Improvement of Corrosion Resistance and Biocompatibility of Biodegradable Mg–Ca Alloy by ALD HfZrO2 Film
Abstract
:1. Introduction
2. Experimental Procedure
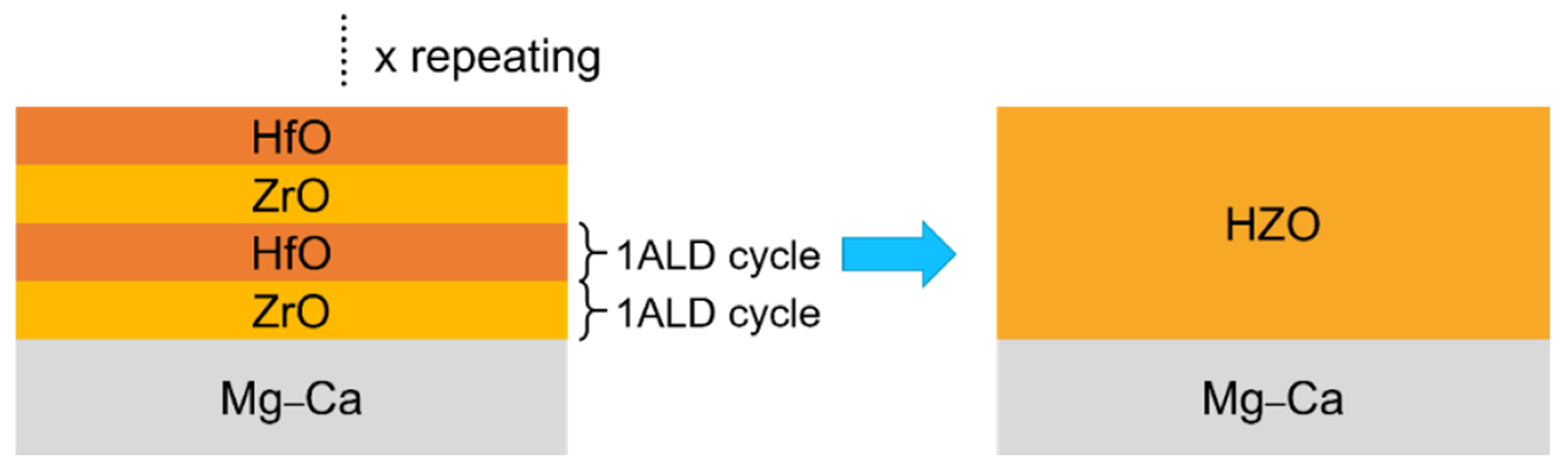
2.1. Sample Preparation
2.2. Characterization of Mg–Ca Alloy and ALD–HZO Film
2.3. Corrosion Evaluation
2.4. Biocompatibility
3. Results and Discussion
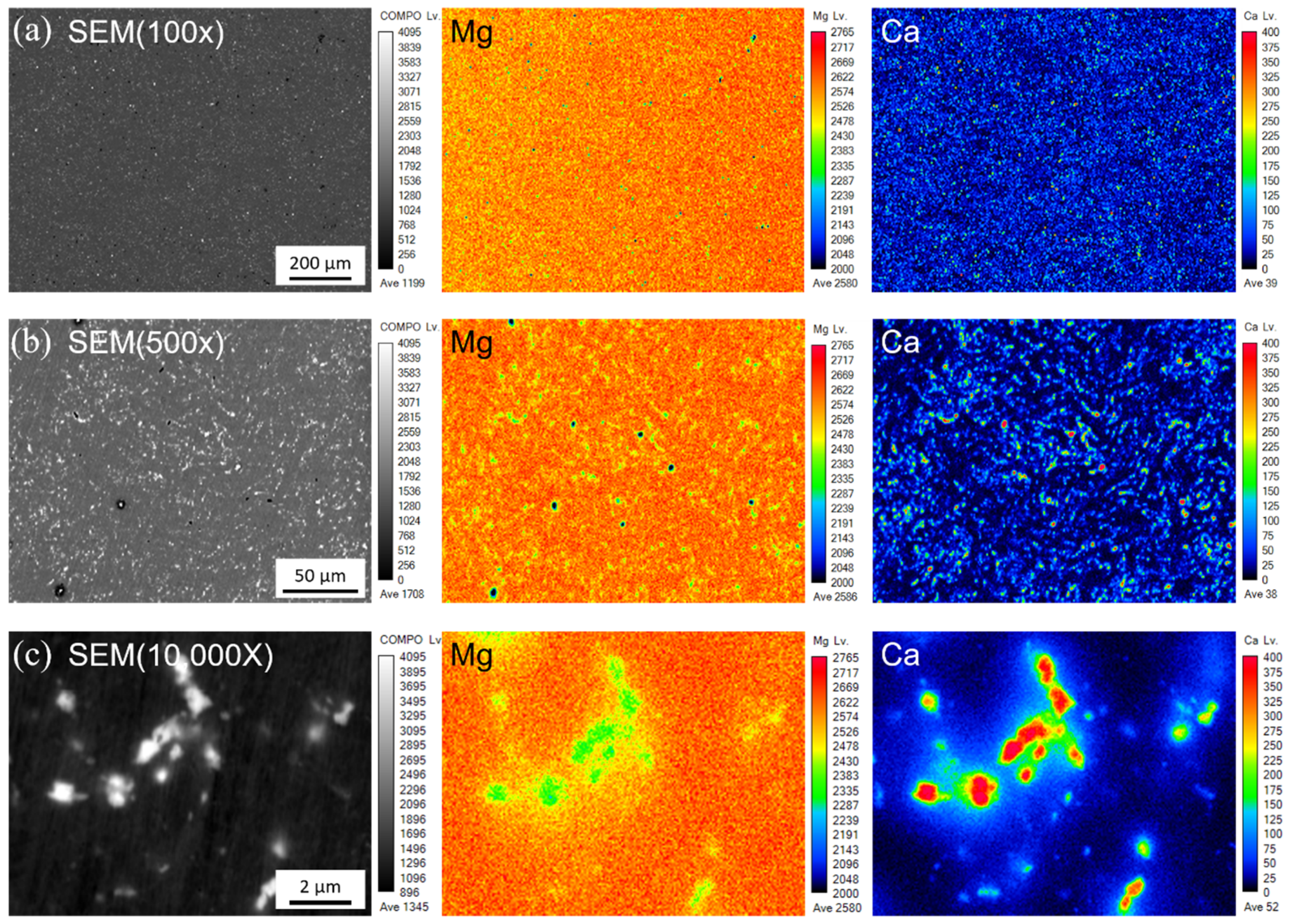
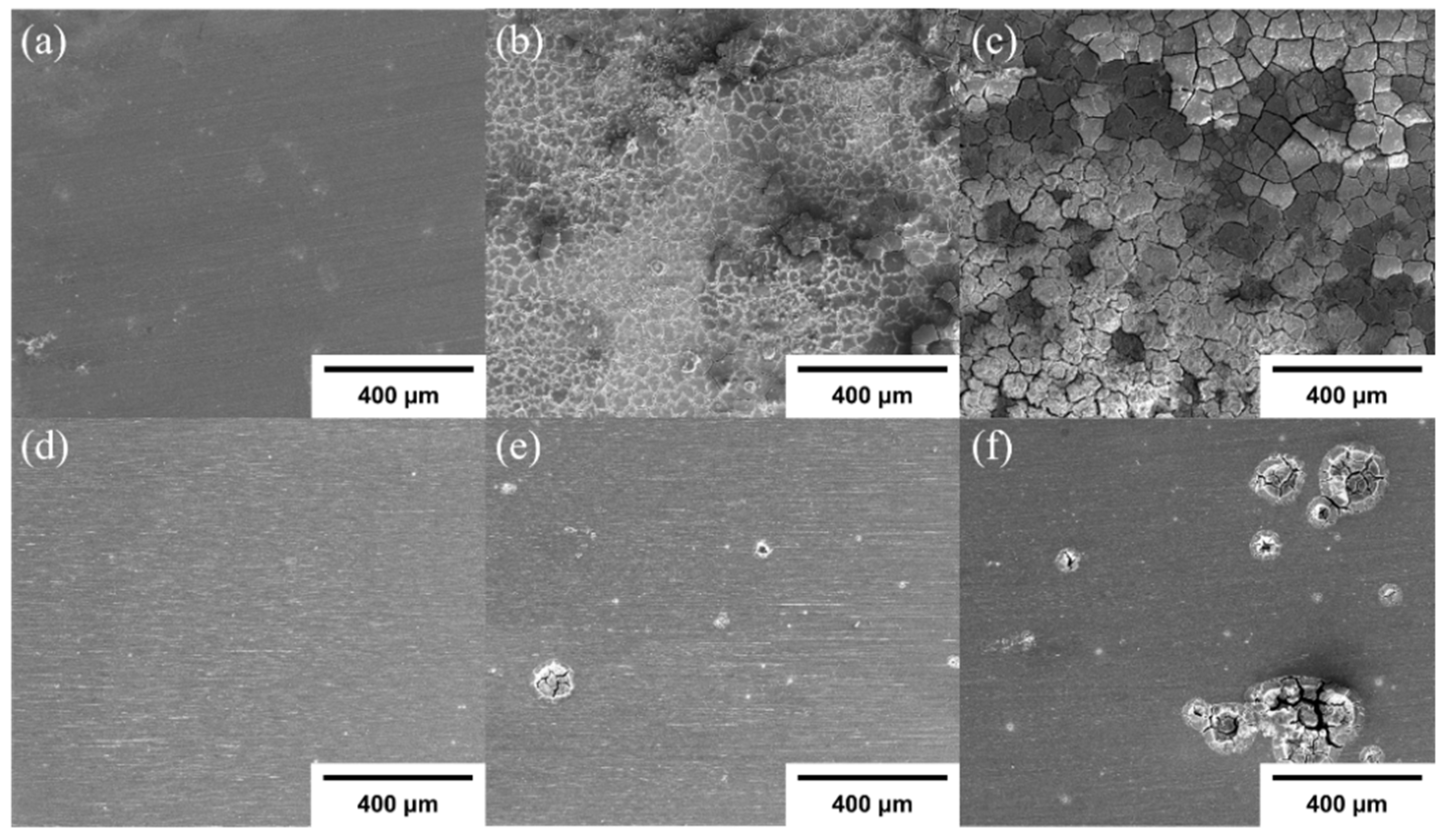
3.1. Precipitate of as-Extruded Mg–Ca Alloy
3.2. Characteristics of HZO Film
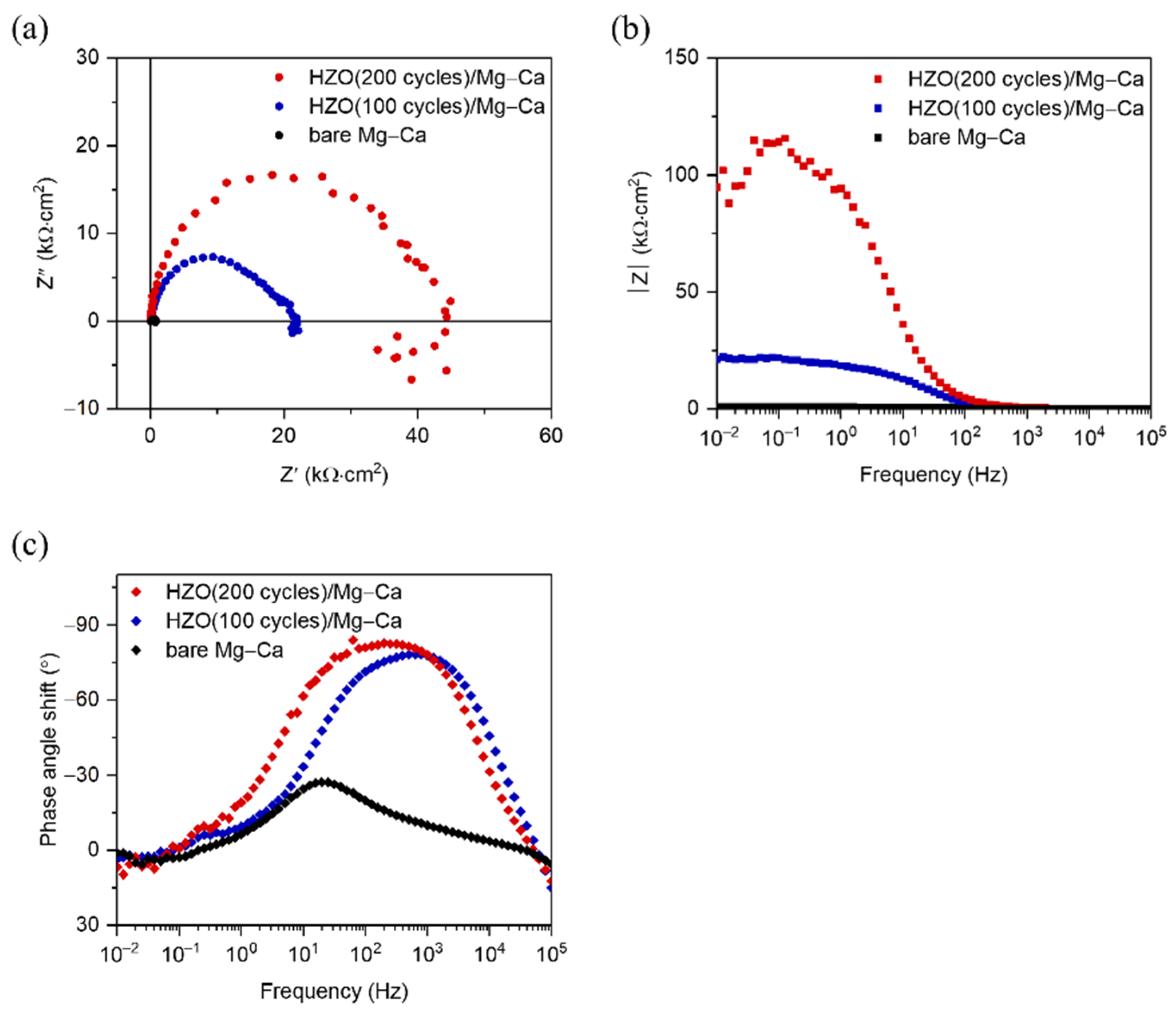
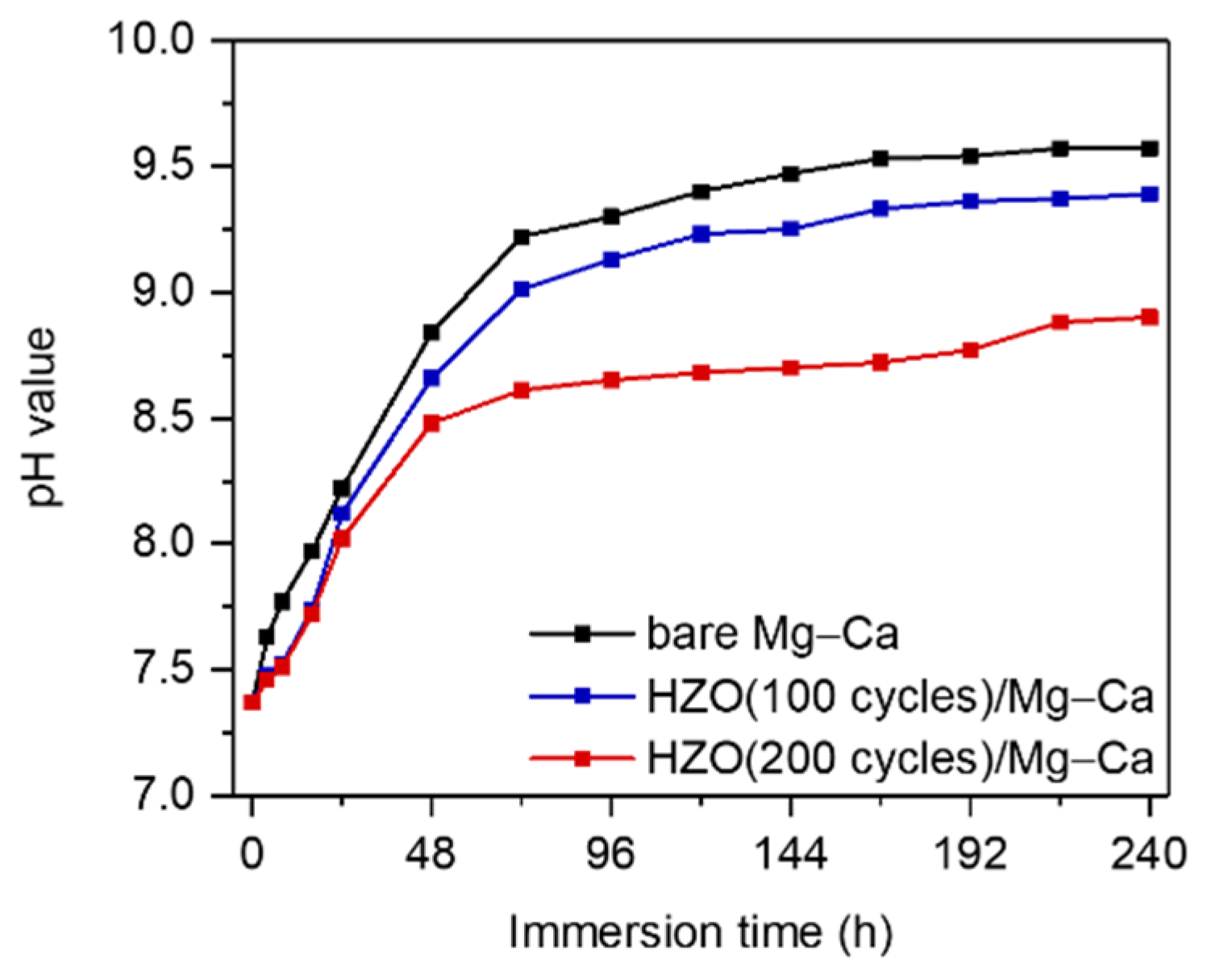
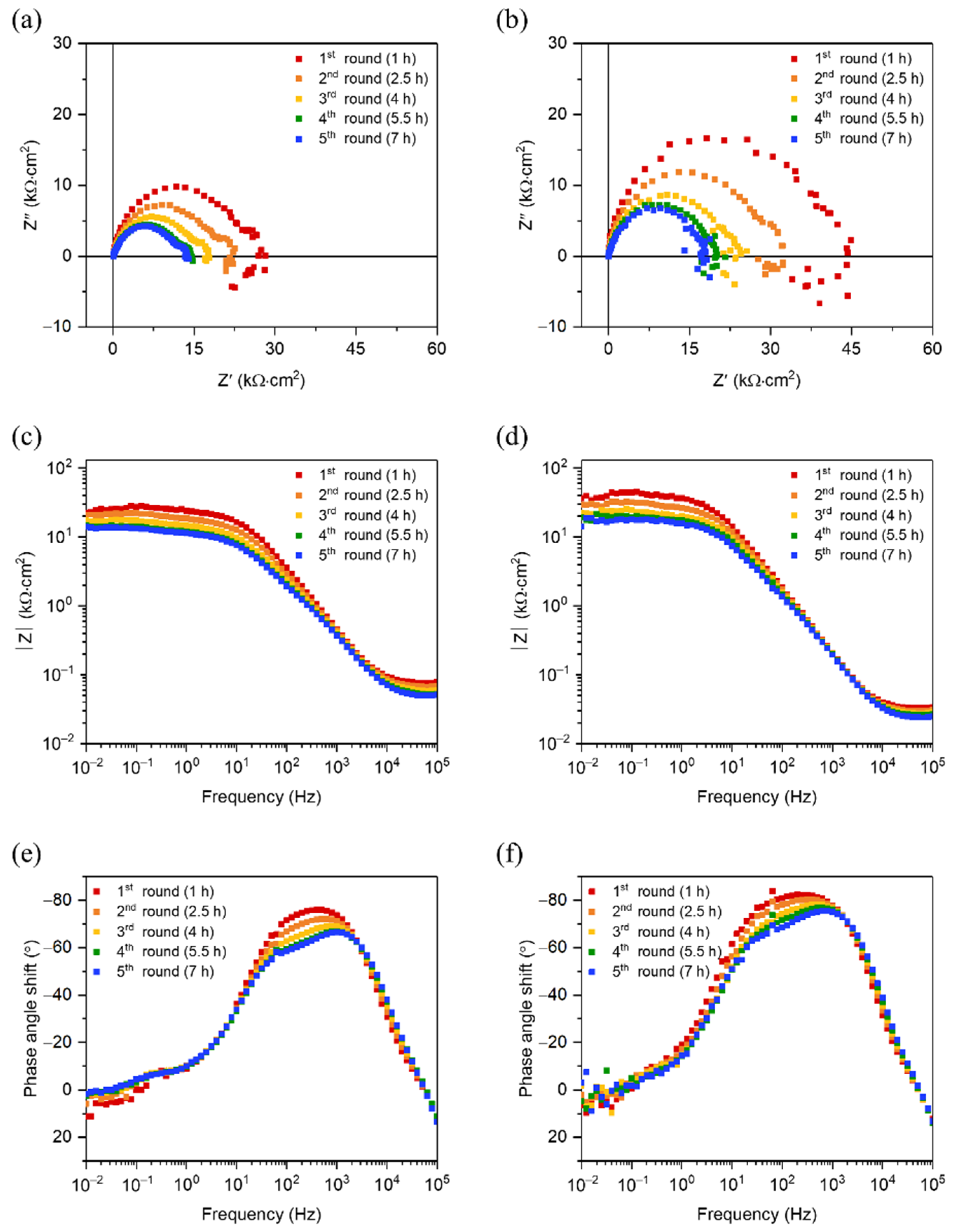
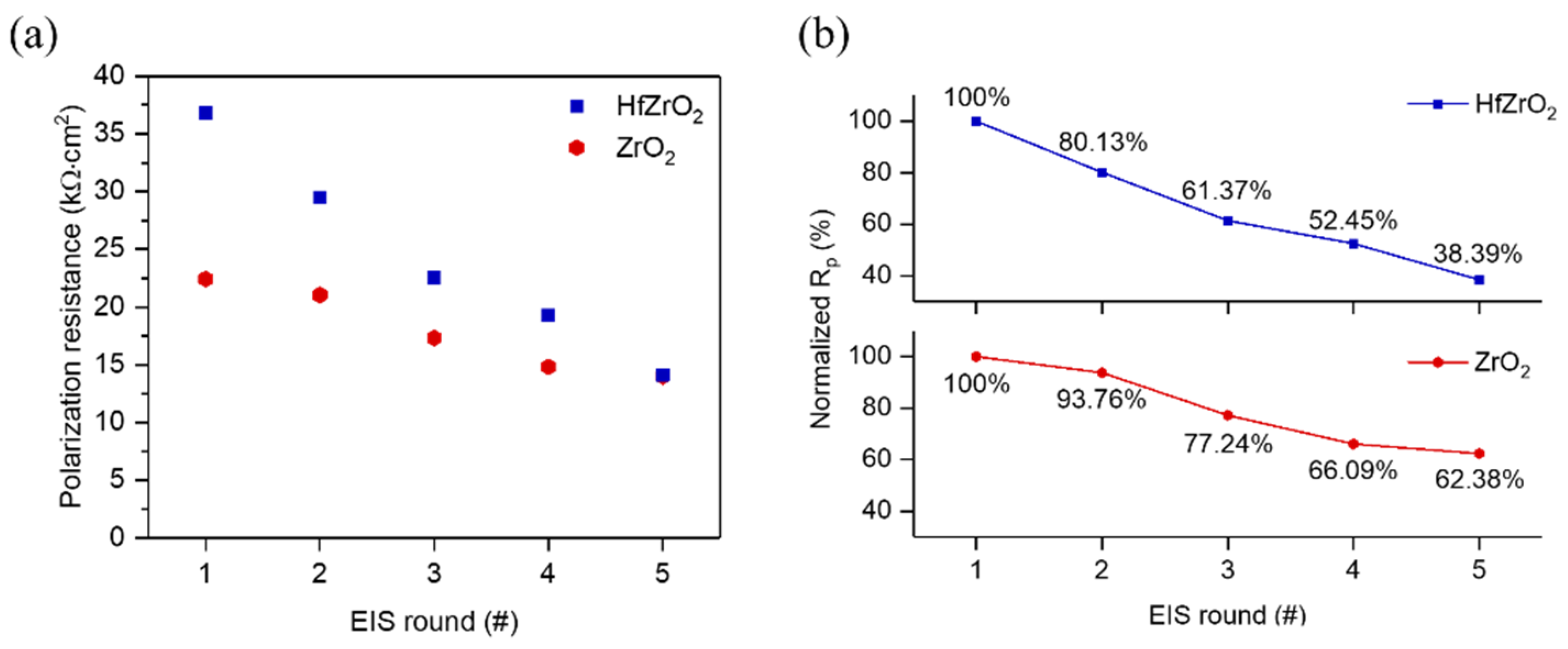
3.3. Corrosion Resistance of HZO Film
3.4. Corrosion Rate of Mg–Ca Alloy with/without HZO Film
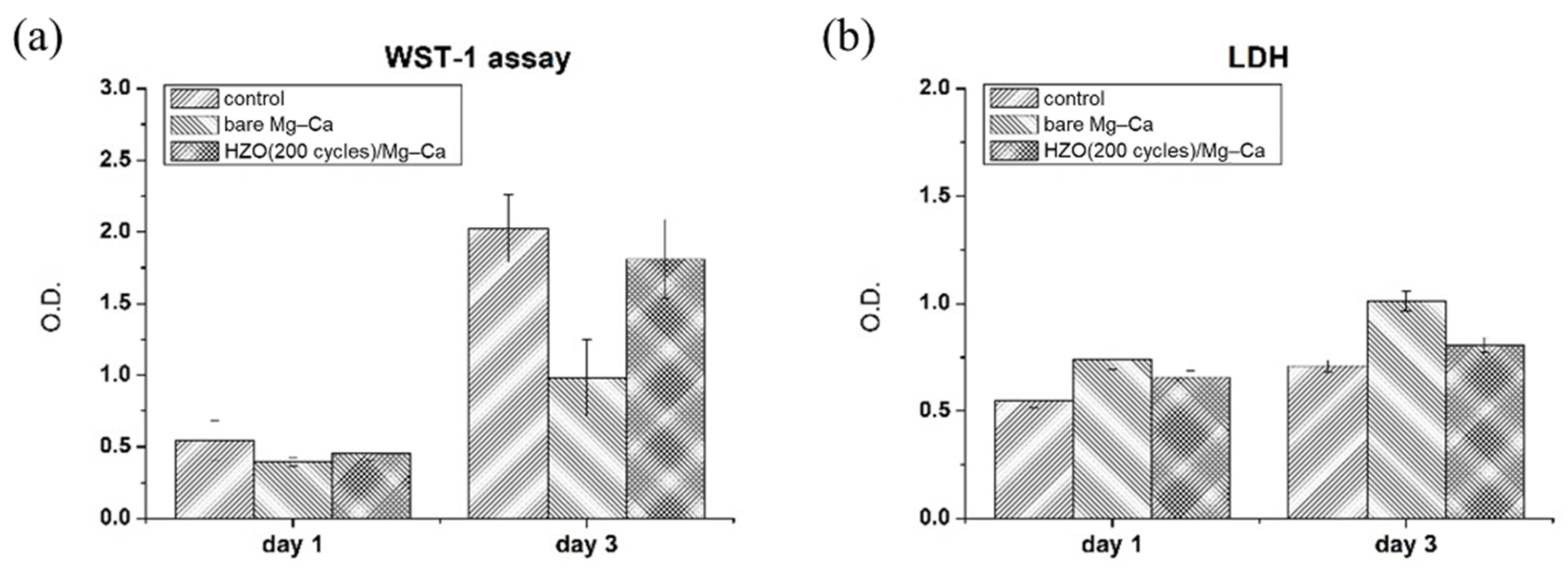
3.5. Biocompatibility of HZO Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/ water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition: Basics, opportunities, and challenges. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 050801. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Yuan, W.; Liu, X.; Zheng, Y.; Cui, Z.; Yang, X.; Pan, H.; Wu, S. Atomic layer deposited ZrO2 nanofilm on Mg–Sr alloy for enhanced corrosion resistance and biocompatibility. Acta Biomater. 2017, 58, 515–526. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion protection of copper using Al2O3, TiO2, ZnO, HfO2, and ZrO2 atomic layer deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; BinAfif, A.; Dadlani, A.; Berto, F.; Torgersen, J. Comparing physiologically relevant corrosion performances of Mg AZ31 alloy protected by ALD and sputter coated TiO. Surf. Coatings Technol. 2020, 395, 125922. [Google Scholar] [CrossRef]
- Fusco, M.; Oldham, C.; Parsons, G. Investigation of the corrosion behavior of atomic layer deposited Al2O3/TiO2 nanolaminate thin films on copper in 0.1 M NaCl. Materials 2019, 12, 672. [Google Scholar] [CrossRef] [Green Version]
- Marin, E.; Lanzutti, A.; Guzman, L.; Fedrizzi, L. Corrosion protection of AISI 316 stainless steel by ALD alumina/titania nanometric coatings. J. Coatings Technol. Res. 2011, 8, 655–659. [Google Scholar] [CrossRef]
- García-Saucedo, C.; Field, J.A.; Otero-Gonzalez, L.; Sierra-Álvarez, R. Low toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiae. J. Hazard. Mater. 2011, 192, 1572–1579. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Alagarsamy, K.; Kamalan Kirubaharan, A.M. Biological and corrosion behavior of m-ZrO2 and t-ZrO2 coated 316L SS for potential biomedical applications. Ceram. Int. 2018, 44, 14940–14946. [Google Scholar] [CrossRef]
- Kim, S.J.; Mohan, J.; Summerfelt, S.R.; Kim, J. Ferroelectric Hf0.5Zr0.5O2 thin films: A review of recent advances. JOM 2019, 71, 246–255. [Google Scholar] [CrossRef]
- Lee, M.H.; Chen, P.G.; Liu, C.; Chu, K.Y.; Cheng, C.C.; Xie, M.J.; Liu, S.N.; Lee, J.W.; Huang, S.J.; Liao, M.H.; et al. Prospects for ferroelectric HfZrOx FETs with experimentally CET = 0.98 nm, SSfor = 42 mV/dec, SSrev = 28 mV/dec, switch-off < 0.2 V, and hysteresis-free strategies. In Proceedings of the 2015 IEEE International Electron Devices Meeting (IEDM), Washington, DC, USA, 7–9 December 2015. [Google Scholar] [CrossRef]
- Cao, R.; Liu, Q.; Liu, M.; Song, B.; Shang, D.; Yang, Y.; Luo, Q.; Wu, S.; Li, Y.; Wang, Y.; et al. Improvement of endurance in HZO-based ferroelectric capacitor using ru electrode. IEEE Electron. Device Lett. 2019, 40, 1744–1747. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.W.; Peuster, M. Development and biocompatibility of a novel corrodible fluoride-coated magnesium-calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications. J. Biomed. Mater. Res. Part A 2009, 93, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Hort, N.; Huong, Y.; Kainer, K.U. Intermetallics in magnesium alloys. Adv. Eng. Mater. 2006, 8, 235–240. [Google Scholar] [CrossRef]
- Onodera, A.; Takesada, M. Ferroelectricity in simple binary crystals. Crystals 2017, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Triyoso, D.H.; Gregory, R.; Park, M.; Wang, K.; Lee, S.I. Physical and electrical properties of atomic-layer-deposited HfxZr1−xO2 with TEMAHf, TEMAZr, and ozone. J. Electrochem. Soc. 2007, 155, 43–46. [Google Scholar] [CrossRef]
- Magaino, S.; Kawaguchi, A.; Hirata, A.; Osaka, T. Spectrum analysis of corrosion potential fluctuations for localized corrosion of type 304 stainless steel. J. Electrochem. Soc. 1987, 134, 2993–2997. [Google Scholar] [CrossRef]
- Yamakawa, K.; Inoue, H. Analysis of corrosion potential fluctuation for stress corrosion cracking. Corros. Sci. 1990, 31, 503–508. [Google Scholar] [CrossRef]
- Sun, H.; Cooke, K.; Eitzinger, G.; Hamilton, P.; Pollet, B. Development of PVD coatings for PEMFC metallic bipolar plates. Thin Solid Film. 2013, 528, 199–204. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, K.F.; Chiu, C.; Semenov, V.I.; Lin, H.C.; Chen, M.J. Effect of atomic layer plasma treatment on TALD-ZrO2 film to improve the corrosion protection of Mg-Ca alloy. Surf. Coatings Technol. 2021, 427, 127811. [Google Scholar] [CrossRef]
- Kong, Y.M.; Bae, C.J.; Lee, S.H.; Kim, H.W.; Kim, H.E. Improvement in biocompatibility of ZrO2–Al2O3 nano-composite by addition of HA. Biomaterials 2005, 26, 509–517. [Google Scholar] [CrossRef]


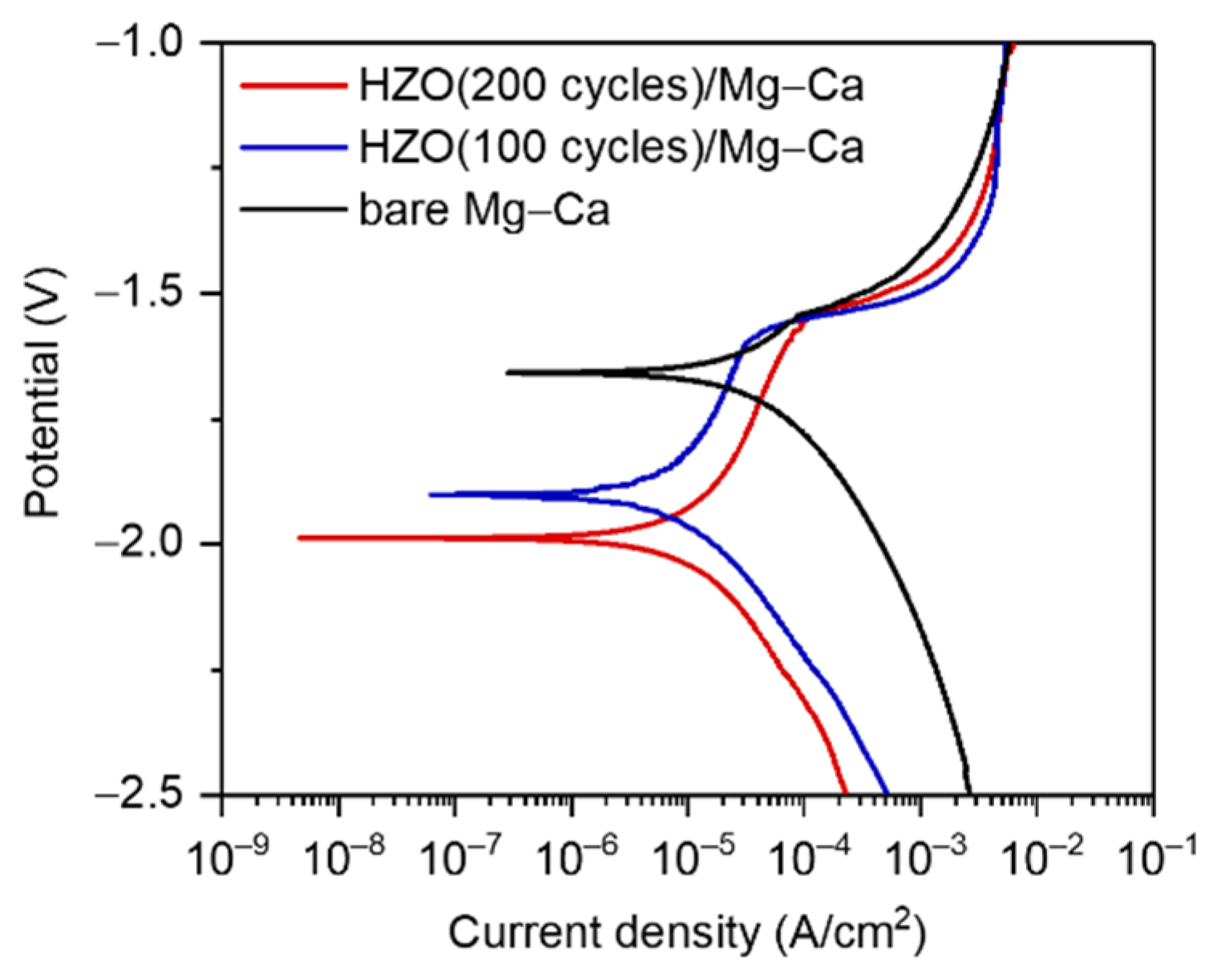
| Sample | icorr (A/cm2) | Ecorr (V) | Epitting (V) | Epitting-Ecorr (V) |
|---|---|---|---|---|
| Bare Mg–Ca alloy | 2.56 × 10−5 | −1.66 | −1.54 | 0.12 |
| HZO(100 cycles)/Mg–Ca | 8.39 × 10−6 | −1.89 | −1.57 | 0.32 |
| HZO(200 cycles)/Mg–Ca | 7.31 × 10−6 | −1.98 | −1.55 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-C.; Lin, K.; Lin, Y.-H.; Yang, K.-C.; Semenov, V.I.; Lin, H.-C.; Chen, M.-J. Improvement of Corrosion Resistance and Biocompatibility of Biodegradable Mg–Ca Alloy by ALD HfZrO2 Film. Coatings 2022, 12, 212. https://doi.org/10.3390/coatings12020212
Lin P-C, Lin K, Lin Y-H, Yang K-C, Semenov VI, Lin H-C, Chen M-J. Improvement of Corrosion Resistance and Biocompatibility of Biodegradable Mg–Ca Alloy by ALD HfZrO2 Film. Coatings. 2022; 12(2):212. https://doi.org/10.3390/coatings12020212
Chicago/Turabian StyleLin, Pi-Chen, Kaifan Lin, Yu-Hsuan Lin, Kai-Chiang Yang, Vladimir Ivanovitch Semenov, Hsin-Chih Lin, and Miin-Jang Chen. 2022. "Improvement of Corrosion Resistance and Biocompatibility of Biodegradable Mg–Ca Alloy by ALD HfZrO2 Film" Coatings 12, no. 2: 212. https://doi.org/10.3390/coatings12020212
APA StyleLin, P.-C., Lin, K., Lin, Y.-H., Yang, K.-C., Semenov, V. I., Lin, H.-C., & Chen, M.-J. (2022). Improvement of Corrosion Resistance and Biocompatibility of Biodegradable Mg–Ca Alloy by ALD HfZrO2 Film. Coatings, 12(2), 212. https://doi.org/10.3390/coatings12020212






