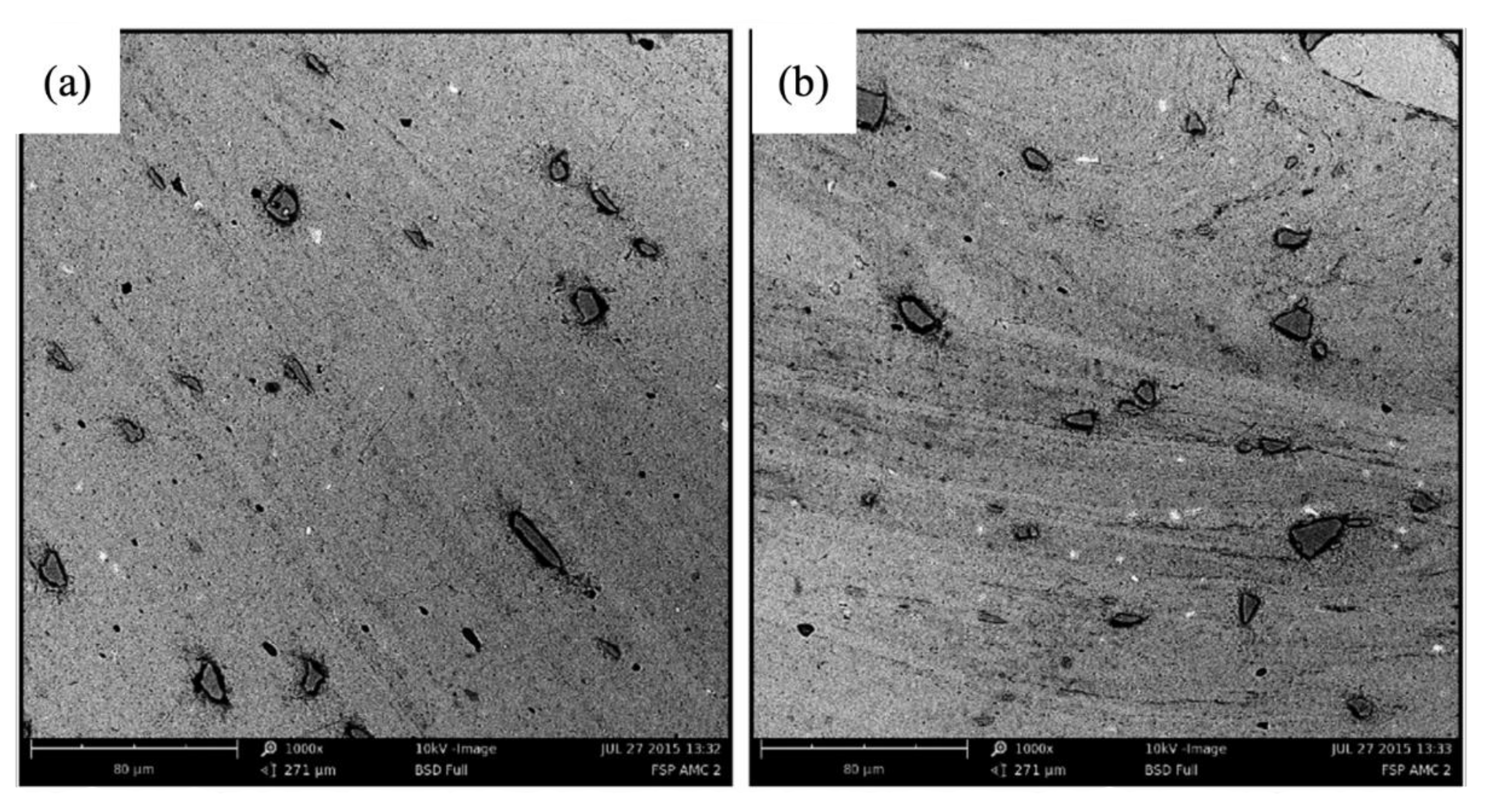
5. Friction Stir Processing
5.1. FSP without Any Additions
5.2. FSP with the Addition of Surface Alloying Elements
5.3. FSP with the Addition of Hard Reinforcement Phases
6. Friction Surfacing
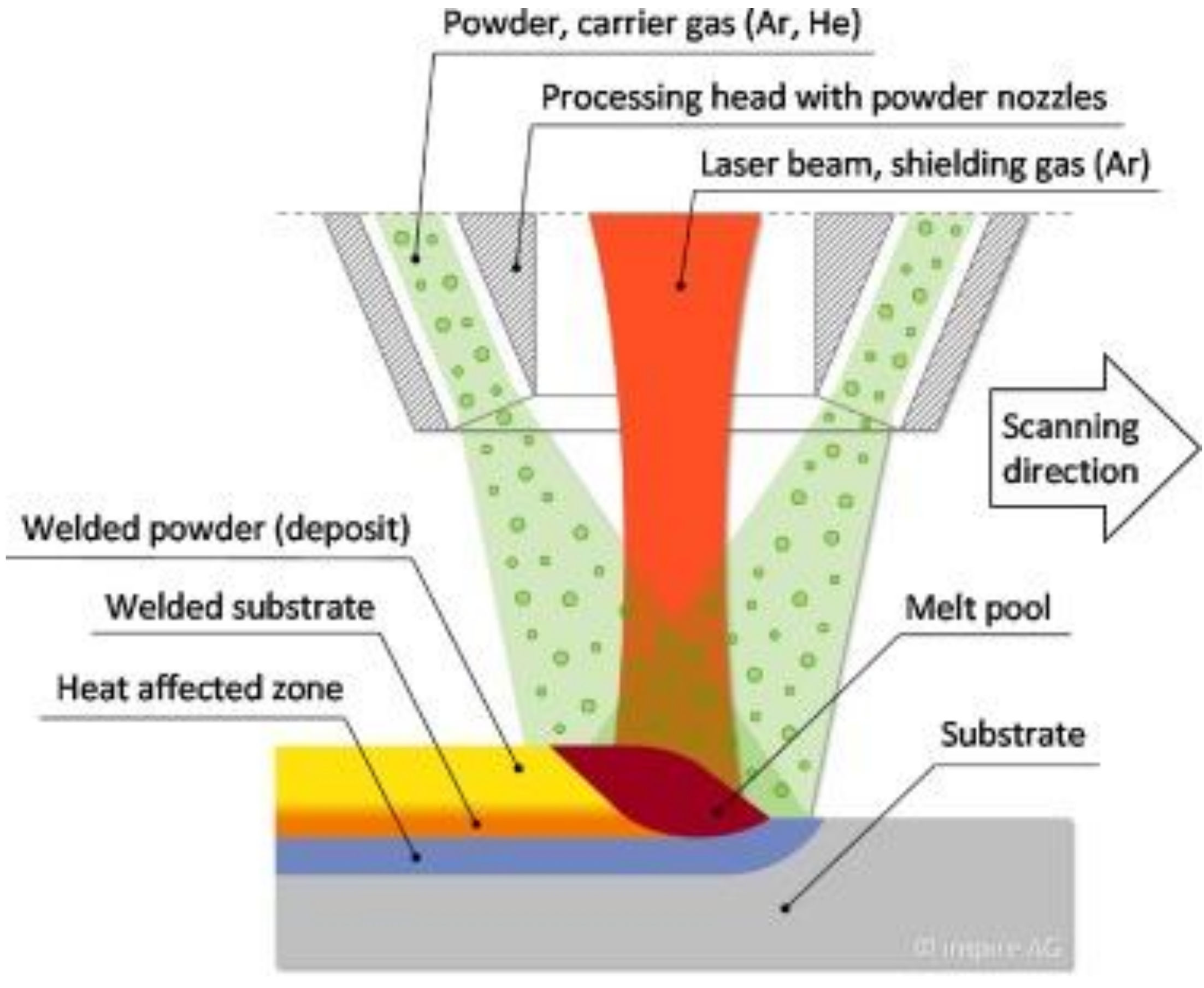
7. Laser Cladding
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| BC | Boron Carbide |
| BN | Boron Nitride |
| CNT | Carbon nanotube |
| DC | Direct Current |
| FSP | Friction Stir Processing |
| FSW | Friction Stir Welding |
| SEM | Scanning Electron Microscopy |
| PEO | Plasma Electrolytic Oxidation |
| UTS | Ultimate Tensile Strength |
| YS | Yield Strength |
References
- Riquelme, A.; Rodrigo, P.; Escalera-Rodríguez, M.D.; Rams, J. Corrosion Resistance of Al/SiC Laser Cladding Coatings on AA6082. Coatings 2020, 10, 673. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Alaneme, K.K.; Chown, L.H. Aluminium Matrix Hybrid Composites: A Review of Reinforcement Philosophies; Mechanical, Corrosion and Tribological Characteristics. J. Mater. Res. Technol. 2015, 4, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhao, G.; Liu, C.; Zuo, L. Effects of Different Tempers on Precipitation Hardening of 6000 Series Aluminium Alloys. Trans. Nonferrous Met. Soc. China 2007, 17, 122–127. [Google Scholar] [CrossRef]
- Tisza, M.; Lukács, Z. High Strength Aluminum Alloys in Car Manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 418, 012033. [Google Scholar] [CrossRef]
- Chi, Y.; Gu, G.; Yu, H.; Chen, C. Laser Surface Alloying on Aluminum and Its Alloys: A Review. Opt. Lasers Eng. 2018, 100, 23–37. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. [Google Scholar] [CrossRef]
- Rahman, M.; Ariffin, A.; Jamaludin, B.N.J.B.N.; Haron, C. Fatigue behaviour of 6000 series aluminium alloys on cylinder block of a free piston linear engine using total life approach. J. Mek. 2006, 21, 1–15. [Google Scholar]
- Grohol, C.M.; Shin, Y.C.; Frank, A. Laser Cladding of Aluminum Alloy 6061 via Off-Axis Powder Injection. Surf. Coat. Technol. 2021, 415, 127099. [Google Scholar] [CrossRef]
- Sweet, G.A.; Brochu, M.; Hexemer, R.L.; Donaldson, I.W.; Bishop, D.P. Consolidation of Aluminum-Based Metal Matrix Composites via Spark Plasma Sintering. Mater. Sci. Eng. A 2015, 648, 123–133. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
- John, M.; Ralls, A.M.; Dooley, S.C.; Thazhathidathil, A.K.V.; Perka, A.K.; Kuruveri, U.B.; Menezes, P.L. Ultrasonic Surface Rolling Process: Properties, Characterization, and Applications. Appl. Sci. 2021, 11, 10986. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent Development in Aluminium Alloys for the Automotive Industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Kaufman, J.G. Introduction to Aluminum Alloys and Tempers; ASM International: Russell Township, OH, USA, 2000; ISBN 978-0-87170-689-8. [Google Scholar]
- Miao, W.F.; Laughlin, D.E. Precipitation Hardening in Aluminum Alloy 6022. Scr. Mater. 1999, 40, 873–878. [Google Scholar] [CrossRef]
- Lang, P.; Povoden-Karadeniz, E.; Falahati, A.; Kozeschnik, E. Simulation of the Effect of Composition on the precipitation in 6xxx Al Alloys during Continuous-Heating DSC. J. Alloys Compd. 2014, 612, 443–449. [Google Scholar] [CrossRef]
- Summers, P.T.; Chen, Y.; Rippe, C.M.; Allen, B.; Mouritz, A.P.; Case, S.W.; Lattimer, B.Y. Overview of Aluminum Alloy Mechanical Properties during and after Fires. Fire Sci. Rev. 2015, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Ilangovan, M.; Boopathy, S.R.; Balasubramanian, V. Microstructure and Tensile Properties of Friction Stir Welded Dissimilar AA6061–AA5086 Aluminium Alloy Joints. Trans. Nonferrous Met. Soc. China 2015, 25, 1080–1090. [Google Scholar] [CrossRef]
- Vahdani, M.; Mirnia, M.J.; Bakhshi-Jooybari, M.; Gorji, H. Electric Hot Incremental Sheet Forming of Ti-6Al-4V Titanium, AA6061 Aluminum, and DC01 Steel Sheets. Int. J. Adv. Manuf. Technol. 2019, 103, 1199–1209. [Google Scholar] [CrossRef]
- ASM Handbook Committee. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Russell Township, OH, USA, 1990. [Google Scholar]
- ASM International. Properties and Selection of Aluminum Alloys; ASM International: Russell Township, OH, USA, 2019. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Corrosion Behaviour of Different Tempers of AA7075 Aluminium Alloy. Electrochim. Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Montes, L.P.; Martinez, M.A.; Michael, J.; Hlava, P.F. The Electrochemical Characteristics of Bulk-Synthesized Al2CuMg. J. Electrochem. Soc. 1999, 146, 4424–4428. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.; Maeva, E.; Leshchinsky, E.; Maev, R.G. Corrosion Protection of Light Alloys Using Low Pressure Cold Spray. J. Therm. Spray Technol. 2012, 21, 304–313. [Google Scholar] [CrossRef]
- Cerchier, P.; Pezzato, L.; Gennari, C.; Moschin, E.; Moro, I.; Dabalà, M. PEO Coating Containing Copper: A Promising Anticorrosive and Antifouling Coating for Seawater Application of AA 7075. Surf. Coat. Technol. 2020, 393, 125774. [Google Scholar] [CrossRef]
- Zhang, P.; Nie, X.; Han, L.; Hu, H. Wear Protection of Al383/SiO2 Metal Matrix Composites by Plasma Electrolytic Oxidation (PEO) Process. SAE Int. J. Mater. Manuf. 2010, 3, 55–62. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Rotshtein, V.P.; Shulov, V.A. Surface Modification and Alloying of Aluminum and Titanium Alloys with Low-Energy, High-Current Electron Beams. J. Met. 2011, 2011, 673685. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Martin, M.C.; Ajayi, O.O. Surface Layer Modification of 6061 Al Alloy by Friction Stir Processing and Second Phase Hard Particles for Improved Friction and Wear Performance. J. Tribol. 2014, 136, 044501. [Google Scholar] [CrossRef]
- Polat, B.D.; Bilici, B.; Afşin, P.; Akyil, C.; Keles, O. A study of taguchi method to optimize 6XXX series aluminium anodic oxide film’s hardness and investigation of corrosion behaviors of oxide films. In Proceedings of the 145th Annual Meeting & Exhibition TMS 2016, Nashville, TN, USA, 14–18 February 2016; Springer: Cham, Switzerland, 2016; pp. 401–408. [Google Scholar]
- Avelar-Batista Wilson, J.C.; Grabowski, A.; Kustosik, K.; Wilson, A.D. Tartaric Acid Cross-Contamination in Post-Cascade Rinses after Sulphuric Acid Anodising (SAA): Effect on Adhesive Bond Strength of AA6060-T6 Alloy. Int. J. Adhes. Adhes. 2018, 81, 30–35. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Tanem, B.S.; Bjørgum, A.; Mårdalen, J.; Hallenstvet, M. Anodising as Pre-Treatment before Organic Coating of Extruded and Cast Aluminium Alloys. Corros. Sci. 2004, 46, 2081–2095. [Google Scholar] [CrossRef]
- Tabrizian, N.; Hansen, H.N.; Hansen, P.E.; Ambat, R.; Møller, P. Influence of Annealing and Deformation on Optical Properties of Ultra Precision Diamond Turned and Anodized 6060 Aluminium Alloy. Surf. Coat. Technol. 2010, 204, 2632–2638. [Google Scholar] [CrossRef]
- Short, E.P.; Bryant, A.J. A Review of Some Defects Appearing on Anodized Aluminium. Trans. IMF 1975, 53, 169–177. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Nilsson, J.-O.; Gustavsson, M.; Crispin, A. Origin of Streaks on Anodised Aluminium Alloy Extrusions. Trans. IMF 2013, 91, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Bononi, M.; Giovanardi, R.; Bozza, A. Pulsed Current Hard Anodizing of Heat Treated Aluminum Alloys: Frequency and Current Amplitude Influence. Surf. Coat. Technol. 2016, 307, 861–870. [Google Scholar] [CrossRef]
- Aggerbeck, M.; Canulescu, S.; Dirscherl, K.; Johansen, V.E.; Engberg, S.; Schou, J.; Ambat, R. Appearance of Anodised Aluminium: Effect of Alloy Composition and Prior Surface Finish. Surf. Coat. Technol. 2014, 254, 28–41. [Google Scholar] [CrossRef]
- Wielage, B.; Nickel, D.; Alisch, G.; Podlesak, H.; Lampke, T. Effects of Pre-Treatment on the Growth Rate and Morphology of Hard Anodic Films on Aluminium (EN AW-6082). Surf. Coat. Technol. 2007, 202, 569–576. [Google Scholar] [CrossRef]
- Gröber, D.; Georgi, W.; Sieber, M.; Scharf, I.; Hellmig, R.J.; Leidich, E.; Lampke, T.; Mayr, P. The Effect of Anodising on the Fatigue Performance of Self-Tapping Aluminium Screws. Int. J. Fatigue 2015, 75, 108–114. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R.; Petković, M.; Kasalica, B.; Belča, I.; Žekić, A.; Zeković, L. Characterization of the Plasma Electrolytic Oxidation of Titanium in Sodium Metasilicate. Appl. Surf. Sci. 2013, 265, 226–233. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L. Investigation of the Formation Mechanisms of Plasma Electrolytic Oxidation Coatings on Mg Alloy AM50 Using Particles. Electrochim. Acta 2016, 196, 680–691. [Google Scholar] [CrossRef]
- Dehnavi, V.; Liu, X.Y.; Luan, B.L.; Shoesmith, D.W.; Rohani, S. Phase Transformation in Plasma Electrolytic Oxidation Coatings on 6061 Aluminum Alloy. Surf. Coat. Technol. 2014, 251, 106–114. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. An Investigation of Oxide Coating Synthesized on an Aluminum Alloy by Plasma Electrolytic Oxidation in Molten Salt. Appl. Sci. 2017, 7, 889. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Jang, Y.-J.; Jung, J.P. Effect of KOH to Na2SiO3 Ratio on Microstructure and Hardness of Plasma Electrolytic Oxidation Coatings on AA 6061 Alloy. J. Mater. Eng. Perform. 2017, 26, 5032–5042. [Google Scholar] [CrossRef]
- Wang, K.; Koo, B.H.; Lee, C.G.; Kim, Y.J.; Lee, S.; Byon, E. Effects of Hybrid Voltages on Oxide Formation on 6061 Al-Alloys During Plasma Electrolytic Oxidation. Chin. J. Aeronaut. 2009, 22, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.J.; Wang, B.B.; Zhao, X.; Zhang, B.B. Robust Micro Arc Oxidation Coatings on 6061 Aluminum Alloys via Surface Thickening and Microvoid Reducing Approach. In Metallurgy Technology and Materials VI, Proceedings of the 6th International Conference on Metallurgy Technology and Materials, Xi’an, China, May 30–31 2018; Trans Tech Publications Ltd.: Baech, Switzerland, 2018; Volume 279, pp. 148–152. [Google Scholar]
- Sowa, M.; Wala, M.; Blacha-Grzechnik, A.; Maciej, A.; Kazek-Kęsik, A.; Stolarczyk, A.; Simka, W. Corrosion Inhibitor-Modified Plasma Electrolytic Oxidation Coatings on 6061 Aluminum Alloy. Materials 2021, 14, 619. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-J.; Al Bosta, M.M.S.; Wu, W.-T. Preparation of Self-Lubricating Composite Coatings through a Micro-Arc Plasma Oxidation with Graphite in Electrolyte Solution. Surf. Coat. Technol. 2014, 259, 318–324. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of Graphene Particles on the Micro-Arc Oxidation Behaviors of 6063 Aluminum Alloy and the Coating Properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Jiang, D.; Xia, X.; Hou, J.; Zhang, X.; Dong, Z. Enhanced Corrosion Barrier of Microarc-Oxidized Mg Alloy by Self-Healing Superhydrophobic Silica Coating. Ind. Eng. Chem. Res. 2019, 58, 165–178. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.; Ntomprougkidis, V.; Migot, S.; Bruyère, S.; Soldera, F.; Belmonte, T.; Henrion, G. Formation of a Metastable Nanostructured Mullite during Plasma Electrolytic Oxidation of Aluminium in “Soft” Regime Condition. Mater. Des. 2019, 180, 107977. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A Review of Recent Work on Discharge Characteristics during Plasma Electrolytic Oxidation of Various Metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Matykina, E.; Arrabal, R.; Mohedano, M.; Mingo, B.; Gonzalez, J.; Pardo, A.; Merino, M.C. Recent Advances in Energy Efficient PEO Processing of Aluminium Alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1439–1454. [Google Scholar] [CrossRef]
- Hong, L.; Wang, D.; Bian, G.-D.; Wang, L.-L.; Hu, S.-G. Self-Lubricating PEO Coating on an Al Alloy Produced by Vacuum Impregnation Post-Treatment. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 965–974. [Google Scholar] [CrossRef]
- Al Bosta, M.M.S.; Ma, K.-J. Suggested Mechanism for the MAO Ceramic Coating on Aluminium Substrates Using Bipolar Current Mode in the Alkaline Silicate Electrolytes. Appl. Surf. Sci. 2014, 308, 121–138. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma Electrolytic Oxidation (PEO) for Production of Anodised Coatings on Lightweight Metal (Al, Mg, Ti) Alloys. Trans. IMF 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Tran, Q.P. Plasma Electrolytic Oxidation Coating On 6061 Al Alloy Using An Electrolyte Without Alkali Ions. Vietnam J. Sci. Technol. 2018, 54, 151. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Song, X.; Zhou, J.; Liu, H.; Yang, Y. The Study on the Overall Plasma Electrolytic Oxidation for 6061–7075 Dissimilar Aluminum Alloy Welded Parts Based on the Dielectric Breakdown Theory. Materials 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treviño, M.; Garza-Montes-de-Oca, N.F.; Pérez, A.; Hernández-Rodríguez, M.A.L.; Juárez, A.; Colás, R. Wear of an Aluminium Alloy Coated by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2012, 206, 2213–2219. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, H.; Liu, S.; Qi, Y.; Liang, J. Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate. Materials 2021, 14, 4037. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.-P.; Chin, T.-S.; Kuo, Y.-C.; Jin, C.-X.; Trung, T.; Tuan, C.V.; Dang, D.Q. Diamond Powder Incorporated Oxide Layers Formed on 6061 Al Alloy by Plasma Electrolytic Oxidation. J. Alloys Compd. 2018, 751, 289–298. [Google Scholar] [CrossRef]
- Byeon, S.S.; Wang, K.; Jung, Y.G.; Koo, B.H. Characteristic of AlON–Al2O3 Coatings on Al6061 Alloy by Electrolytic Plasma Processing in Aluminate and Nitride Electrolytes. Surf. Coat. Technol. 2010, 204, 3196–3199. [Google Scholar] [CrossRef]
- Madhavi, Y.; Narasaiah, N.; Jyothirmayi, A.; Rama Krishna, L. Influence of Surface-Roughness on the Corrosion-Fatigue Behavior of MAO Coated 6061-T6 Al Alloy Assessed in NaCl Medium. Surf. Coat. Technol. 2021, 414, 127102. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Du, H.Q.; Zhao, Y.W. Corrosion Behavior and Structure of Plasma Electrolytic Oxidation Coated Aluminum Alloy. Int. J. Electrochem. Sci. 2017, 12, 6788–6800. [Google Scholar] [CrossRef]
- Malayoglu, U.; Tekin, K.C.; Malayoglu, U.; Shrestha, S. An Investigation into the Mechanical and Tribological Properties of Plasma Electrolytic Oxidation and Hard-Anodized Coatings on 6082 Aluminum Alloy. Mater. Sci. Eng. A 2011, 528, 7451–7460. [Google Scholar] [CrossRef]
- Shrestha, S.; Dunn, B.D. Plasma electrolytic oxidation and anodising of aluminium alloys for spacecraft applications. In Surface Engineering of Light Alloys; Woodhead Publishing: Sawston, UK, 2010; Available online: https://www.sciencedirect.com/science/article/pii/B978184569537850018X?via%3Dihub (accessed on 2 January 2022).
- Yürektürk, Y.; Muhaffel, F.; Baydoğan, M. Characterization of Micro Arc Oxidized 6082 Aluminum Alloy in an Electrolyte Containing Carbon Nanotubes. Surf. Coat. Technol. 2015, 269, 83–90. [Google Scholar] [CrossRef]
- Yan, H.; Wang, J.; Meng, C.; Wang, X.; Song, S.; Fan, X.; Zhang, L.; Li, H.; Li, W.; Zhu, M. Towards Long-Term Corrosion and Wear Protection of Al Alloy: Synergy of Ti3C2Tx Flake and Micro-Arc Oxidation Coating. Corros. Sci. 2020, 174, 108813. [Google Scholar] [CrossRef]
- Ralls, A.M.; Kasar, A.K.; Menezes, P.L. Friction Stir Processing on the Tribological, Corrosion, and Erosion Properties of Steel: A Review. J. Manuf. Mater. Process. 2021, 5, 97. [Google Scholar] [CrossRef]
- Kumar Rajak, D.; Pagar, D.D.; Menezes, P.L.; Eyvazian, A. Friction-Based Welding Processes: Friction Welding and Friction Stir Welding. J. Adhes. Sci. Technol. 2020, 34, 2613–2637. [Google Scholar] [CrossRef]
- Kurt, A.; Uygur, I.; Cete, E. Surface Modification of Aluminium by Friction Stir Processing. J. Mater. Process. Technol. 2011, 211, 313–317. [Google Scholar] [CrossRef]
- Rathee, S.; Maheshwari, S.; Siddiquee, A.N.; Srivastava, M. A Review of Recent Progress in Solid State Fabrication of Composites and Functionally Graded Systems Via Friction Stir Processing. Crit. Rev. Solid State Mater. Sci. 2018, 43, 334–366. [Google Scholar] [CrossRef]
- Girish, G.; Anandakrishnan, V. A Study on the Microstructure, Hardness, and Tribological Behavior of Aluminum-Based Metal–Matrix Composite Fabricated through Recursive Friction Stir Processing. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 235, 671–683. [Google Scholar] [CrossRef]
- Singh, P.; Kandikonda, H.R. Friction Stir Processing of Aluminum Alloys. Indian J. Eng. Mater. Sci. 2021, 28, 16. [Google Scholar]
- Mehta, K.M.; Badheka, V.J. Effect of Friction Stir Processing Passes on Wear Properties of Al-6061-T6 Alloy. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 199. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, Q.; Qin, Q.; Wu, Y.; Su, X. Effect of the Processing Parameters of Friction Stir Processing on the Microstructure and Mechanical Properties of 6063 Aluminum Alloy. Mater. Sci. Eng. A 2019, 751, 70–79. [Google Scholar] [CrossRef]
- Patel, V.V.; Badheka, V.; Kumar, A. Friction Stir Processing as a Novel Technique to Achieve Superplasticity in Aluminum Alloys: Process Variables, Variants, and Applications. Metallogr. Microstruct. Anal. 2016, 5, 278–293. [Google Scholar] [CrossRef]
- Shafiei Zarghani, A.; Kashani Bozorg, S.F.; Zarei-Hanzaki, A. Ultrafine Grained 6082 Aluminum Alloy Fabricated by Friction Stir Processing. Int. J. Mod. Phys. B 2008, 22, 2874–2878. [Google Scholar] [CrossRef]
- Kumar, S. Ultrasonic Assisted Friction Stir Processing of 6063 Aluminum Alloy. Arch. Civ. Mech. Eng. 2016, 16, 473–484. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Prakash, M.; Arun, N.; Jeyakumar, A.A. The Effect of the Number of Passes in Friction Stir Processing of Aluminum Alloy (AA6082) and Its Failure Analysis. Appl. Surf. Sci. 2019, 491, 420–431. [Google Scholar] [CrossRef]
- Al-Fadhalah, K.; Asi, F. Aging Behavior of Aluminum Alloy 6082 Subjected to Friction Stir Processing. Crystals 2018, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Sarvaiya, J.; Singh, D. Determination of defect-free working range of friction stir processing for AA6082-T6. In Recent Advances in Smart Manufacturing and Materials, Proceedings of the International Conference on Evolution in Manufacturing (ICEM 2020), Online, 10–12 December 2020; Agrawal, R., Jain, J.K., Yadav, V.S., Manupati, V.K., Varela, L., Eds.; Springer: Singapore, 2021; pp. 327–339. [Google Scholar]
- Mane, K.M.; Hosmani, S.S. Friction Stir Surface Processing of Al 6061 Alloy: Role of Surface Alloying with Copper and Heat-Treatment. Trans. Indian Inst. Met. 2018, 71, 1411–1425. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Dinaharan, I.; Palanivel, R.; Sathiskumar, R. Effect of Friction Stir Processing on Microstructure and Tensile Behavior of AA6061/Al3Fe Cast Aluminum Matrix Composites. J. Alloys Compd. 2019, 785, 531–541. [Google Scholar] [CrossRef]
- Miranda, R.M.; Gandra, J.; Vilaça, P. Surface modification by friction based processes. In Modern Surface Engineering Treatments; IntechOpen: London, UK, 2013; ISBN 978-953-51-1149-8. [Google Scholar]
- Choi, D.-H.; Kim, Y.-I.; Kim, D.-U.; Jung, S.-B. Effect of SiC Particles on Microstructure and Mechanical Property of Friction Stir Processed AA6061-T4. Trans. Nonferrous Met. Soc. China 2012, 22, s614–s618. [Google Scholar] [CrossRef]
- Eftekharinia, H.; Amadeh, A.A.; Khodabandeh, A.; Paidar, M. Microstructure and Wear Behavior of AA6061/SiC Surface Composite Fabricated via Friction Stir Processing with Different Pins and Passes. Rare Met. 2020, 39, 429–435. [Google Scholar] [CrossRef]
- Salehi, M.; Saadatmand, M.; Aghazadeh Mohandesi, J. Optimization of Process Parameters for Producing AA6061/SiC Nanocomposites by Friction Stir Processing. Trans. Nonferrous Met. Soc. China 2012, 22, 1055–1063. [Google Scholar] [CrossRef]
- Mehdi, H.; Mishra, R.S. Effect of Multi-Pass Friction Stir Processing and SiC Nanoparticles on Microstructure and Mechanical Properties of AA6082-T6. Adv. Ind. Manuf. Eng. 2021, 3, 100062. [Google Scholar] [CrossRef]
- Salehi, M.; Farnoush, H.; Mohandesi, J.A. Fabrication and Characterization of Functionally Graded Al–SiC Nanocomposite by Using a Novel Multistep Friction Stir Processing. Mater. Des. 2014, 63, 419–426. [Google Scholar] [CrossRef]
- Zuhailawati, H.; Halmy, M.N.; Almanar, I.P.; Dhindaw, B.K. Friction Stir Processed of 6061-T6 Aluminum Alloy Reinforced with Silica from Rice Husk Ash. Adv. Mater. Res. 2014, 1024, 227–230. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Heidarpour, A.; Jalilvand, M.M.; Roknian, M. Effect of Friction Stir Processing on the Microhardness, Wear and Corrosion Behavior of Al6061 and Al6061/SiO2 Nanocomposites. J. Mater. Eng. Perform. 2019, 28, 4826–4837. [Google Scholar] [CrossRef]
- Devaraju, A.; Kumar, A.; Kumaraswamy, A.; Kotiveerachari, B. Influence of Reinforcements (SiC and Al2O3) and Rotational Speed on Wear and Mechanical Properties of Aluminum Alloy 6061-T6 Based Surface Hybrid Composites Produced via Friction Stir Processing. Mater. Des. 2013, 51, 331–341. [Google Scholar] [CrossRef]
- Qu, J.; Xu, H.; Feng, Z.; Frederick, D.A.; An, L.; Heinrich, H. Improving the Tribological Characteristics of Aluminum 6061 Alloy by Surface Compositing with Sub-Micro-Size Ceramic Particles via Friction Stir Processing. Wear 2011, 271, 1940–1945. [Google Scholar] [CrossRef]
- Naresh, P.; Kumar, A.; Krishna Kishore, M. Influence of Nano Reinforcement Volume Percentage on Fabrication of Surface Nanocomposite by FSP. Mater. Sci. Forum 2017, 879, 1369–1374. [Google Scholar] [CrossRef]
- Guo, J.F.; Liu, J.; Sun, C.N.; Maleksaeedi, S.; Bi, G.; Tan, M.J.; Wei, J. Effects of Nano-Al2O3 Particle Addition on Grain Structure Evolution and Mechanical Behaviour of Friction-Stir-Processed Al. Mater. Sci. Eng. A 2014, 602, 143–149. [Google Scholar] [CrossRef]
- Kheirkhah, S.; Imani, M.; Aliramezani, R.; Zamani, M.H.; Kheilnejad, A. Microstructure, Mechanical Properties and Corrosion Resistance of Al6061/BN Surface Composite Prepared by Friction Stir Processing. Surf. Topogr. Metrol. Prop. 2019, 7, 035002. [Google Scholar] [CrossRef]
- Patel, S.K.; Singh, V.P.; Kuriachen, B. Modification of Aluminium Alloy Surface Composite Reinforced with ZrO2 Particles Fabricated Through Friction Stir Processing. In Advances in Additive Manufacturing and Joining, Proceedings of the 7th International and 28th All India Manufacturing Technology, Design and Research conference, Chennai, India, 13–15 December 2018; Shunmugam, M.S., Kanthababu, M., Eds.; Springer: Singapore, 2020; pp. 579–586. [Google Scholar]
- Rathee, S.; Maheshwari, S.; Siddiquee, A.N.; Srivastava, M. Distribution of Reinforcement Particles in Surface Composite Fabrication via Friction Stir Processing: Suitable Strategy. Mater. Manuf. Process. 2018, 33, 262–269. [Google Scholar] [CrossRef]
- Palanivel, R.; Dinaharan, I.; Laubscher, R.F.; Davim, J.P. Influence of Boron Nitride Nanoparticles on Microstructure and Wear Behavior of AA6082/TiB2 Hybrid Aluminum Composites Synthesized by Friction Stir Processing. Mater. Des. 2016, 106, 195–204. [Google Scholar] [CrossRef]
- Vatankhah Barenji, R.; Khojastehnezhad, V.M.; Pourasl, H.H.; Rabiezadeh, A. Wear Properties of Al–Al2O3/TiB2 Surface Hybrid Composite Layer Prepared by Friction Stir Process. J. Compos. Mater. 2016, 50, 1457–1466. [Google Scholar] [CrossRef]
- Anvari, S.R.; Karimzadeh, F.; Enayati, M.H. Wear Characteristics of Al–Cr–O Surface Nano-Composite Layer Fabricated on Al6061 Plate by Friction Stir Processing. Wear 2013, 304, 144–151. [Google Scholar] [CrossRef]
- Anandha Kumar, C.J.; Gopi, S.; Kumar, S.S.; Mohan, D.G. Mechanical, Metallurgical and Tribological Properties of Friction Stir Processed Aluminium Alloy 6061 Hybrid Surface Composites. Surf. Topogr. Metrol. Prop. 2021, 9, 045019. [Google Scholar] [CrossRef]
- Dinesh, D.; Megalingam, A.; Rajamurugan, G.; Arundeep, M.; Tajdeen, A. Wear and Microstructure Characteristics of Friction Stir Processed Al6063/B4C+SiO2 Composites. Adv. Asp. Eng. Res. 2021, 3, 140–150. [Google Scholar] [CrossRef]
- Bararpour, S.M.; Aval, H.J.; Jamaati, R. An Experimental and Theoretical Investigation of Thermo-Mechanical Issues in Friction Surfacing of Al-Mg Aluminium Alloys: Material Flow and Residual Stress. Modelling Simul. Mater. Sci. Eng. 2020, 28, 035003. [Google Scholar] [CrossRef]
- Jujare, T.; Kumar, A.; Kailas, S.V.; Bhat, K.U. Friction Surfacing of Mild Steel by Copper: A Feasibility Study. Procedia Mater. Sci. 2014, 5, 1300–1307. [Google Scholar] [CrossRef] [Green Version]
- Gandra, J.; Miranda, R.M.; Vilaça, P. Performance Analysis of Friction Surfacing. J. Mater. Process. Technol. 2012, 212, 1676–1686. [Google Scholar] [CrossRef]
- Kallien, Z.; Rath, L.; Roos, A.; Klusemann, B. Experimentally Established Correlation of Friction Surfacing Process Temperature and Deposit Geometry. Surf. Coat. Technol. 2020, 397, 126040. [Google Scholar] [CrossRef]
- Ehrich, J.; Roos, A.; Hanke, S. Effect of Mg and Si content in aluminum alloys on friction surfacing processing behavior. In Light Metals 2019, Proceedings of the TMS 2019 Annual Meeting and Exhibition, San Antonio, TX, USA, 10–14 March 2019; Chesonis, C., Ed.; Springer: Cham, Switzerland, 2019; pp. 357–363. [Google Scholar]
- Krohn, H.; Hanke, S.; Beyer, M.; dos Santos, J.F. Influence of External Cooling Configuration on Friction Surfacing of AA6082 T6 over AA2024 T351. Manuf. Lett. 2015, 5, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Gandra, J.; Pereira, D.; Miranda, R.M.; Silva, R.J.C.; Vilaça, P. Deposition of AA6082-T6 over AA2024-T3 by Friction Surfacing—Mechanical and Wear Characterization. Surf. Coat. Technol. 2013, 223, 32–40. [Google Scholar] [CrossRef]
- Gandra, J.; Vigarinho, P.; Pereira, D.; Miranda, R.M.; Velhinho, A.; Vilaça, P. Wear Characterization of Functionally Graded Al–SiC Composite Coatings Produced by Friction Surfacing. Mater. Des. 2013, 52, 373–383. [Google Scholar] [CrossRef]
- Vijaya Kumar, B.; Madhusudhan Reddy, G.; Mohandas, T. Identification of Suitable Process Parameters for Friction Surfacing of Mild Steel with AA6063 Aluminium Alloy. Int. J. Adv. Manuf. Technol. 2014, 74, 433–443. [Google Scholar] [CrossRef]
- Yuvaraj, N. Improving the Wear Properties of Aluminum 6082 Alloy by Surface Compositing with Zro2 Ceramic Particles Via Friction Stir Processing. Mater. Sci. Res. India 2018, 15, 68–74. [Google Scholar] [CrossRef]
- John, M.; Kalvala, P.R.; Misra, M.; Menezes, P.L. Peening Techniques for Surface Modification: Processes, Properties, and Applications. Materials 2021, 14, 3841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, P.; Lan, Q.; Meng, G.; Ren, Y.; Yang, Z.; Xu, P.; Liu, Z. Recent Research and Development Status of Laser Cladding: A Review. Opt. Laser Technol. 2021, 138, 106915. [Google Scholar] [CrossRef]
- Zhou, J.; Han, X.; Li, H.; Liu, S.; Yi, J. Investigation of Layer-by-Layer Laser Remelting to Improve Surface Quality, Microstructure, and Mechanical Properties of Laser Powder Bed Fused AlSi10Mg Alloy. Mater. Des. 2021, 210, 110092. [Google Scholar] [CrossRef]
- de Oliveira, U.; Ocelík, V.; De Hosson, J.T.M. Analysis of Coaxial Laser Cladding Processing Conditions. Surf. Coat. Technol. 2005, 197, 127–136. [Google Scholar] [CrossRef]
- Henri, P.; Jonne, N.; Sebastian, T.; Jari, T.; Steffen, N.; Petri, V. Laser cladding with coaxial wire feeding. In Proceedings of the International Congress on Applications of Lasers & Electro Optics (ICALEO), Anaheim, CA, USA, 23–27 September 2012; pp. 1196–1201. [Google Scholar] [CrossRef]
- Wirth, F.; Wegener, K. A Physical Modeling and Predictive Simulation of the Laser Cladding Process. Addit. Manuf. 2018, 22, 307–319. [Google Scholar] [CrossRef]
- Vilar, R. Laser Cladding. J. Laser Appl. 1999, 11, 64–79. [Google Scholar] [CrossRef]
- Li, M.; Han, B.; Song, L.; He, Q. Enhanced Surface Layers by Laser Cladding and Ion Sulfurization Processing towards Improved Wear-Resistance and Self-Lubrication Performances. Appl. Surf. Sci. 2020, 503, 144226. [Google Scholar] [CrossRef]
- Farahmand, P.; Liu, S.; Zhang, Z.; Kovacevic, R. Laser Cladding Assisted by Induction Heating of Ni–WC Composite Enhanced by Nano-WC and La2O3. Ceram. Int. 2014, 40, 15421–15438. [Google Scholar] [CrossRef]
- Zarezadeh Mehrizi, M.; Shamanian, M.; Saidi, A.; Shoja-Razazvi, R.; Beigi, R. Evaluation of Oxidation Behavior of Laser Clad CoWSi–WSi2 Coating on Pure Ni Substrate at Different Temperatures. Ceram. Int. 2015, 41, 9715–9721. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Wang, R.; Wei, D.; Cai, M.; Fu, Y. Microstructure of Laser-Clad Ni60 Cladding Layers Added with Different Amounts of Rare-Earth Oxides on 6063 Al Alloys. J. Alloys Compd. 2018, 740, 1099–1107. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zeng, Z.; Fu, Y. Effect of Rare-Earth on Friction and Wear Properties of Laser Cladding Ni-Based Coatings on 6063Al. J. Alloys Compd. 2017, 727, 278–285. [Google Scholar] [CrossRef]
- Menezes, P.L.; Nosonovsky, M.; Kailas, S.V.; Lovell, M.R. Friction and wear. In Tribology for Scientists and Engineers: From Basics to Advanced Concepts; Menezes, P.L., Nosonovsky, M., Ingole, S.P., Kailas, S.V., Lovell, M.R., Eds.; Springer: New York, NY, USA, 2013; pp. 43–91. ISBN 978-1-4614-1945-7. [Google Scholar]








| Alloy Type and Temper | Composition (wt%) | Mechanical Properties |
|---|---|---|
| 6005A-T6 | Si: 0.6–0.9, Fe: 0.3–0.6, Cu: ≤ 0.1, Mn: ≤ 0.1, Mg: 0.4–0.6, Cr: ≤ 0.01, Zn ≤ 0.1, Ti ≤ 0.1 Al: balance | YS: 200 MPa, UTS: 250 MPa, Elong: 8% |
| 6061-T6 | Si: 0.4–0.8, Fe: ≤ 0.7, Cu: 0.15–0.4, Mn: ≤ 0.15, Mg: 0.8–1.2, Cr: 0.04–0.35, Zn ≤ 0.25, Ti ≤ 0.15 Al: balance | YS: 276 MPa, UTS: 310 MPa, Elong: 12% |
| 6063-T6 | Si: 0.2–0.6, Fe: ≤ 0.35, Cu: ≤ 0.1, Mn: ≤ 0.1, Mg: 0.45–0.9, Cr: ≤ 0.10, Zn ≤ 0.15, Ti ≤ 0.1 Al: balance | YS: 214 MPa, UTS: 241 MPa, Elong: 12% |
| 6082-T6 | Si: 0.7–1.3, Fe: ≤ 0.5, Cu: ≤ 0.1, Mn: ≤ 0.4–1.0, Mg: 0.6–1.2, Cr: ≤ 0.25, Zn ≤ 0.2, Ti ≤ 0.1 Al: balance | YS: 260 MPa, UTS: 310 MPa, Elong: 10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, K.U.; Panemangalore, D.B.; Kuruveri, S.B.; John, M.; Menezes, P.L. Surface Modification of 6xxx Series Aluminum Alloys. Coatings 2022, 12, 180. https://doi.org/10.3390/coatings12020180
Bhat KU, Panemangalore DB, Kuruveri SB, John M, Menezes PL. Surface Modification of 6xxx Series Aluminum Alloys. Coatings. 2022; 12(2):180. https://doi.org/10.3390/coatings12020180
Chicago/Turabian StyleBhat, Kuruveri Udaya, Devadas Bhat Panemangalore, Spandana Bhat Kuruveri, Merbin John, and Pradeep L. Menezes. 2022. "Surface Modification of 6xxx Series Aluminum Alloys" Coatings 12, no. 2: 180. https://doi.org/10.3390/coatings12020180






