Enhanced Electrocatalytic CO2 Reduction of Bismuth Nanosheets with Introducing Surface Bismuth Subcarbonate
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Sample Characterizations
2.3. Electrochemical Measurements
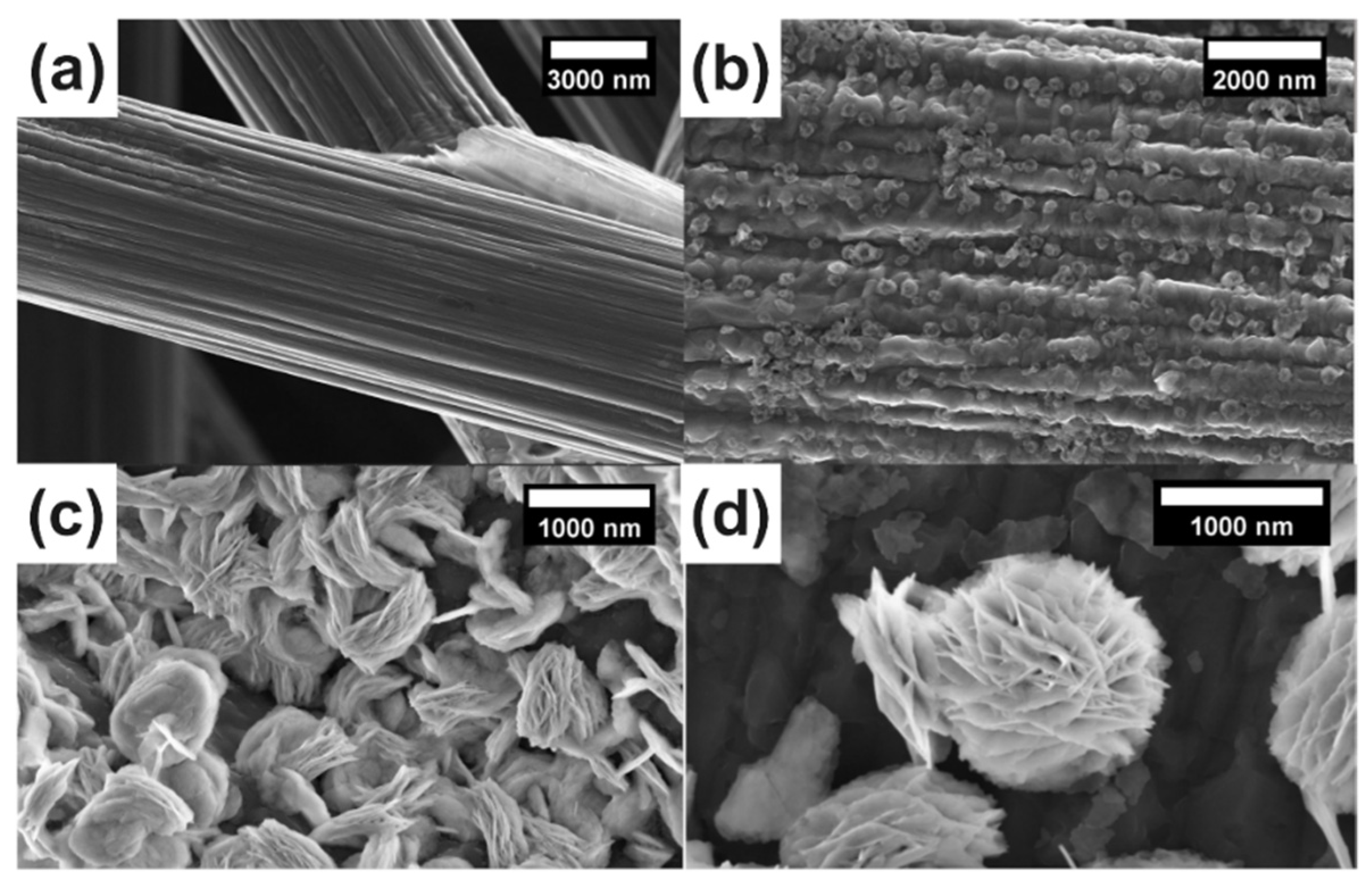
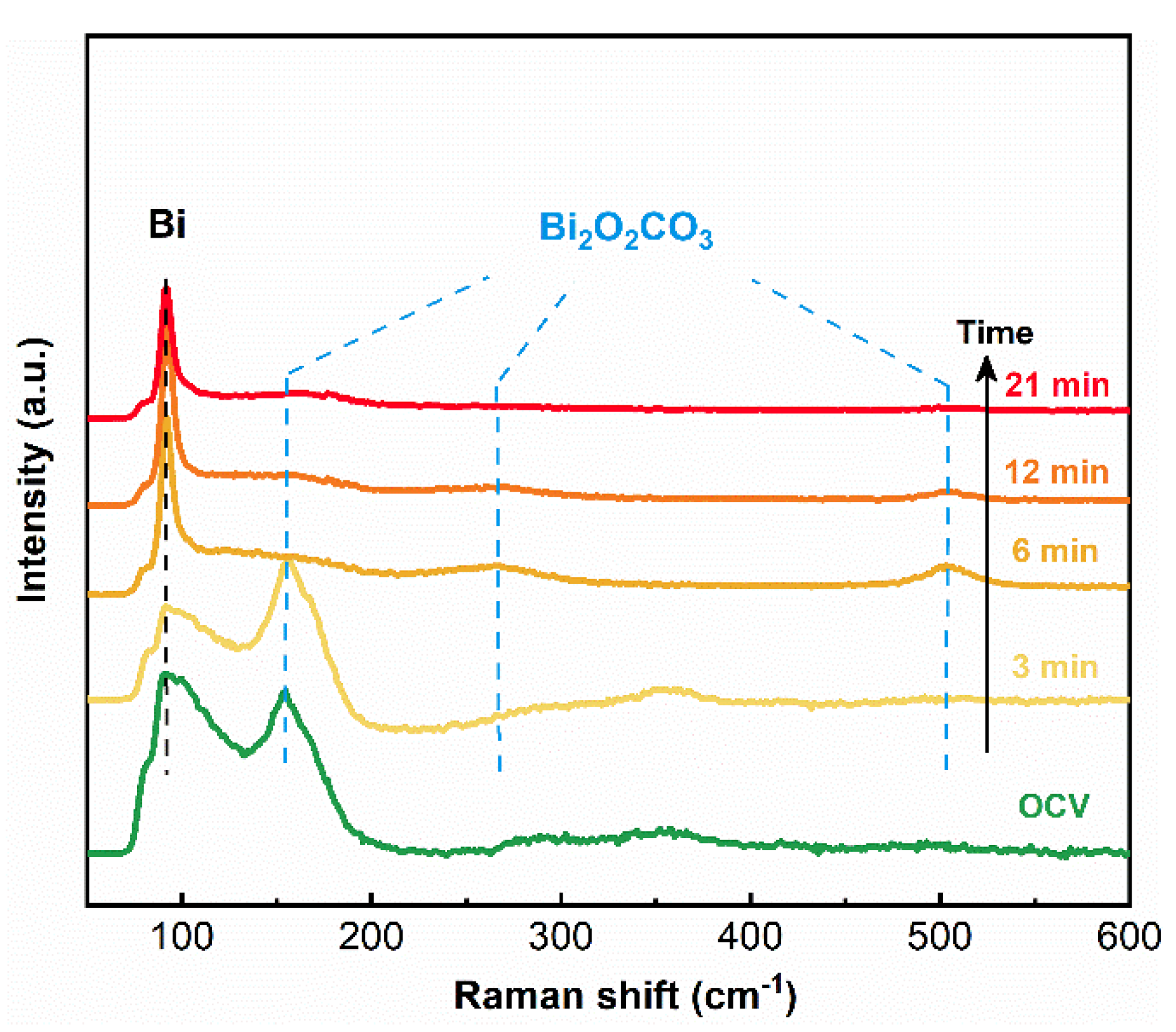
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.N.; Dinh, C.T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 2020, 49, 7488–7504. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; Chang, S.X.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, C.; Yu, X.; Yao, Y.; Li, Z.; Wu, C.; Yao, W.; Zou, Z. Extraterrestrial artificial photosynthetic materials for in-situ resource utilization. Natl. Sci. Rev. 2021, 8, nwab104. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yuan, T.; Chen, S.; Li, H.; Hu, C.; Dong, H.; Wu, B.; Wang, T.; Li, J.; Ozin, G.A.; et al. Effect of bicarbonate on CO2 electroreduction over cathode catalysts. Fundam. Res. 2021, 1, 432–438. [Google Scholar] [CrossRef]
- Löwe, A.; Schmidt, M.; Bienen, F.; Kopljar, D.; Wagner, N.; Klemm, E. Optimizing Reaction Conditions and Gas Diffusion Electrodes Applied in the CO2 Reduction Reaction to Formate to Reach Current Densities up to 1.8 A cm–2. ACS Sustain. Chem. Eng. 2021, 9, 4213–4223. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Ren, Y.; Cui, S.; Li, W.; Qiu, J. Recent advances in innovative strategies for the CO2 electroreduction reaction. Energy Environ. Sci. 2021, 14, 765–780. [Google Scholar] [CrossRef]
- Claassens, N.J.; Cotton, C.A.R.; Kopljar, D.; Bar-Even, A. Making quantitative sense of electromicrobial production. Nat. Catal. 2019, 2, 437–447. [Google Scholar] [CrossRef]
- Pribyl-Kranewitter, B.; Beard, A.; Gîjiu, C.; Dinculescu, D.; Schmidt, T. Influence of low-temperature electrolyser design on economic and environmental potential of CO and HCOOH production: A techno-economic assessment. Renew. Sustain. Energy Rev. 2021, 154, 111807. [Google Scholar] [CrossRef]
- Oh, W.; Rhee, C.K.; Han, J.W.; Shong, B. Atomic and Molecular Adsorption on the Bi(111) Surface: Insights into Catalytic CO2 Reduction. J. Phys. Chem. C 2018, 122, 23084–23090. [Google Scholar] [CrossRef]
- Duan, Y.X.; Zhou, Y.T.; Yu, Z.; Liu, D.X.; Wen, Z.; Yan, J.M.; Jiang, Q. Boosting production of HCOOH from CO2 electroreduction via Bi/CeOx. Angew. Chem. Int. Ed. Engl. 2021, 133, 8880–8884. [Google Scholar] [CrossRef]
- Suominen, M.; Kallio, T. What We Currently Know about Carbon-Supported Metal and Metal Oxide Nanomaterials in Electrochemical CO2 Reduction. ChemElectroChem 2021, 8, 2397–2406. [Google Scholar] [CrossRef]
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2020, 10, 1902338. [Google Scholar] [CrossRef]
- Hara, K.; Kudo, A.; Sakata, T. Electrochemical reduction of carbon dioxide under high pressure on various electrodes in an aqueous electrolyte. J. Electroanal. Chem. 1995, 391, 141–147. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.; Li, Y.; Li, Y. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Fan, M.; Prabhudev, S.; Garbarino, S.; Qiao, J.; Botton, G.A.; Harrington, D.; Tavares, A.C.; Guay, D. Uncovering the nature of electroactive sites in nano architectured dendritic Bi for highly efficient CO2 electroreduction to formate. Appl. Catal. B Environ. 2020, 274, 119031. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, M.; Rao, X.; Liu, Y.; Zhang, J. Electrochemical reduction of carbon dioxide (CO2): Bismuth-based electrocatalysts. J. Mater. Chem. A 2021, 9, 13770–13803. [Google Scholar] [CrossRef]
- Fan, T.; Ma, W.; Xie, M.; Liu, H.; Zhang, J.; Yang, S.; Huang, P.; Dong, Y.; Chen, Z.; Yi, X. Achieving high current density for electrocatalytic reduction of CO2 to formate on bismuth-based catalysts. Cell Rep. Phys. Sci. 2021, 2, 100353. [Google Scholar] [CrossRef]
- Al-Tamreh, S.A.; Ibrahim, M.H.; El-Naas, M.H.; Vaes, J.; Pant, D.; Benamor, A.; Amhamed, A. Electroreduction of Carbon Dioxide into Formate: A Comprehensive Review. ChemElectroChem 2021, 8, 3207–3220. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Quan, F.; Huang, J.; Jia, F.; Zhang, L. Selective electro-reduction of CO2 to formate on nanostructured Bi from reduction of BiOCl nanosheets. Electrochem. Commun. 2014, 46, 63–66. [Google Scholar] [CrossRef]
- Lee, C.W.; Hong, J.S.; Yang, K.D.; Jin, K.; Lee, J.H.; Ahn, H.-Y.; Seo, H.; Sung, N.-E.; Nam, K.T. Selective Electrochemical Production of Formate from Carbon Dioxide with Bismuth-Based Catalysts in an Aqueous Electrolyte. ACS Catal. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Pastor, A.; Laurent, S.; Brun, E.; Sicard-Roselli, C.; Port, M. Metallic bismuth nanoparticles: Towards a robust, productive and ultrasound assisted synthesis from batch to flow-continuous chemistry. Ultrason. Sonochemistry 2019, 56, 167–173. [Google Scholar] [CrossRef]
- Zhang, D.; Tao, Z.; Feng, F.; He, B.; Zhou, W.; Sun, J.; Xu, J.; Wang, Q.; Zhao, L. High efficiency and selectivity from synergy: Bi nanoparticles embedded in nitrogen doped porous carbon for electrochemical reduction of CO2 to formate. Electrochim Acta 2020, 334, 135563. [Google Scholar] [CrossRef]
- Wang, D.; Chang, K.; Zhang, Y.; Wang, Y.; Liu, Q.; Wang, Z.; Ding, D.; Cui, Y.; Pan, C.; Lou, Y.; et al. Unravelling the electrocatalytic activity of bismuth nanosheets towards carbon dioxide reduction: Edge plane versus basal plane. Appl. Catal. B Environ. 2021, 299, 120693. [Google Scholar] [CrossRef]
- Kuang, Z.; Peng, C.; Li, C.; Yao, H.; Zhou, X.; Chen, H. Efficient electrocatalytic CO2 conversion into formate with AlxBiyOz nanorods in a wide potential window. Catal. Sci. Technol. 2021, 11, 7704–7711. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Y.; Zhang, T.; Li, X.; Zhang, H.; Chen, X. Bismuth nanodendrites as a high performance electrocatalyst for selective conversion of CO2 to formate. J. Mater. Chem. A 2016, 4, 13746–13753. [Google Scholar] [CrossRef]
- Fan, K.; Jia, Y.; Ji, Y.; Kuang, P.; Zhu, B.; Liu, X.; Yu, J. Curved Surface Boosts Electrochemical CO2 Reduction to Formate via Bismuth Nanotubes in a Wide Potential Window. ACS Catal. 2020, 10, 358–364. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lei, T.; Liu, Y.; Qiao, J. Enhancing CO2 electrolysis to formate on facilely synthesized Bi catalysts at low overpotential. Appl. Catal. B Environ. 2017, 218, 46–50. [Google Scholar] [CrossRef]
- Sui, P.F.; Xu, C.; Zhu, M.N.; Liu, S.; Liu, Q.; Luo, J.L. Interface-Induced Electrocatalytic Enhancement of CO2 -to-Formate Conversion on Heterostructured Bismuth-Based Catalysts. Small 2022, 18, e2105682. [Google Scholar] [CrossRef]
- Gao, T.; Wen, X.; Xie, T.; Han, N.; Sun, K.; Han, L.; Wang, H.; Zhang, Y.; Kuang, Y.; Sun, X. Morphology effects of bismuth catalysts on electroreduction of carbon dioxide into formate. Electrochim. Acta 2019, 305, 388–393. [Google Scholar] [CrossRef]
- Kim, S.; Dong, W.J.; Gim, S.; Sohn, W.; Park, J.Y.; Yoo, C.J.; Jang, H.W.; Lee, J.-L. Shape-controlled bismuth nanoflakes as highly selective catalysts for electrochemical carbon dioxide reduction to formate. Nano Energy 2017, 39, 44–52. [Google Scholar] [CrossRef]
- An, X.; Li, S.; Hao, X.; Du, X.; Yu, T.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. The In Situ morphology transformation of bismuth-based catalysts for the effective electroreduction of carbon dioxide. Sustain. Energy Fuels 2020, 4, 2831–2840. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; Vega-Verduga, C.; Díaz, D.; Zumeta-Dubé, I. Transformation of Bismuth and β-Bi2O3 Nanoparticles into (BiO)2CO3 and (BiO)4(OH)2CO3 by Capturing CO2: The Role of Halloysite Nanotubes and “Sunlight” on the Crystal Shape and Size. Cryst. Growth Des. 2018, 18, 4334–4346. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Ma, Z. Facile Formation of Bi2O2CO3/Bi2MoO6 Nanosheets for Visible Light-Driven Photocatalysis. ACS Omega 2019, 4, 3871–3880. [Google Scholar] [CrossRef] [Green Version]
- Lv, W.; Bei, J.; Zhang, R.; Wang, W.; Kong, F.; Wang, L.; Wang, W. Bi2O2CO3 Nanosheets as Electrocatalysts for Selective Reduction of CO2 to Formate at Low Overpotential. ACS Omega 2017, 2, 2561–2567. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-Q.; Shahini, E.; Gao, M.-R.; Gong, L.; Sui, P.-F.; Tang, T.; Zeng, H.; Luo, J.-L. Bi2O3 Nanosheets Grown on Carbon Nanofiber with Inherent Hydrophobicity for High-Performance CO2 Electroreduction in a Wide Potential Window. ACS Nano 2021, 15, 17757–17768. [Google Scholar] [CrossRef]
- Deng, P.; Wang, H.; Qi, R.; Zhu, J.; Chen, S.; Yang, F.; Zhou, L.; Qi, K.; Liu, H.; Xia, B.Y. Bismuth Oxides with Enhanced Bismuth–Oxygen Structure for Efficient Electrochemical Reduction of Carbon Dioxide to Formate. ACS Catal. 2019, 10, 743–750. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Jiang, W.; Liu, Z.; Zhang, J.; Gao, L.; Yao, W. Sub-2 nm ultra-thin Bi2O2CO3 nanosheets with abundant Bi-O structures toward formic acid electrosynthesis over a wide potential window. Nano Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, P.; Jia, J.; Zhang, S.; Zheng, X.; Zhang, L.; Zhang, B.; Chen, J.; Hao, W.; Chen, G.; et al. Supercritical CO2-constructed intralayer [Bi2O2]2+ structural distortion for enhanced CO2 electroreduction. J. Mater. Chem. A Mater. 2020, 8, 13320–13327. [Google Scholar] [CrossRef]
- Dutta, A.; Montiel, I.Z.; Kiran, K.; Rieder, A.; Grozovski, V.; Gut, L.; Broekmann, P. A Tandem (Bi2O3 → Bimet) Catalyst for Highly Efficient ec-CO2 Conversion into Formate: Operando Raman Spectroscopic Evidence for a Reaction Pathway Change. ACS Catal. 2021, 11, 4988–5003. [Google Scholar] [CrossRef]
- Li, L.; Ma, D.-K.; Qi, F.; Chen, W.; Huang, S. Bi nanoparticles/Bi2O3 nanosheets with abundant grain boundaries for efficient electrocatalytic CO2 reduction. Electrochim. Acta 2019, 298, 580–586. [Google Scholar] [CrossRef]
- Bertin, E.; Garbarino, S.; Roy, C.; Kazemi, S.; Guay, D. Selective electroreduction of CO2 to formate on Bi and oxide-derived Bi films. J. CO2 Util. 2017, 19, 276–283. [Google Scholar] [CrossRef]
- Liu, S.; Lu, X.F.; Xiao, J.; Wang, X.; Lou, X.W. (David) Bi2O3 Nanosheets Grown on Multi-Channel Carbon Matrix to Catalyze Efficient CO2 Electroreduction to HCOOH. Angew. Chem. 2019, 131, 13966–13971. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Ling, Y.; Li, F.; Bond, A.M.; Zhang, J. Controllable Synthesis of Few-Layer Bismuth Subcarbonate by Electrochemical Exfoliation for Enhanced CO2 Reduction Performance. Angew. Chem. Int. Ed. 2018, 57, 13283–13287. [Google Scholar] [CrossRef]
- Zhao, M.; Gu, Y.; Gao, W.; Cui, P.; Tang, H.; Wei, X.; Zhu, H.; Li, G.; Yan, S.; Zhang, X.; et al. Atom vacancies induced electron-rich surface of ultrathin Bi nanosheet for efficient electrochemical CO2 reduction. Appl. Catal. B Environ. 2020, 266, 118625. [Google Scholar] [CrossRef]
- Wang, J.; Mao, J.; Zheng, X.; Zhou, Y.; Xu, Q. Sulfur boosting CO2 reduction activity of bismuth subcarbonate nanosheets via promoting proton-coupled electron transfer. Appl. Surf. Sci. 2021, 562, 150197. [Google Scholar] [CrossRef]
- Puppin, L.G.; Khalid, M.; da Silva, G.T.T.; Ribeiro, C.; Varela, H.; Lopes, O.F. Electrochemical reduction of CO2 to formic acid on Bi2O2CO3/carbon fiber electrodes. J. Mater. Res. 2020, 35, 272–280. [Google Scholar] [CrossRef]
- Ramler, J.; Lichtenberg, C. Molecular Bismuth Cations: Assessment of Soft Lewis Acidity. Chem.—A Eur. J. 2020, 26, 10250–10258. [Google Scholar] [CrossRef]
- Ramler, J.; Wüst, L.; Rempel, A.; Wolz, L.; Lichtenberg, C. Bismuth Atoms in Hydrocarbon Ligands: Bismepines as Rigid, Ditopic Arene Donors in Coordination Chemistry. Organometallics 2021, 40, 832–837. [Google Scholar] [CrossRef]
- Yan, T.; Li, N.; Wang, L.; Ran, W.; Duchesne, P.N.; Wan, L.; Nguyen, N.T.; Wang, L.; Xia, M.; Ozin, G.A. Bismuth atom tailoring of indium oxide surface frustrated Lewis pairs boosts heterogeneous CO2 photocatalytic hydrogenation. Nat. Commun. 2020, 11, 6095. [Google Scholar] [CrossRef]
- Rayner-Canham, G.; Overton, T. Descriptive Inorganic Chemistry, 4th ed.; W. H. Freeman Basingstoke; Palgrave Macmillan: New York, NY, USA, 2006. [Google Scholar]
- Tobon- Zapata, G.E.; Etcheverry, S.B.; Baran, E.J. Vibrational spectrum of bismuth subcarbonate. J. Mater. Sci. Lett. 1997, 16, 656–657. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Zhang, X.; Williams, T.; Easton, C.D.; Bond, A.M.; Zhang, J. Electrochemical reduction of CO2 on defect-rich Bi derived from Bi2S3with enhanced formate selectivity. J. Mater. Chem. A 2018, 6, 4714–4720. [Google Scholar] [CrossRef]
- Rajamani, A.R.; Jothi, S.; Kumar, M.D.; Srikaanth, S.; Singh, M.K.; Otero-Irurueta, G.; Ramasamy, D.; Datta, M.; Rangarajan, M. Effects of Additives on Kinetics, Morphologies and Lead-Sensing Property of Electrodeposited Bismuth Films. J. Phys. Chem. C 2016, 120, 22398–22406. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Hu, B.; Zhao, J.; Jiang, W.; Feng, D.; Zhang, C.; Yao, W. Enhanced Electrocatalytic CO2 Reduction of Bismuth Nanosheets with Introducing Surface Bismuth Subcarbonate. Coatings 2022, 12, 233. https://doi.org/10.3390/coatings12020233
Liu S, Hu B, Zhao J, Jiang W, Feng D, Zhang C, Yao W. Enhanced Electrocatalytic CO2 Reduction of Bismuth Nanosheets with Introducing Surface Bismuth Subcarbonate. Coatings. 2022; 12(2):233. https://doi.org/10.3390/coatings12020233
Chicago/Turabian StyleLiu, Shiyuan, Botao Hu, Junkai Zhao, Wenjun Jiang, Deqiang Feng, Ce Zhang, and Wei Yao. 2022. "Enhanced Electrocatalytic CO2 Reduction of Bismuth Nanosheets with Introducing Surface Bismuth Subcarbonate" Coatings 12, no. 2: 233. https://doi.org/10.3390/coatings12020233
APA StyleLiu, S., Hu, B., Zhao, J., Jiang, W., Feng, D., Zhang, C., & Yao, W. (2022). Enhanced Electrocatalytic CO2 Reduction of Bismuth Nanosheets with Introducing Surface Bismuth Subcarbonate. Coatings, 12(2), 233. https://doi.org/10.3390/coatings12020233





