SiC- and Ag-SiC-Doped Hydroxyapatite Coatings Grown Using Magnetron Sputtering on Ti Alloy for Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating and Specimens
2.2. Coatings Characterization
2.2.1. Elemental and Phase Composition
2.2.2. Surface Roughness, Morphology and Wettability
2.2.3. Electrochemical Analysis
2.2.4. Mechanical Properties
2.2.5. Tribological Properties
2.2.6. In Vitro Evaluation of Cytocompatibility by Indirect Cytocompatibility Studies
3. Results and Discussion
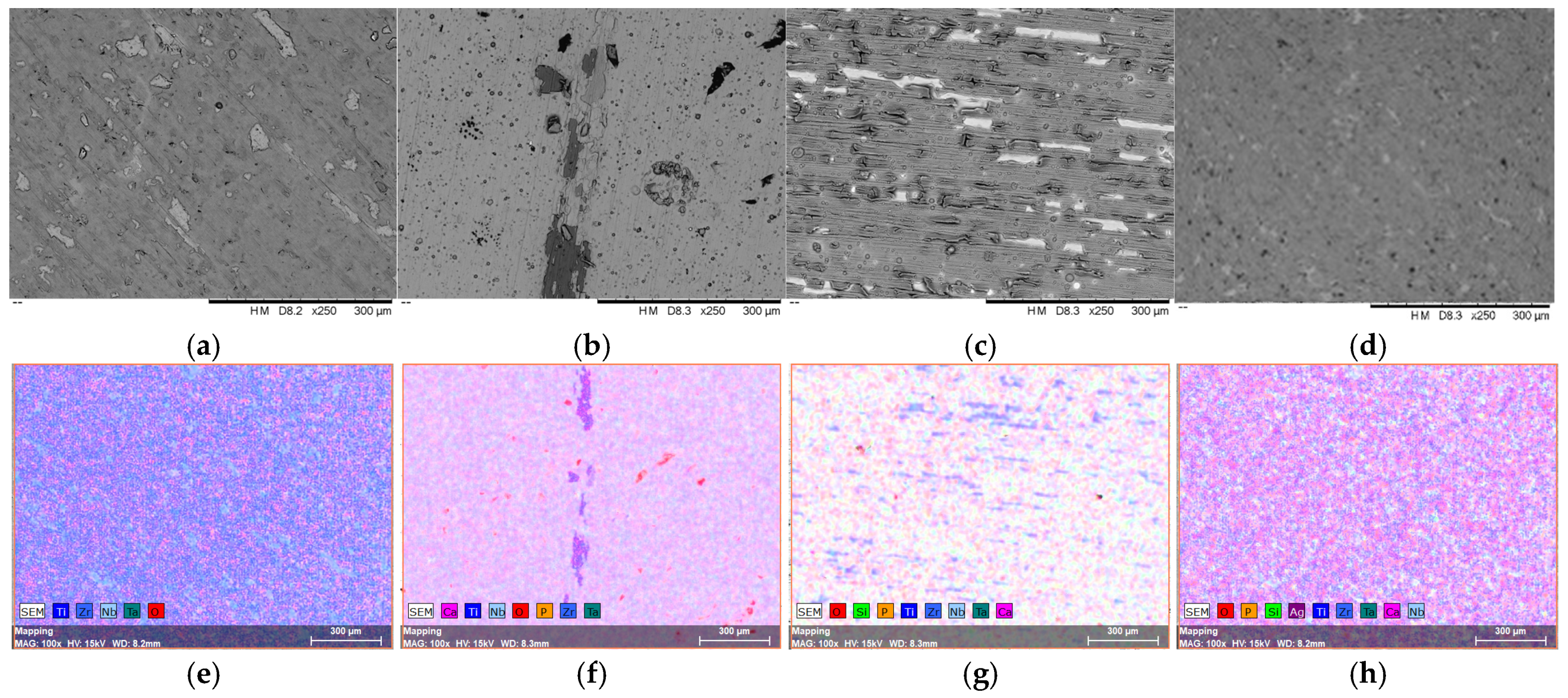
3.1. Surface Morphology, Elemental and Phase Composition of the Investigated Samples
3.2. Surface Morphology and Wettability
3.3. Corrosion Resistance
3.4. Mechanical Properties
3.5. Tribology Tests
3.6. In Vitro Evaluation of the Coatings
Cytocompatibility of Newly Developed Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-alloys and surface modifications to improve the mechanical properties and the biological response to orthopedic and dental implants: A review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Chen, L.Y.; Wang, L. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Pham, M.H.; Mehta, V.A.; Tuchman, A.; Hsieh, P.C. Material science in cervical total disc replacement. BioMed Res. Int. 2015, 2015, 719123. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomater. Silver Jubil. Compend. 2000, 21, 175–189. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Niinomi, M. Low modulus titanium alloys for inhibiting bone atrophy. In Biomaterials Science and Engineering; Pignatello, R., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 249–268. ISBN 978-953-51-4432-8. [Google Scholar]
- Calderon Moreno, J.M.; Vasilescu, E.; Drob, P.; Osiceanu, P.; Vasilescu, C.; Drob, S.I.; Popa, M. Surface and electrochemical characterization of a new ternary titanium based alloy behaviour in electrolytes of varying pH. Corros. Sci. 2013, 77, 52–63. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Lee, H.; Wu, J.; Chao, C.; Chang, Y.; Du, J. A study of low Young’s modulus Ti-15Ta-15Nb alloy using TEM analysis. Materials 2020, 13, 5694. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, W.; Wang, J.; Ren, L.; Zhao, X.; Ma, C. A novel strategy for developing α + β dual-phase titanium alloys with low Young’s modulus and high yield strength. J. Mater. Sci. Technol. 2021, 76, 122–128. [Google Scholar] [CrossRef]
- Cinca, I.; Nocivin, A.; Raducanu, D.; Gloriant, T.; Gordin, D.M.; Dan, I.; Caprarescu, A.; Cojocaru, V.D. XRD and nano-indentation testing of thermo-mechanical processed Ti-29Nb-9Ta-10Zr alloy. Met. Mater. 2016, 53, 17–26. [Google Scholar] [CrossRef][Green Version]
- Vladescu, A.; Braic, V.; Balaceanu, M.; Braic, M.; Parau, A.C.; Ivanescu, S.; Fanara, C. Characterization of the Ti-10Nb-10Zr-5Ta alloy for biomedical applications. Part 1: Microstructure, mechanical properties, and corrosion resistance. J. Mater. Eng. Perform. 2013, 22, 2389–2397. [Google Scholar] [CrossRef]
- Braic, V.; Balaceanu, M.; Braic, M.; Vitelaru, C.; Titorencu, I.; Pruna, V.; Parau, A.C.; Fanara, C.; Vladescu, A. Characterization of the Ti-10Nb-10Zr-5Ta alloy for biomedical applications. Part 2: Wettability, tribological performance and biocompatibility. J. Mater. Eng. Perform. 2014, 23, 326–332. [Google Scholar] [CrossRef]
- Calderon Moreno, J.M.; Vasilescu, C.; Drob, S.I.; Neacsu, E.I.; Popa, M. Evaluation of the microstructural, mechanical and anti-corrosive properties of a new ternary Ti-15Zr-5Nb alloy in simulated oral environment. Mater. Corros. 2014, 65, 703–714. [Google Scholar] [CrossRef]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical behaviours of the bone-implant interface: A review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef] [PubMed]
- Geesink, R.G.T.; De Groot, K.; Klein, C.P.A.T. Bonding of bone to apatite-coated implants. J. Bone Jt. Surg. Ser. B 1988, 70, 17–22. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Sulong, A.B.; Ghani, S.A.C.; Ghazalli, Z. Hydroxyapatite-based coating on biomedical implant. In Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; Thirumalai, J., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 69–88. [Google Scholar]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (HAp) for Biomedical Applications; Mucalo, M., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 143–157. ISBN 978-1-78242-033-0. [Google Scholar]
- Charalambous, C.P. Calcium phosphate ceramics as hard tissue prosthetics. Class. Pap. Orthop. 2014, 419–421. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Liang, H.; Shi, B.; Fairchild, A.; Cale, T. Applications of plasma coatings in artificial joints: An overview. Vacuum 2004, 73, 317–326. [Google Scholar] [CrossRef]
- Dudek, A. Surface properties in titanium with hydroxyapatite coating. Opt. Appl. 2009, 39, 825–831. [Google Scholar]
- Song, K.; Huang, H.; Lu, M.; Yang, A.; Weng, J.; Duan, K. Hydrothermal preparation and characterization of Zn, Si, Mg, Fe doped hydroxyapatite. J. Inorg. Mater. 2021, 36, 1091. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Noor, A.F.M.; Krishnasamy, S.; Alengaram, U.J. Effect of multi-ions doping on the properties of carbonated hydroxyapatite bioceramic. Ceram. Int. 2019, 45, 3473–3477. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.R.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Bratschitsch, G.; Puchwein, P.; Zollner-Schwetz, I.; Sadoghi, P.; Radl, R.; Leithner, A.; Leitner, L. Spinal surgery site infection leading to implant loosening is influenced by the number of prior operations. Glob. Spine J. 2020, 1–6. [Google Scholar] [CrossRef]
- Hallab, N.J. A review of the biologic effects of spine implant debris: Fact from fiction. SAS J. 2009, 3, 143–160. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into biological apatite: Physiochemical properties and preparation approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef]
- Vladescu, A.; Mihai Cotrut, C.; Ak Azem, F.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R.; et al. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef]
- Yang, L.; Mao, Z. Effect of SiC particle contents and size on the microstructure and dissolution of SiC-hydroxyapatite coatings. Coatings 2021, 11, 1166. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 1–53. [Google Scholar] [CrossRef]
- Coe, S.C.; Wadge, M.D.; Felfel, R.M.; Ahmed, I.; Walker, G.S.; Scotchford, C.A.; Grant, D.M. Production of high silicon-doped hydroxyapatite thin film coatings via magnetron sputtering: Deposition, characterisation, and in vitro biocompatibility. Coatings 2020, 10, 190. [Google Scholar] [CrossRef]
- Hollinger, M.A. Toxicology aspects of topical silver pharmaceuticals. Crit. Rev. Toxicol. 1996, 26, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5574. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial silver. Met.-Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Vik, H.; Andersen, K.J.; Julshamn, K.; Todnem, K. Neuropathy caused by silver absorption from arthroplasty cement. Lancet 1985, 1, 872. [Google Scholar] [CrossRef]
- Vladescu, A.; Braic, M.; Azem, F.A.; Titorencu, I.; Braic, V.; Pruna, V.; Kiss, A.; Parau, A.C.; Birlik, I. Effect of the deposition temperature on corrosion resistance and biocompatibility of the hydroxyapatite coatings. Appl. Surf. Sci. 2015, 354, 373–379. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Braic, L.; Vranceanu, D.M.; Kiss, A.; Dinu, M.; Balaceanu, M.; Braic, V.; Vladescu, A. Influence of the annealing treatment on the structure, morphology, and corrosion resistance of sputtered Zr-Ti-Si-O coatings used for biomedical applications. Mater. Corros. 2017, 68, 552–559. [Google Scholar] [CrossRef]
- Braic, L.; Zoita, N.C. Influence of the deposition time and temperature on the texture of InN thin films grown by RF-magnetron sputtering. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 2013–2017. [Google Scholar]
- Cotrut, C.-M.; Braic, V.; Balaceanu, M.; Titorencu, I.; Braic, M.; Parau, A.C. Corrosion resistance, mechanical properties and biocompatibility of Hf-containing ZrCN coatings. Thin Solid Films 2013, 538, 48–55. [Google Scholar] [CrossRef]
- Agarwal, B.K. X-ray Spectroscopy—An Introduction, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 978-3-540-38668-1. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Lyman, C.E.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-ray Microanalysis, 3rd ed.; Springer: Boston, MA, USA, 2003; ISBN 978-1-4615-0215-9. [Google Scholar]
- International Organization for Standardization. ISO 10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- Mitran, V.; Ion, R.; Miculescu, F.; Necula, M.G.; Mocanu, A.C.; Stan, G.E.; Antoniac, I.V.; Cimpean, A. Osteoblast cell response to naturally derived calcium phosphate-based materials. Materials 2018, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Perez, B.; Matamoros-Veloza, Z.; Rendon-Angeles, J.C.; Yanagisawa, K.; Onda, A.; Pérez-Terrazas, J.E.; Mejia-Martínez, E.E.; Burciaga Díaz, O.; Rodríguez-Reyes, M. Synthesis of silicon-substituted hydroxyapatite using hydrothermal process. Boletín Sociedad Española Cerámica Vidrio 2020, 59, 50–64. [Google Scholar] [CrossRef]
- Supova, M. Substituted hydroxyapatites for biomedical applications: A review. Ceramics International 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grosse und der Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen. Nachrichten Gesellschaft Wissenschaften Gottingen Mathematisch-Physikalische Klasse 1918, 26, 90–100. [Google Scholar]
- Raducanu, D.; Vasilescu, E.; Cojocaru, V.D.; Cinca, I.; Drob, P.; Vasilescu, C.; Drob, S.I. Mechanical and corrosion resistance of a new nanostructured Ti-Zr-Ta-Nb alloy. J. Mech. Behav. Biomed. Mater. 2011, 4, 1421–1430. [Google Scholar] [CrossRef]
- Hynowska, A.; Pellicer, E.; Fornell, F.; González, S.; van Steenberge, N.; Suriñach, S.; Gebert, A.; Calin, M.; Eckert, J.; Baró, M.D.; et al. Nanostructured b-phase Ti-39.3Nb-13.3Zr-10.7Ta alloys for biomedical applications: Microstructure benefits on the mechanical and corrosion performances. Mater. Sci. Eng. C 2012, 32, 2418–2425. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Cotrut, C.M.; Bramowicz, M.; Titorencu, I.; Kulesza, S.; Kiss, A.; Berbecaru, A.; Pruna, V.; Branzei, M.; Vladescu, A. Osseointegration of sputtered SiC-added hydroxyapatite for orthopaedic applications. Ceram. Int. 2016, 42, 10085–10093. [Google Scholar] [CrossRef]
- Morgan, H.; Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P.; Anderson, P. Preparation and characterisation of monoclinic hydroxyapatite and its precipitated carbonate apatite intermediate. Biomaterials 2000, 21, 617–627. [Google Scholar] [CrossRef]
- Elliott, J.C.; Mackie, P.E.; Young, R.A. Monoclinic hydroxyapatite. Science 1973, 180, 1055–1057. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates, 1st ed.; Book Series Studies in Inorganic Chemistry; Elsevier Science: Amsterdam, The Netherlands, 1994; ISBN 9781483290317. [Google Scholar]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Vladescu, A.; Birlik, I.; Braic, V.; Toparli, M.; Celik, E.; Ak Azem, F. Enhancement of the mechanical properties of hydroxyapatite by SiC addition. J. Mech. Behav. Biomed. Mater. 2014, 40, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ouadfel, M.A.; Keffous, A.; Brighet, A.; Gabouze, N.; Hadjersi, T.; Cheriet, A.; Kechouane, M.; Boukezzata, A.; Boukennous, Y.; Belkacem, Y.; et al. Si-rich a-Si 1-x C x thin films by d.c. magnetron co-sputtering of silicon and silicon carbide: Structural and optical properties. Appl. Surf. Sci. 2013, 265, 94–100. [Google Scholar] [CrossRef]
- Mirzaee, M.; Vaezi, M.; Palizdar, Y. Synthesis and characterization of silver doped hydroxyapatite nanocomposite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Mater. Sci. Eng. C 2016, 69, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Braic, M.; Kiss, A.; Moga, M.; Pozna, E.; Pana, I.; Vladescu, A. Influence of Ag content on the antibacterial properties of SiC doped hydroxyapatite coatings. Ceram. Int. 2016, 42, 1801–1811. [Google Scholar] [CrossRef]
- Rau, J.V.; Fosca, M.; Cacciotti, I.; Laureti, S.; Bianco, A.; Teghil, R. Nanostructured Si-substituted hydroxyapatite coatings for biomedical applications. Thin Solid Films 2013, 543, 167–170. [Google Scholar] [CrossRef]
- Nakata, K.; Kubo, T.; Numako, C.; Onoki, T.; Nakahira, A. Synthesis and characterization of silicon-doped hydroxyapatite. Mater. Trans. 2009, 50, 1046–1049. [Google Scholar] [CrossRef]
- Vladescu, A.; Padmanabhan, S.C.; Ak Azem, F.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.A.; Braic, V. Mechanical properties and biocompatibility of the sputtered Ti doped hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef]
- Surmeneva, M.A.A.; Vladescu, A.; Surmenev, R.A.A.; Pantilimon, C.M.M.; Braic, M.; Cotrut, C.M.M. Study on a hydrophobic Ti-doped hydroxyapatite coating for corrosion protection of a titanium based alloy. RSC Adv. 2016, 6, 87665–87674. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, C.; Hong, Y.; Zhang, X. A review of protein adsorption on bioceramics. Interface Focus 2012, 2, 259–277. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; Ortega-Andreu, M.; Alonso-Carro, G. Total hip arthroplasty. In Musculoskeletal Aspects of Haemophilia; Blackwell Science: Hoboken, NJ, USA, 2008; ISBN 9780470693872. [Google Scholar]
- Braic, M.; Vladescu, A.; Braic, V.; Cotrut, C.M.; Stanciu, D. Corrosion behaviour of Ti–10Nb–10Zr–5Ta alloys in artificial saliva solution with fluoride content. Mater. Corros. 2015, 66, 1331–1337. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Definition of the transfer coefficient in electrochemistry (IUPAC Recommendations 2014). Pure Appl. Chem. 2014, 86, 259–262. [Google Scholar] [CrossRef]
- Gostin, P.F.; Gebert, A.; Schultz, L. Comparison of the corrosion of bulk amorphous steel with conventional steel. Corros. Sci. 2010, 52, 273–281. [Google Scholar] [CrossRef]
- Stern, M.; Geary, L. Electrochemical polarization: I. A theoritical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Elsener, B.; Rota, A.; Böhni, H. Impedance study on the corrosion of PVD and CVD titanium nitride coatings. Mater. Sci. Forum 1991, 44, 29–38. [Google Scholar] [CrossRef]
- Dinu, M.; Hauffman, T.; Cordioli, C.; Vladescu, A.; Braic, M.; Hubin, A.; Cotrut, C.M.C.M. Protective performance of Zr and Cr based silico-oxynitrides used for dental applications by means of potentiodynamic polarization and odd random phase multisine electrochemical impedance spectroscopy. Corros. Sci. 2017, 115, 118–128. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptioug, A.; Kurtukova, M.O.; et al. Influence of the electrolyte’s pH on the properties of electrochemically deposited hydroxyapatite coating on additively manufactured Ti64 alloy. Sci. Rep. 2017, 7, 16819. [Google Scholar] [CrossRef]
- Pintaude, G. Introduction of the ratio of the hardness to the reduced elastic modulus for abrasion. In Tribology—Fundamentals to Advancements; Gegener, J., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 217–230. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, H.J.; Oh, I.H.; Lee, B.T. Effects of Ag-doping on microstructure and mechanical properties of hydroxyapatite films. Key Eng. Mater. 2005, 277, 113–118. [Google Scholar] [CrossRef]
- Sliney, H.E. The use of silver in self-lubricating coatings for extreme temperatures. ASLE Trans. 1986, 29, 370–376. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Burnett, P.J.; Rickerby, D.S. The relationship between hardness and scratch adhession. Thin Solid Films 1987, 154, 403–416. [Google Scholar] [CrossRef]















| Coating | P(HA) [W] | P(SiC) [W] | I(Ag) [mA] | Ubias [V] | tdeposition (min) |
|---|---|---|---|---|---|
| HA | 2 × 120 | - | - | −30 | 260 |
| SiC-HA | 2 × 120 | 1 × 15 | - | −30 | 240 |
| Ag-SiC-HA | 2 × 120 | 1 × 15 | 2 | −30 | 235 |
| Element | Ti | Nb | Zr | Ta |
|---|---|---|---|---|
| Composition (wt.%) | 74.9 ± 3.1 | 9.7 ± 1.6 | 9.9 ± 1.4 | 5.4 ± 0.6 |
| Coating | Elemental Composition (at. %) | (Ca + Ag)/(P + Si) | |||||
|---|---|---|---|---|---|---|---|
| Ca | P | O | Si | C | Ag | ||
| HA | 21.11 ± 0.68 | 12.54 ± 0.43 | 66.34 ± 4.60 | - | - | - | 1.68 |
| SiC-HA | 19.79 ± 0.61 | 4.45 ± 0.13 | 60.60 ± 4.53 | 7.37 ± 0.25 | 7.80 ± 0.24 | - | 1.67 |
| Ag-SiC-HA | 20.54 ± 0.75 | 5.22 ± 0.15 | 58.43 ± 4.20 | 7.57 ± 0.22 | 7.06 ± 0.23 | 1.08 ± 0.12 | 1.69 |
| Sample | Eoc (mV) | Ei=0 (mV) | icorr (µA/cm2) | Rp (kΩ) | P | Pe (%) |
|---|---|---|---|---|---|---|
| TNZT | −122 | −317 | 226.309 | 1.506 | - | - |
| HA | −234 | −156 | 5.834 | 28.464 | 0.049 | 97.4 |
| SiC-HA | −216 | −222 | 4.884 | 31.323 | 0.046 | 97.8 |
| Ag-SiC-HA | −73 | −340 | 0.4365 | 177.558 | 0.008 | 99.8 |
| Sample | H (GPa) | Er (GPa) | H/Er | H3/Er2 (GPa) | H2/2Er (GPa) |
|---|---|---|---|---|---|
| HA | 7.351 ± 0.201 | 98.245 | 0.075 | 0.041 | 0.275 |
| SiC-HA | 12.394 ± 0.381 | 126.131 | 0.098 | 0.120 | 0.609 |
| Ag-SiC-HA | 10.628 ± 0.213 | 119.545 | 0.089 | 0.084 | 0.472 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pana, I.; Braic, V.; Vladescu, A.; Ion, R.; Parau, A.C.; Zoita, N.C.; Dinu, M.; Kiss, A.E.; Cimpean, A.; Braic, M. SiC- and Ag-SiC-Doped Hydroxyapatite Coatings Grown Using Magnetron Sputtering on Ti Alloy for Biomedical Application. Coatings 2022, 12, 195. https://doi.org/10.3390/coatings12020195
Pana I, Braic V, Vladescu A, Ion R, Parau AC, Zoita NC, Dinu M, Kiss AE, Cimpean A, Braic M. SiC- and Ag-SiC-Doped Hydroxyapatite Coatings Grown Using Magnetron Sputtering on Ti Alloy for Biomedical Application. Coatings. 2022; 12(2):195. https://doi.org/10.3390/coatings12020195
Chicago/Turabian StylePana, Iulian, Viorel Braic, Alina Vladescu, Raluca Ion, Anca Constantina Parau, Nicolae Catalin Zoita, Mihaela Dinu, Adrian Emil Kiss, Anisoara Cimpean, and Mariana Braic. 2022. "SiC- and Ag-SiC-Doped Hydroxyapatite Coatings Grown Using Magnetron Sputtering on Ti Alloy for Biomedical Application" Coatings 12, no. 2: 195. https://doi.org/10.3390/coatings12020195
APA StylePana, I., Braic, V., Vladescu, A., Ion, R., Parau, A. C., Zoita, N. C., Dinu, M., Kiss, A. E., Cimpean, A., & Braic, M. (2022). SiC- and Ag-SiC-Doped Hydroxyapatite Coatings Grown Using Magnetron Sputtering on Ti Alloy for Biomedical Application. Coatings, 12(2), 195. https://doi.org/10.3390/coatings12020195






.jpg)



