Abstract
The effects of the grain boundary precipitation on intergranular corrosion behavior were investigated by exfoliation tests and complementary techniques like scanning electron microscope (SEM), optical profilometry (OP), transmission electron microscope together with energy dispersive spectroscopy (TEM-EDS), and atomic force microscope (AFM). The results reveal the influencing mechanism of intergranular corrosion behavior from grain boundary precipitates (GBPs). The potential discrepancy between GBPs and adjacent areas induces corrosion cavity germination along the grain boundary. Furthermore, the increase of both active Mg and Zn content in GBPs improve the potential difference, which aggravates the intergranular corrosion cavity germination. However, the increment of noble Cu content in GBP is beneficial to reduce the potential difference. On the other side, the distribution of the continuous precipitates in the grain boundary region helps the initial corrosion cavities to connect, which improves the growth of intergranular corrosion cracks. Additionally, discontinuous GBPs and precipitation free zone (PFZ) hinder the spread and connection of intergranular corrosion cavities. Therefore, 7050 aluminum alloy forming different grain boundary precipitation characteristics after different aging treatments shows different resistance to intergranular corrosion: peak-aging < under-aging < over-aging.
1. Introduction
7050 Al alloys as one high-strength Al alloys are extensively used for the aerospace industry owing to their precipitation hardening behavior [1,2,3]. The precipitation sequence of 7050 Al alloys is solid solution → GP zone → η’ phase → η phase (MgZn2). However, 7050 Al alloys are also sensitive to exfoliation due to the precipitation, which limits its aerospace application [4,5]. Exfoliation corrosion causes spalling or blistering on the surface of the material, which significantly reduces the strength, fracture toughness, and fatigue properties of the material, as well as the service life of the structural parts [6]. Exfoliation mainly exhibits classical intergranular corrosion (IGC), which proceeds parallel to the surface, and usually along with grain boundaries producing a layered corrosion appearance [7,8,9,10]. Many researchers found that the susceptibility of 7050 Al alloys to corrosion is closely related to precipitation behavior in the matrix [11,12,13], which is mainly ascribed to the formation of galvanic cells at microstructure. Lu et al. [14] studied the effect of microstructure on the exfoliation corrosion resistance of Al-Zn-Mg alloys, finding that the presence of abundant η phases on high-angle grain boundaries reduced the exfoliation corrosion resistance. Birbilis [15] reported that the step change of pitting rate corresponds with the S-phase size range from 3 to 8 nm of the Al-Cu-Mg alloys, for which secondary precipitation stops acting as distinct electrochemical entities. Interestingly, the Cu-rich precipitates are considered as local cathodes, which facilitate driving local anodic dissolution of the near matrix alloy [16]. Wang et al. [17] revealed that the pitting potential decreased from peak-aged to over-aged tempers in 7055 alloy thanks to the reduction of Cu content in matrix with precipitates growing. To improve the corrosion resistance of Al-Zn-Mg-Cu alloys, over-aging treatment such as T73 tempering is often used to coarsen the grains, which leads to the discrete distribution of the grain boundary η-MgZn2 phase [18,19]. Based on previous investigations, to improve the pitting resistance of 7A85 Al alloys, some advanced technologies are developed to adjust precipitation behavior, like non-isothermal aging and re-aging RRA [20,21]. However, Xie et al. [22] found that more precipitates appeared inside the grains and grain boundaries of the RRA samples, leading to RRA treated Al-Zn-Mg-Cu-Zr alloy samples with lower exfoliation corrosion resistance than natural aging and single-stage aged alloy samples.
In precipitation hardening Al alloys, the bulk potential is mainly dictated by the bulk component of the alloy and not decided by microstructure of the matrix, since the aging process does not alter the bulk component [23]. By the way, the corresponding Ecorr is more or less unchanged and reproducible within a window of about 100 mV. However, in the grain boundary region, a significant composition difference between coarse precipitates and free precipitation zone (PFZ) results in local galvanic cells corrosion. In 3.5% NaCl solution, the corrosion potential of η-MgZn2 phase is −1.05 VSEC. In contrast, PFZs are generally considered to be pure Al with a corrosion potential of −0.68 VSEC [24]. Galvanic relationships among discrete microstructural elements of 7050 Al alloys can be deduced from a table of corrosion potentials of MgZn2 phases under various corrosion environments. Therefore, intergranular corrosion of 7050 Al alloys is closely affected by grain boundary precipitates (GBPs), and its susceptibility to intergranular corrosion improves with the composition of GBPs. Wang et al. [25] studied the effect of solution treatment temperature on the intergranular corrosion of peak-aged Al-Zn-Mg-Cu alloys, through the microstructure analysis and simulation of solute segregation and precipitation at grain boundaries, finding that peak-aged alloy grain boundaries η-Mg (Zn, Cu)2 precipitation phase increased Cu content in the range of 465–485 °C, resulting in decreased IGC susceptibility of peak-aged alloys. The authors believe that it is related to the electrochemical driving force of the grain boundary microcouples between the η-Mg (Zn, Cu)2 phase and PFZs. However, dating the influence of precipitation in the grain boundary region on intergranular corrosion during the exfoliation process has not been deeply investigated. As we know, various studies on aluminum alloys indicated that solute elements are deposited and aggregated in the grain boundary region after aging [21,23]. Grain boundary chemistry may be playing an important part in corrosion nucleation. The intergranular corrosion process is controlled by the potential discrepancy between anodic sites (precipitates at grain boundary) and cathodic sites (solute element depleted aera) in the grain boundary region. In addition, Charitidou [26] proposed that metal hydride, like Mg-hydride, forms at the grain boundary and is responsible for intergranular corrosion in aluminum alloys. In an effort to reveal the connection mechanism between Mg–H interaction and alloy embrittlement. Huang et al. [27] indicated that intergranular H-embrittlement crack growth rates increase with the concentration of solid solution Mg on the grain boundary.
In this study, we systematically investigated the effect of grain boundary precipitates on the intergranular corrosion behavior of 7050 aluminum alloys. Currently, most of the related articles, which just speculate on the potential discrepancy caused by different precipitates morphology, hardly supply the quantitative data. In this paper, it is important to investigate the influence mechanism on intergranular corrosion of 7050 Al alloys from precipitation through quantitatively analyzing alloy element distribution and electric-potential discrepancy in the grain boundary region, which helps us to deeply understand the correlation between the grain boundary precipitation and intergranular corrosion behavior. Based on these experiments, the optimum heat treatment process for 7050 Al alloys with excellent intergranular corrosion resistance could be obtained.
2. Material and Experimental Procedure
To investigate the role of different grain boundary precipitates on intergranular corrosion during the exfoliation process, the samples cut from a commercial 7050 Al alloys (composition: Zn 5.82 wt.%, Mg 2.22 wt.%, Cu 2.14 wt.%, Zr 0.09 wt.%, Fe 0.09 wt.%, Si 0.03 wt.%) are heat-treated for three kinds of tempers (under-aging, peak-aging, and over-aging). The details of the heat-treatment process for 7050 Al alloy samples and their corresponding hardness in this work are listed in Table 1. Subsequently, these aged samples immersed in exfoliation corrosion (EXCO) solution (4.0 M NaCl + 0.5 M KNO3 + 0.1 M HNO3 aqueous solution) at 25 ± 2 °C and pH = 0.4 according to ASTM G34 [28], since the EXCO test is easy to form classical intergranular corrosion. The total testing time was 48 h.

Table 1.
Heat-treatment methods used for 7050 Al alloy samples and their corresponding hardness. (UA, PA, and OA correspond to under-aging, peak-aging, and over-aging, respectively).
After immersion for different hours, the samples were cut and prepared for metallographic examination by an optical microscope (Zeiss OM, Carl Zeiss, Oberkochen, Germany) and scanning electron microscope (Zeiss Supra 55 SEM, Carl Zeiss, Oberkochen, Germany). Furthermore, the corrosion 3D-morphology for each studied sample was characterized by optical profilometry (Veeco Contour OP, Bruker, MA, USA). The corrosion depth is measured by optical profilometry and an optical microscope.
To precisely research the influence of grain boundary precipitates on intergranular corrosion, the morphology and composition of grain boundary precipitates were finely characterized by transmission electron microscope (Tecnai G2 F30 TEM, FEI Company, Hillsboro, USA) together with energy dispersive spectroscopy (Bruker, Madison, WI, USA), operated at 300 kV. TEM samples were prepared by twinjet polishing in the electrolyte mixture (methanol 70% + nitric acid 30%) at −20 °C and 15 V. For EDS analysis of the composition, each piece of data was the arithmetic mean of three measurements. Moreover, a commercial atomic force microscope (Nanoscope IIIa, Bruker, Madison, WI, USA) is used to acquire information about the shape, position, and local potential discrepancy of precipitates with the sub-micrometer resolution, working in tapping mode. The potential is determined during the second scan when an AC voltage is applied to the tip for creating an oscillating dipole at the tip. The oscillation of the cantilever caused by an external electric field is detected by the normal AFM detection scheme. A map of the relative potential of the surface concerning that of the CoCr tip is determined. In this research, all potential mapping was performed in air at room temperature.
3. Results and Discussion
3.1. Corrosion Morphology
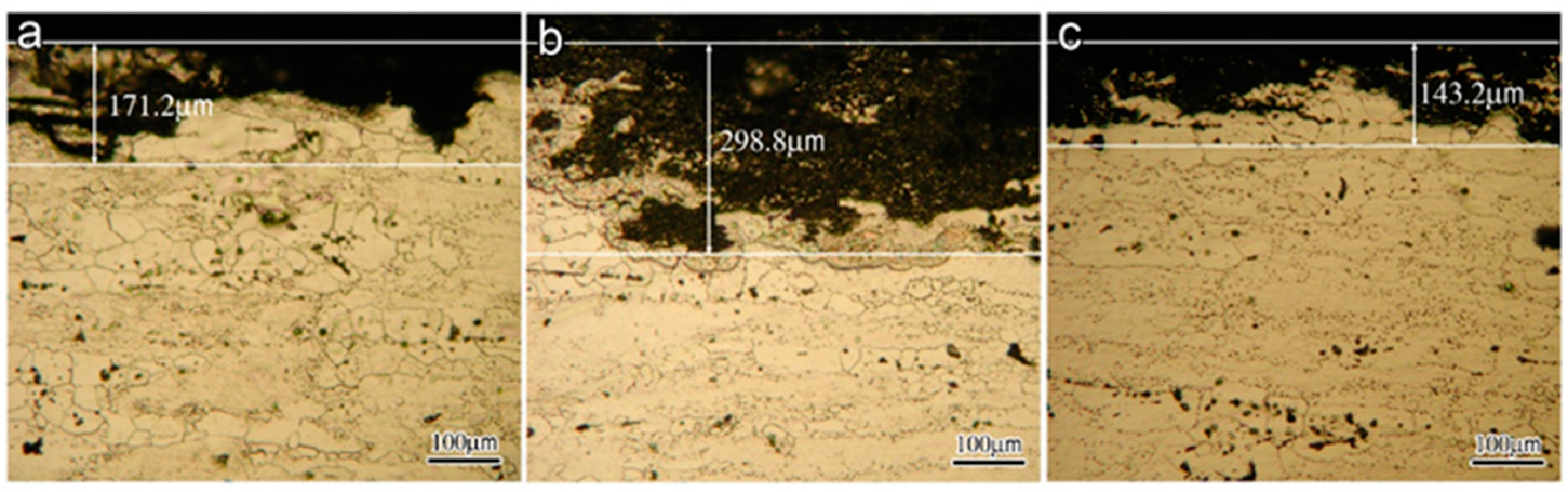
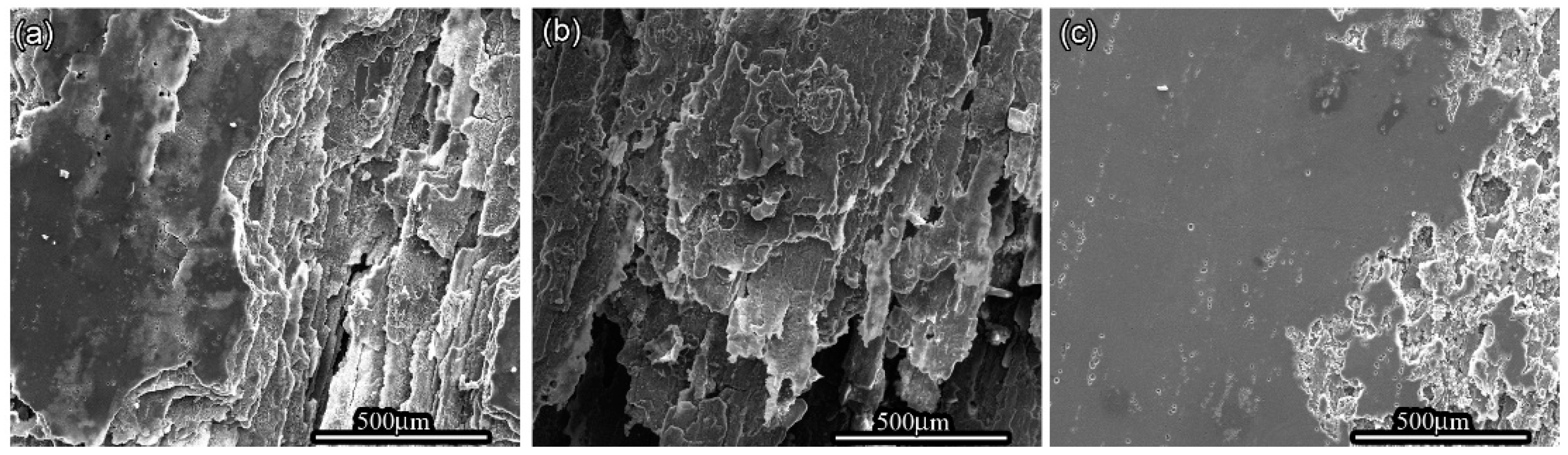
OM and SEM images exhibit the cross-section and surface corrosion morphology of every sample, after immersing in EXCO solution for 48 h, as shown in Figure 1 and Figure 2. Exfoliation in every sample exhibits obvious intergranular corrosion. Several deep local cavities appear in these samples, and many corroded areas are apparent with extensive intergranular penetration and fall-out. Figure 1 clearly shows that the penetrated thickness of the peak aged sample is deepest among the three samples, and the attack path is similar along the grain boundary. There are obvious lamellar peeling products in the three samples, and in every sample, the penetrated area is not uniform, as shown in Figure 2.
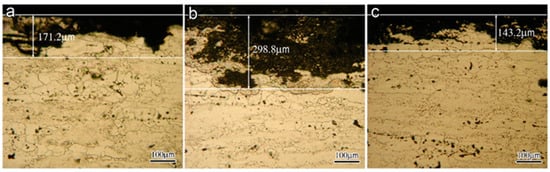
Figure 1.
OM Morphology of the different aged samples in EXCO solution after 48 h (a) under-aged sample; (b) peak-aged sample; (c) over-aged sample.
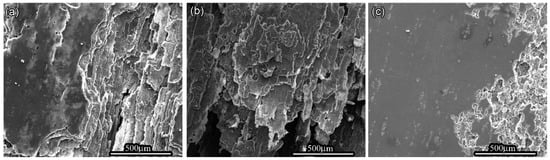
Figure 2.
Morphology of the different aged 7050 samples in EXCO solution after 48 h (a) under-aged sample; (b) peak-aged sample; (c) over-aged sample.
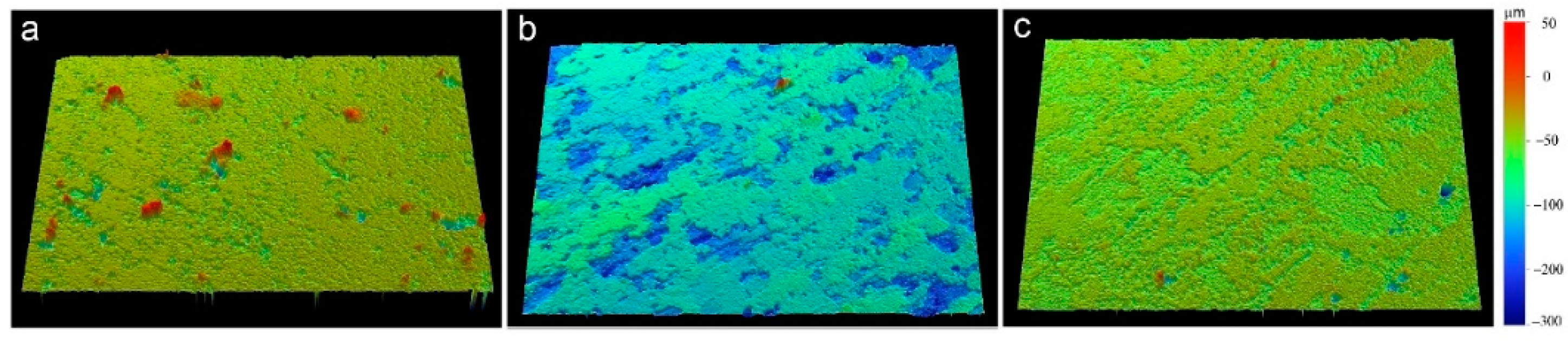
After immersing the EXCO test, the 3D-morphology of different aged samples is shown in Figure 3, and the color stands for height. The surface of the peak-aged sample is much rougher than the other samples. The results suggest that the peak-aged sample was heavily corroded after immersion. Five areas were randomly selected for each sample and analyzed by optical profilometry to generate statistical data of the corrosion depth and area. The corrosion depth falls in a range of 50–260 μm. On average, corrosion depth in the peak-aged sample is deeper and wider than that in the other samples.

Figure 3.
3D-Morphology of the different aged samples in EXCO solution after 48 h (a) under-aged sample; (b) peak-aged sample; (c) over-aged sample.
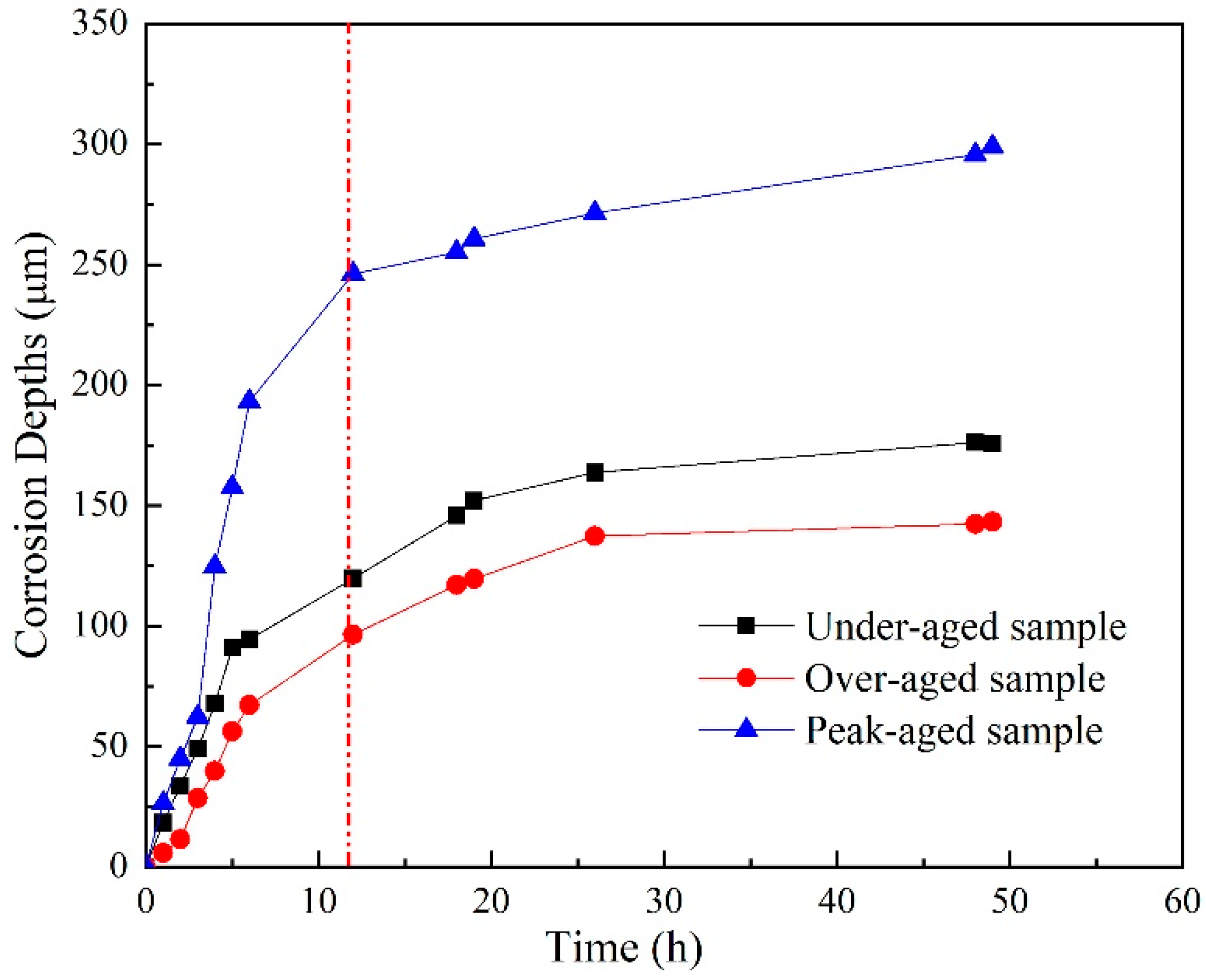
Based on the above OM and OP results of sample immersing in EXCO solution for different hours, corrosion depth curves of the different aged samples were acquired, as shown in Figure 4. In the initial stage of corrosion, the corrosion depth increases fast, and the penetration rate of the peak-aged sample exhibits the fastest in the three samples. However, the penetration rate slows down after immersion for 12 h. In summary, the three aged samples exhibit typical intergranular corrosion during the immersion process, and the exfoliation level is peak-aged sample > under-aged sample > over-aged sample.
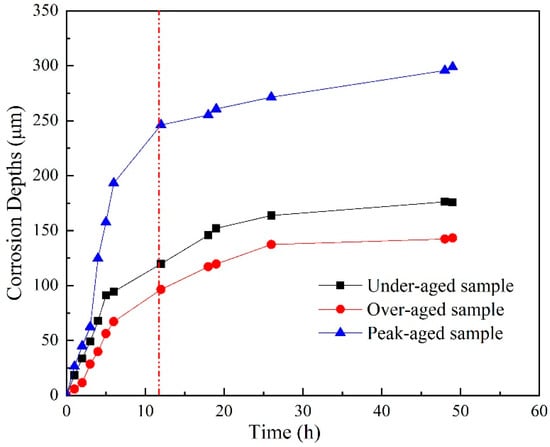
Figure 4.
Corrosion depth curves of the different aged samples immersion in EXCO solution after different hours.
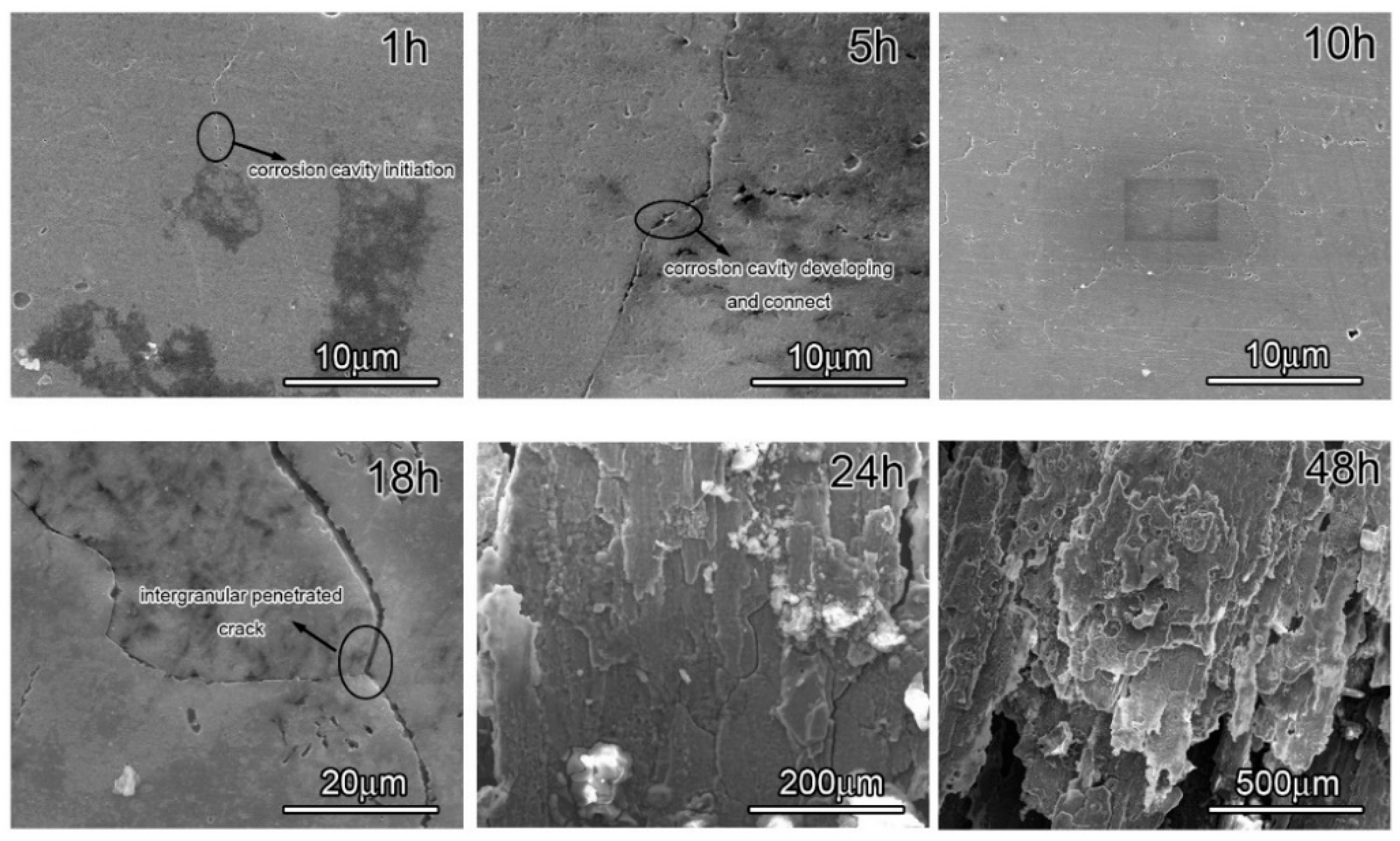
The attack detail of 7050 samples was characterized through observing peak aged sample immersion in EXCO solution for different hours, since the development of intergranular corrosion in the three samples is similar, and the morphology evolution is displayed in Figure 5. The images clearly show that some tiny corrosion cavities are about 100–300 nm and discontinuously distributed along the grain boundary in the sample, at the beginning. Subsequently, the corrosion cavities gradually grow up and increase, and some of them connect into a continuous line with increasing immersion time so that these continuous corrosion cavities become one intergranular penetrated crack. The corrosion crack blunting takes place due to micro-cavities formation and coalescence. Finally, the extensive intergranular penetration and grain fall-out were presented in the samples after an immersion test. Based on the above corrosion morphology evolution, Figure 5 suggests that the initiation and development of exfoliation prefer to arise along the grain boundary, which is closely related to grain boundary precipitation. In summary, in 7050 Al alloys, corrosion cavities are firstly present, and then intergranular penetration develops during the exfoliation process. Additionally, this phenomenon is also proved by other papers [29,30,31].
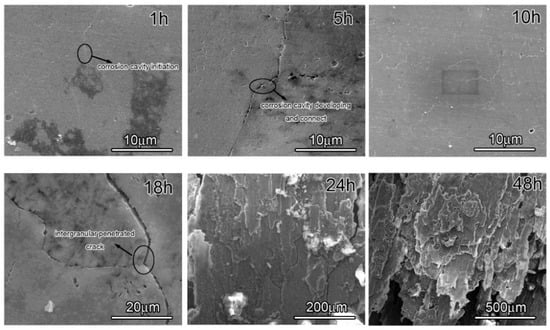
Figure 5.
Morphology of the peak-aged sample in EXCO solution for different amounts of time.
3.2. Grain Boundary Precipitates Feature
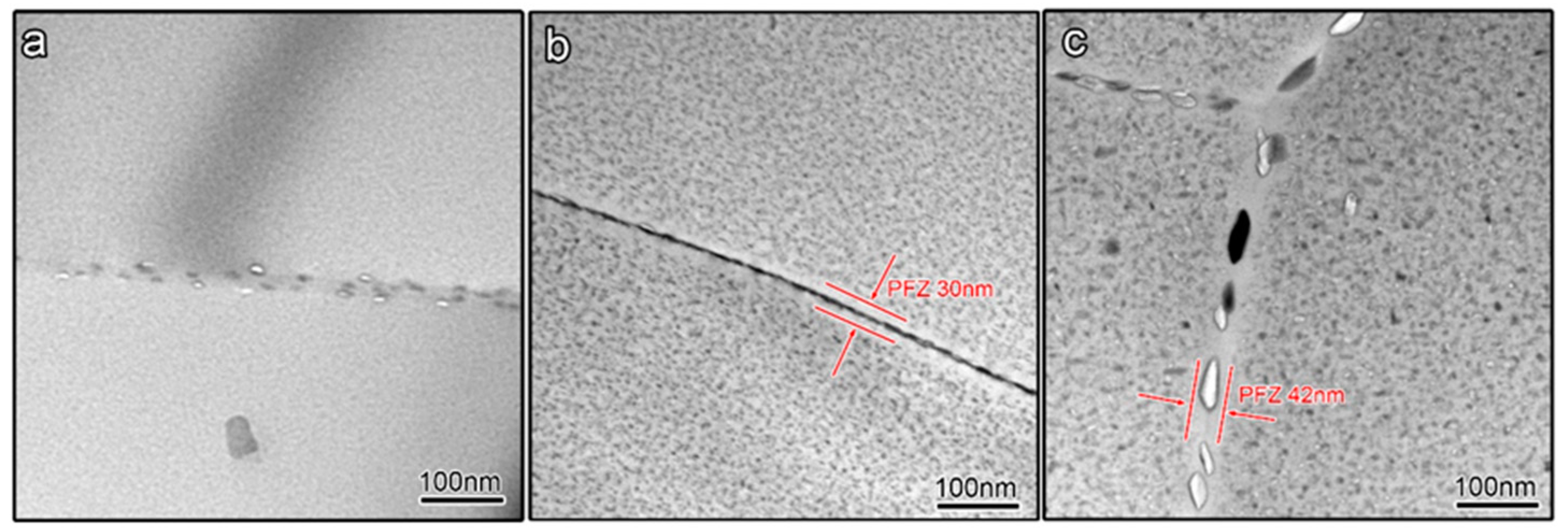
In 7xxx series Al alloys, the corrosion cavities prefer to conceive along the grain boundary, which is mainly controlled by the precipitates feature in the grain boundary region [32]. The precipitates feature in the grain boundary region of three kinds of aged samples exhibits differences, as shown in Figure 6. The GBPs of the under-aged sample and peak-aged sample are tiny and densely distributed to form a continuous line. However, the GBPs of the over-aged sample are large and discontinuously distributed. In addition, the PFZ is just presented in the peak-aged sample and over-aged sample, and the PFZ in the over-aged sample is a little wider than that in the peak-aged sample. The accurate size of GBPs and PFZ is listed in Table 2. Interestingly, the above results suggest that, in the peak-aged sample, the size of initial corrosion cavities is close to the size of GBPs, through comparative analysis of Figure 5 and Figure 6.
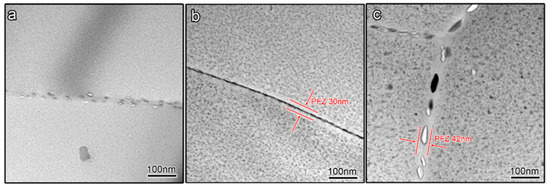
Figure 6.
Morphology of precipitates in grain boundary region of different aged samples (a) under-aged sample; (b) peak-aged sample; (c) over-aged sample.

Table 2.
GBP size, space between GBPs and PFZ width of the 7050 samples with different tempers.
More importantly, the precipitates grow up through alloy element diffusion during the aging process, so different size GBPs result in the heterogeneity of chemical composition in the grain boundary region. It is well known that the heterogeneity of chemistry components induces the initiation of corrosion cavities in the grain boundary region. Birbilis [15] reported that lower Cu or higher Mg, Zn contents result in an apparent potential discrepancy between MgZn(2−x)Cux precipitates and the Al matrix composing some micro galvanic cells [33], thereby the electrochemical corrosion rate is obviously along the grain boundary. To prove the above view, the alloy elements change in the grain boundary region was characterized by transmission electron microscopy with a Nono-EDS model as shown in Figure 7, and the potential discrepancy cross-grain boundary region was tested by AFM as shown in Figure 8.
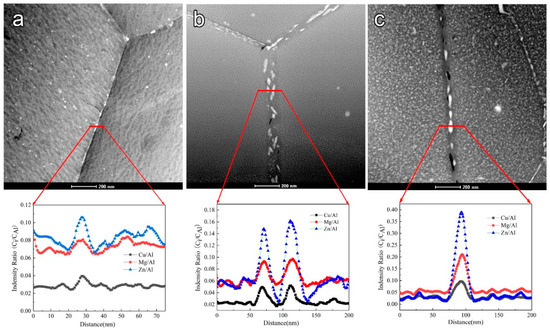
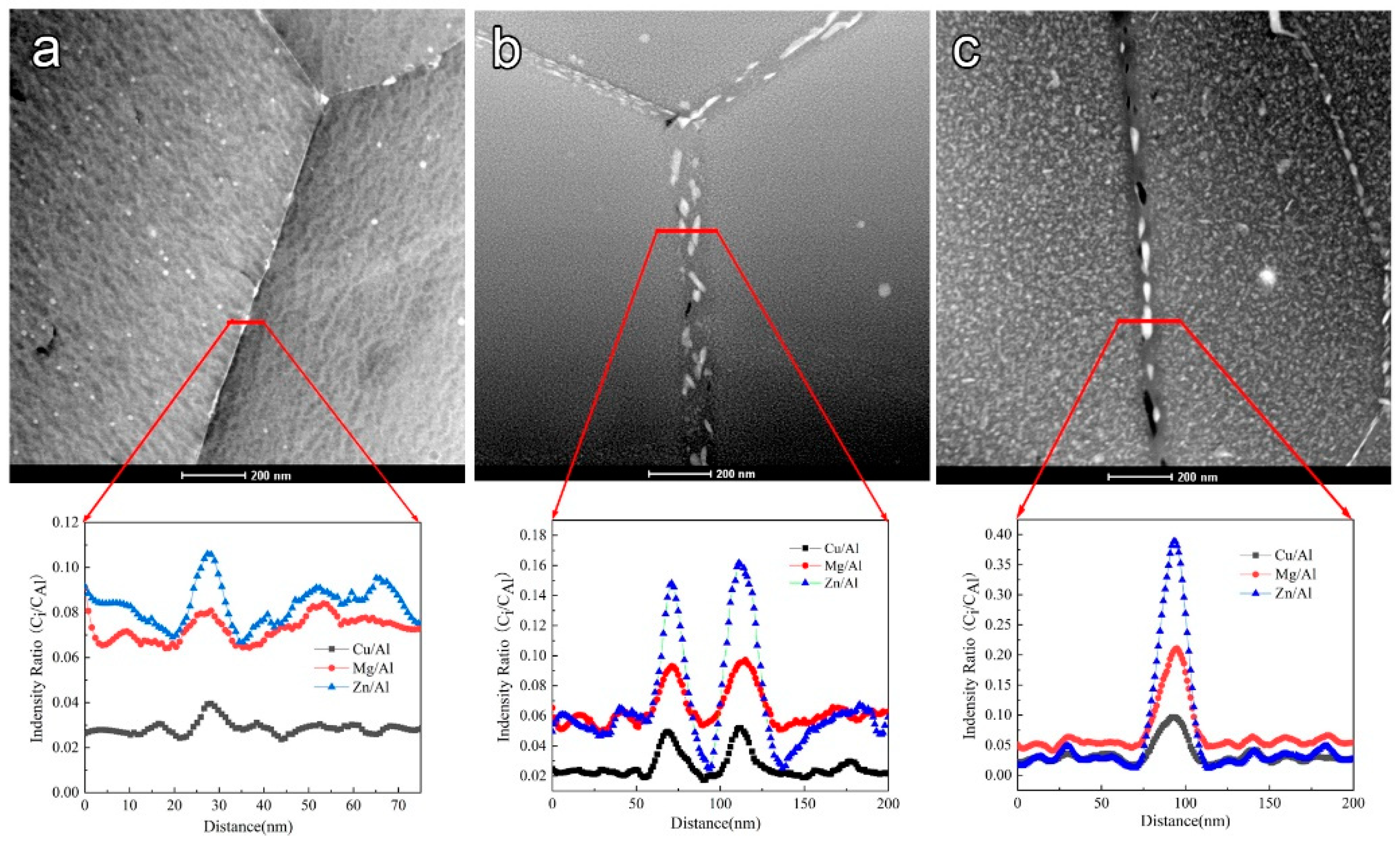
Figure 7.
HAADF images and EDS line profiles of different aged samples (a) under-aged sample; (b) peak-aged sample; (c) over-aged sample.
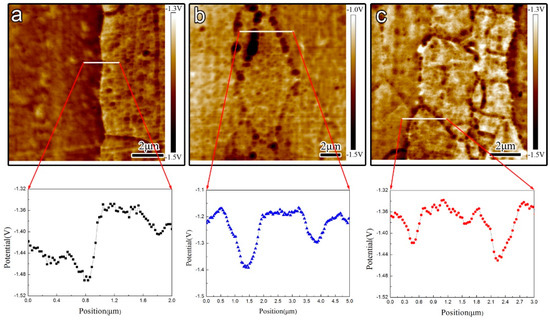
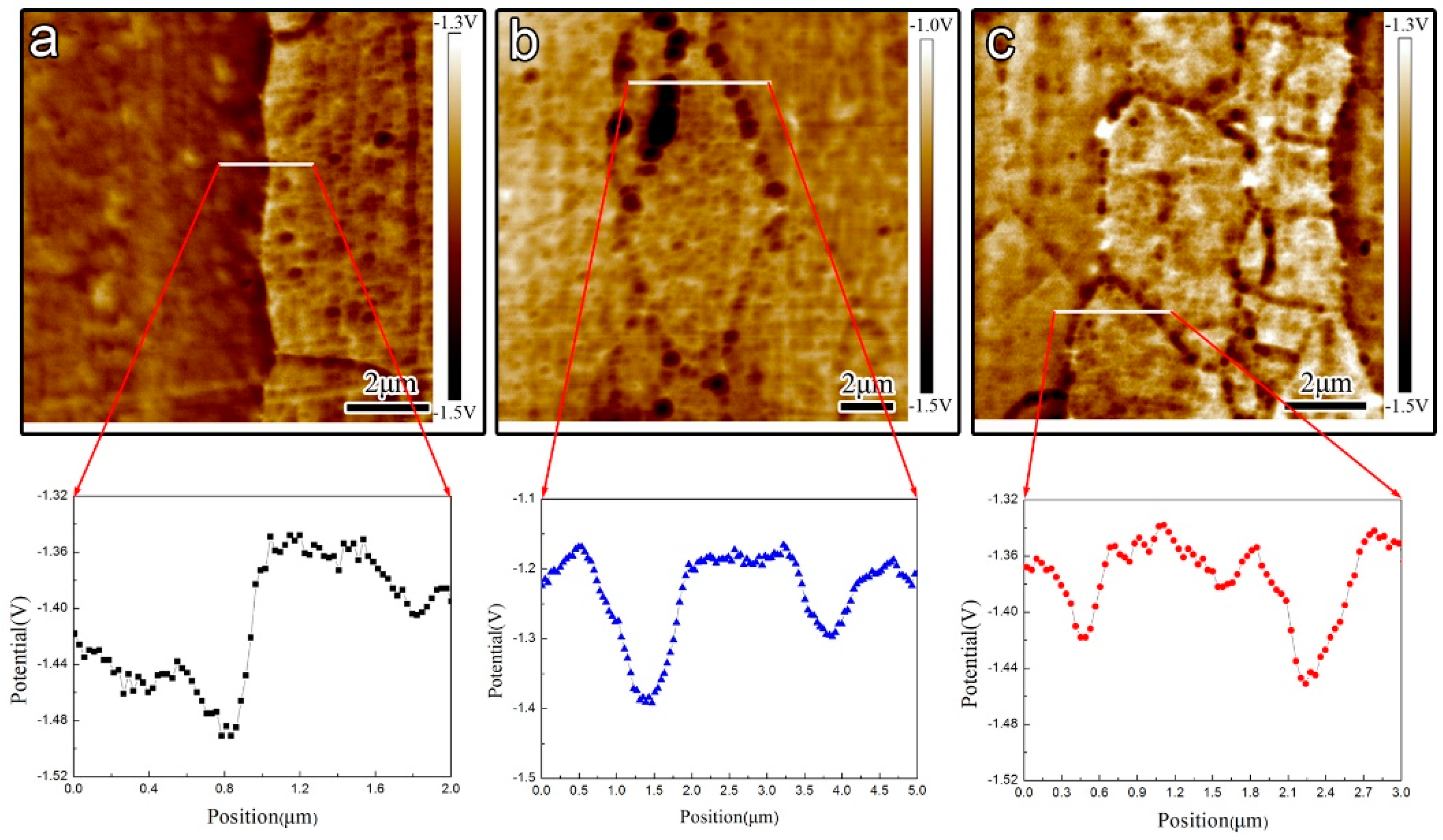
Figure 8.
AFM volta potential map and dual section analysis along the white line in polished samples respectively (a) under-aged sample; (b) peak-aged sample; (c) over-aged sample.
Figure 7 clearly shows the size of the precipitates in the grain boundary, based on the corresponding elements in HAADF mode, so that the composition of precipitates is different from the matrix. The intensity radio (Ci/CAl) curves reflect the composition fluctuation in the grain boundary region. Figure 7 also clearly indicates the distribution of the different elements in the grain boundary region of the three different aged samples. The composition profiles across the red line clearly show that the Zn, Mg, and Cu elements are enriched at GBP, and alloy elements lack PFZ. It is well known that Zn and Mg are active elements, but Cu is a noble element. For comparison purposes, Buchheit [34] reported that the Ecorr of MgZn2 precipitates is −1003 mVSCE, and the Ecorr of Al2Cu precipitates is −665 mVSCE. Frankel [35] also reported the Ecorr of precipitates to improve with Cu increase in Al alloys. The composition and potential of GBPs in samples with different tempers are listed in Table 3. In general, the intensity radio (Ci/CAl) of Cu, Mg, Zn elements is Cu < Mg < Zn in the three kinds of aged samples, which is corresponding to its contents. The alloy elements’ contents of GBPs increase with elevating precipitation levels. Especially, in the over-aged sample, the intensity radio (Ci/CAl) of Cu elements is much higher than the other samples.

Table 3.
Indensity radio (Ci/CAl) and average potential discrepancy between GBP and matrix in samples with different tempers.
3.3. Influence Mechanism on Intergranular Corrosion of GBPs
The mechanism of intergranular corrosion is usually electrochemical corrosion, and the main reason is that the potential difference between grain and grain boundary constitutes a micro-battery, which leads to corrosion along the grain boundary. In a corrosive environment, the enrichment of solute atoms and the precipitation of secondary phases easily occur at the grain boundaries. The precipitation phase generally acts as the cathode, while the grain boundary has a certain chemical inhomogeneity and becomes the anode. As the corrosion process progresses, the oxide film on the surface of the sample is destroyed, and the exposed aluminum matrix is continuously corroded, forming a large number of corrosion pits and corrosion products. Among them, the precipitation phase and its surroundings are preferentially corroded, and annular corrosion pits are formed around the precipitation phase. As the corrosion degree increases, the corrosion tip is more likely to spread along the grain boundary, thereby forming intergranular corrosion. Meanwhile, IGC is also affected by a series of microstructures, such as the size and spacing of the precipitated phases, the width of the PFZ, and the solute concentration gradient [35,36,37,38,39].
The results of AFM prove the potential discrepancy between GBP and matrix, which is consistent with the research of Buchheit [34] who reported that the open circuit potential (OCP) of bulk MgZn2 was about 100 mV more active than the Al matrix, as shown in Figure 8. Thus, GBPs as anodic is easy to attack and form initial corrosion cavity. However, in these three different aged samples, their potential discrepancy between GBPs and matrix is not significantly different. Furthermore, the potential discrepancy between GBPs and matrix in the over-aged sample is just a little lower than that in the peak-aged sample, since the Cu content of GBPs increases. The different microstructural elements of precipitates in 7050 Al alloys can form different galvanic corrosion attacks so that the corrosion cavity along the grain boundary is easy to germinate due to potential discrepancy between GBPs and matrix.
The composition and size of GBPs control the primary intergranular corrosion cavity during the exfoliation process. Studies have shown that a large amount of Cu and a small amount of Zn and Mg are enriched in GBP, which will reduce the peel corrosion sensitivity [33,35,40,41]. The potential of GBPs decreases with Zn and Mg content increasing. However, as a noble element, Cu will increase the potential of GBPs, reducing the potential difference between the matrix and GBPs and making it difficult for corrosion cracks to sprout. High Cu content can inhibit the anodic reaction rate at the crack tip and the cathode formation of hydrogen. Meng reported that, in the free corrosion environment of aerated chloride solutions, the enrichment Cu on the surface of Al alloys results in corrosion potential, and the polarization resistance increases, which obstructs the oxygen reduction reaction [42]. In addition, the composition of GBP significantly affects intergranular H-embrittlement during an intergranular corrosion process in 7xxx series Al alloys, such as Mg element being prone to capturing H in the grain boundary region, but Cu degrading intergranular H-embrittlement [43]. According to several papers [16,17,20], increasing the Mg content of GBPs effectively induces the hydrogen generation at the crack-tips inter-granular stress corrosion process. Due to the high Cu content of GBPs, the over-aged samples are less prone to hydrogen embrittlement. It is easy for the peak-aged specimen to generate hydrogen brittleness because of the low Cu content of GBPs.
The distribution of GBPs decided by the aging process is another key point for intergranular corrosion behavior. During the intergranular corrosion process, GBPs vary in size and grain boundary of dispersion, which obviously affect the initial corrosion cavities spread and connection. Zhang et al. [44]. imposed multi-stage aging heat treatment on the Al-Zn-Mg-Cu alloy, finding that the η phase on the grain boundary was coarsened and discontinued and further distributed, which improves the EXCO resistance of the alloy. In the peak-aged samples and under-aged samples, GBPs exhibit continuous distribution since these precipitates are tiny and dense. Therefore, in the under-aged and peak-aged samples, the linear penetration along grain boundary is easy to form, since initial corrosion cavities induced by the tiny and dense GBPs can easily contact each other. However, in the over-aged sample, these coarse and sparse GBPs, which separate from mutual, lead to discontinuous large interspace at the grain boundary, so that the anodic corrode along the grain boundary is discontinued by these spaces between the GBPs. In addition, the coarse GBPs can avoid atomic H diffusion and retard intergranular corrosion H-brittleness [29]. When coarse GBPs capture more atomic H, hydrogen gas is generated and emanated from the crystal boundary area, so that the low surplus atomic H content in the crystal boundary is hard to induce H-brittleness [45,46]. Corrosion morphology of three kinds of aged samples offers clear evidence, as displayed in Figure 1 and Figure 2, and there are fewer intergranular cracks in the over-aged sample than that in the other samples. Based on the above results and discussion, the peak-aged sample is easiest to induce intergranular corrosion crack growth during the exfoliation process in the three kinds of aged samples due to tiny and dense GBPs continuously distributed at the grain boundary.
In addition, the free precipitation zone (PFZ) in the grain boundary region is also closely related to intergranular corrosion. PFZ, whose Ecorr is −570 mVSCE, is more positive than the Al matrix due to the lack of alloy elements in PFZ [47]. The greater potential discrepancy between GBPs and PFZ may be easier for inducing initial corrosion cavities than that between GBPs and Al matrix, so that, at the beginning of intergranular corrosion, PFZ may accelerate corrosion germination. However, PFZ acts as one isolation area between grain boundary and matrix, hindering initial corrosion cavities along the grain boundary to connect the matrix and grow up. In addition, the mass corrosion products stack along grain boundaries, which leads to the wedging stress to lift the exterior grains endlessly during the intergranular corrosion process, but the soft PFZ can effectively release the wedge stress from the corrosion products. This view is supported by a lot of relevant literature [48,49] and is very compatible with our exfoliation results of the different aged samples. Li [49] also demonstrated that increasing width PFZs can enhance the exfoliation resistance in the Al–Zn–Mg alloy. Li et al. [50] found that the adjacent GB precipitated phase clusters can act as the cathode phase and accelerate the dissolution of PFZ, which is the anode phase. The formation of PFZ [35,51], accompanied by coarsening and discontinuous GBPs, affects the EXCO resistance of the studied alloys. The PFZ region is a solute depletion region that inhibits anodic dissolution. With the prolongation of the aging temperature and aging time, the width of the PFZ region will be further widened, which improves the exfoliation corrosion resistance of the alloy as shown in Figure 1 and Figure 6. Therefore, the OA sample has the most excellent corrosion resistance.
Through the above corrosion tests as well as the observation and analysis of the microstructure, it can be found that the main factors affecting the corrosion resistance of the alloy are as follows. On the one hand, the corrosion resistance of the alloy is affected by the size and distribution of GBPs and the width of PFZ. The corrosion resistance of 7050 aluminum alloy increases with the increase of the size and spacing of GBP as well as the widening of PFZ. This is because the coarsening of GBPs can reduce the potential difference between the matrix and the grains near the grain boundary, slow down the tendency of anodic dissolution, and reduce the corrosion sensitivity of the alloy. On the other hand, the elemental composition of GBPs leads to the potential difference between GBPs and their surrounding or internal grains, which affects the corrosion resistance of the alloy. The increase of Cu content in GBPs in the alloy can reduce the potential difference between the grain boundary and the aluminum matrix, and inhibit the initiation and propagation of corrosion cracks. Thus, the corrosion resistance of the alloy is improved. According to the grain boundary characteristics of 7050 alloy under different aging conditions, as shown in Figure 6, the corrosion resistance of the over-aged alloy is better than that of under-aged alloy and peak-aged alloy.
4. Conclusions
The exfoliation rank of different aged 7050 Al alloys is peak-aging > under-aging > over-aging, through immersing in the EXCO solution, since the precipitation feature in the grain boundary region exerts a significant influence on intergranular corrosion behavior during the exfoliation process. In 7050 Al alloys, the potential discrepancy between GBP and matrix, which depends on the alloy content of GBPs, leads to galvanic cells corrosion along the grain boundary. Increasing Mg and Zn content can increase the susceptibility of anodic dissolution along the grain boundary, but increasing Cu content is beneficial to inhibit the anodic dissolution of GBPs. With precipitates growing up, increasing the separation distance of GBPs will prohibit the quick propagation and connection of corrosion cavities along the grain boundary during the exfoliation process. The coarse and discontinuously distributed precipitates can delay intergranular corrosion development in 7050 Al alloys. Increasing PFZ width is beneficial for enhancing the intergranular corrosion resistance of 7050 Al alloys, since soft PFZ can not only obstruct initial corrosion cavities along the grain boundary to connect the matrix and grow, but also effectively release the wedge stress from the corrosion products.
Author Contributions
Conceptualization, J.C.; Methodology, Y.Q. and R.L.; Data Curation, H.C.; Writing—Original draft preparation. C.W. and B.W.; Visualization, Investigation. J.C.; Supervision, Writing—Reviewing and Editing, J.C. and L.Z. All authors have read and agreed to the published version of the manuscript..
Funding
This work is financially supported by the National Natural Science Foundation of China (Grant Nos. 51871057, 51901044, and 51501040), and the Fujian Natural Science Foundation (2019J01227 and 2020J01352).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Xiao, Q.F.; Huan, J.W.; Jiang, Y.G.; Jiang, F.Q.; Wu, Y.F.; Xu, G.F. Effects of minor Sc and Zr additions on mechanical properties and microstructure evolution of Al-Zn-Mg-Cu alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 1429–1438. [Google Scholar] [CrossRef]
- Chen, J.F.; Zou, L.C.; Yu, Y.; Li, Q.; Chen, Y.L. Microstructure evolution of 7050 Al alloy during age-forming. Mater. Charact. 2015, 102, 114–121. [Google Scholar] [CrossRef]
- Özer, G.; Karaaslan, A. Relationship of RRA heat treatment with exfoliation corrosion, electrical conductivity and microstructure of AA7075 alloy. Mater. Corros. 2017, 68, 1260–1267. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of recrystallization on intergranular fracture and corrosion of Al-Zn-Mg-Cu-Zr alloy. J. Alloys Compd. 2015, 622, 166–173. [Google Scholar] [CrossRef]
- Chubb, J.P.; Morad, T.A.; Hockenhull, B.S. The effect of exfoliation corrosion on the fracture and fatigue behaviour of 7178-T6 aluminium. Int. J. Fatigue 1995, 17, 49–54. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhang, J.L.; Liu, G.; Liang, Y.J. Effects of pre-treatments on aging precipitates and corrosion resistance of a creep-aged Al-Zn-Mg-Cu alloy. Mater. Des. 2015, 83, 866–875. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, K.; Xu, D.K.; Cai, X.; Qiao, Y.X.; Sheng, L.Y. Anisotropic corrosion behavior of hot-rolled Mg-8 wt.%Li alloy. J. Mater. Sci. Technol. 2020, 53, 102–111. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Sun, J.; Han, E.H. Effect of grain structure on the stress corrosion cracking (SCC) behavior of an as-extruded Mg-Zn-Zr alloy. Corros. Sci. 2019, 157, 347–356. [Google Scholar] [CrossRef]
- Marlaud, T.; Malki, B.; Henon, C.; Deschamps, A.; Baroux, B. Relationship between alloy composition, microstructure and exfoliation corrosion in Al-Zn-Mg-Cu alloys. Corros. Sci. 2011, 53, 3139–3149. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Y.; Kou, L.Y.; Zhang, P.; Zou, Z.K.; Zhu, X.J. Effect of corrosion on mechanical properties of 2024 aluminum alloy. Chin. J. Nonferrous Met. 2021, 31, 10–22. [Google Scholar] [CrossRef]
- Chen, J.F.; Zhan, X.F.; Zou, L.C.; Yu, Y.; Li, Q. Effect of precipitate state on the stress corrosion behavior of 7050 aluminum alloy. Mater. Charact. 2016, 114, 1–8. [Google Scholar] [CrossRef]
- Liang, W.J.; Pan, Q.L.; He, Y.B.; Li, Y.C.; Zhou, Y.C.; Lu, C.G. Effect of aging on the mechanical properties and corrosion susceptibility of an Al-Cu-Li-Zr alloy containing Sc. Rare Met. 2008, 27, 146–152. [Google Scholar] [CrossRef]
- Lu, X.; Han, X.; Du, Z.; Wang, G.; Lu, L.; Lei, J.; Zhou, T. Effect of microstructure on exfoliation corrosion resistance in an Al-Zn-Mg alloy. Mater. Charact. 2018, 135, 167–174. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Weyland, M.; Hutchinson, C.R. The effect of precipitate size on the yield strength-pitting corrosion correlation in Al-Cu-Mg alloys. Acta Mater. 2010, 58, 5941–5948. [Google Scholar] [CrossRef]
- Knight, S.P.; Birbilis, N.; Muddle, B.C.; Trueman, A.R.; Lynch, S.P. Correlations between intergranular stress corrosion cracking, grain-boundary microchemistry and grain-boundary electrochemistry for Al-Zn-Mg-Cu alloys. Corros. Sci. 2010, 52, 4073–4080. [Google Scholar] [CrossRef]
- Wang, S.S.; Huang, W.; Yang, L.; Jiang, J.T.; Chen, J.F.; Dai, S.L.; Seidman, D.N.; Frankel, G.S.; Zhen, L. Effect of Cu content and aging conditions on pitting corrosion damage of 7xxx series aluminum alloys. J. Electrochem. Soc. 2015, 162, 150–160. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, B.; Zhang, Y.; Zhu, B.; Liu, H.; He, X. Effect of heat treatment on the microstructure and mechanical properties of the spray-deposited Al-10.8Zn-2.8Mg-1.9Cu alloy. Mater. Sci. Eng. A. 2008, 486, 648–652. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Q.; Shi, Y.; Wang, Y.; Li, B. Influence of aging temperature on corrosion behavior of Al-Zn-Mg-Sc-Zr alloy. Mater. Des. 2014, 55, 551–559. [Google Scholar]
- Jiang, J.T.; Tang, Q.J.; Yang, L.; Zhen, L. Non-isothermal ageing of an Al-8Zn-2Mg-2Cu alloy for enhanced properties. J. Mater. Process. Technol. 2016, 227, 110–116. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.F.; Peng, X.Y.; Liu, S.C.; Deng, Y.; Liang, X.P. Effect of non-isothermal aging on microstructure and properties of Al-5.87Zn-2.07Mg-2.42Cu alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 2899–2908. [Google Scholar] [CrossRef]
- Xie, L.; Lei, Q.; Wang, M.; Sheng, X. Effects of aging mechanisms on the exfoliation corrosion behavior of a spray deposited Al-Zn-Mg-Cu-Zr aluminum alloy. J. Mater. Res. 2017, 32, 1105–1117. [Google Scholar] [CrossRef]
- Chen, J.F.; Zhen, L.; Jiang, J.T.; Yang, L.; Shao, W.Z.; Zhang, B.Y. Microstructures and mechanical properties of age-formed 7050 aluminum alloy. Mater. Sci. Eng. A 2012, 539, 115–123. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Zhang, B.; Wang, J.; Han, E.H.; Ke, W.; Okafor, P.C. SVET and SIET study of galvanic corrosion of Al/MgZn2 in aqueous solutions at different pH. J. Electrochem. Soc. 2018, 165, C180–C194. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, H.; Li, H. Effect of solution-treating temperature on the intergranular corrosion of a peak-aged Al-Zn-Mg-Cu alloy. J. Mater. Sci. Technol. 2020, 9, 6497–6511. [Google Scholar] [CrossRef]
- Charitidou, E.; PapapolAYmerou, G.; Haidemenopoulos, G.N.; Hasiotis, N.; Bontozoglou, V. Characterization of trapped hydrogen in exfoliation corroded aluminum alloy 2024. Scr. Mater. 1999, 41, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.P.; Chen, K.H.; Li, S.; Song, M. Influence of high-temperature pre-precipitation on local corrosion behaviors of Al-Zn-Mg alloy. Scr. Mater. 2007, 56, 305–308. [Google Scholar] [CrossRef]
- ASTM G34-01; Standard Test Method for Exfoliation Corrosion Susceptibility in 2xxx and 7xxx Series Aluminum Alloys (EXCO Test). ASTM International: West Conshohocken, PA, USA, 2013.
- Chen, J.F.; Frankel, G.S.; Jiang, J.T.; Shao, W.Z.; Zhen, L. Effect of age-forming on corrosion properties of an Al-Zn-Mg-Cu alloy. Mater. Corros. 2014, 65, 670–677. [Google Scholar] [CrossRef]
- Song, F.X.; Zhang, X.M.; Liu, S.D.; Tan, Q.; Li, D.F. The effect of quench rate and overageing temper on the corrosion behaviour of AA7050. Corros. Sci. 2014, 78, 276–286. [Google Scholar] [CrossRef]
- Song, F.X.; Zhang, X.M.; Liu, S.D.; Tan, Q.; Li, D.F. Exfoliation corrosion behavior of 7050-T6 aluminum alloy treated with various quench transfer time. Trans. Nonferrous Met. Soc. China 2014, 24, 2258–2265. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Investigation and discussion of characteristics for intermetallic phases common to aluminum alloys as a function of solution pH. J. Electrochem. Soc. 2008, 155, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, P.; Frankel, G.S. A Study of Corrosion and Pitting Initiation of AA2024-T3 Using Atomic Force Microscopy. J. Electrochem. Soc. 2002, 149, 239–247. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys. J. Electrochem. Soc. 2005, 152, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Frankel, G.S. Effect of Cu content on corrosion behavior of 7xxx series aluminum alloys. J. Electrochem. Soc. 2004, 151, B271–B283. [Google Scholar] [CrossRef]
- Maitra, S.; English, G. Environmental factors affecting localized corrosion of 7075-T7351 aluminum alloy plate. Metall. Trans. A 1982, 13, 161–166. [Google Scholar]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al–Zn–Mg–Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raja, V. Influence of heat treatment and scandium addition on the electrochemical polarization behavior of Al–Zn–Mg–Cu–Zr alloy. Metall. Trans. A. 2007, 38, 2843–2852. [Google Scholar] [CrossRef]
- Ramgopal, T.; Schmutz, P.; Frankel, G.S. Electrochemical behavior of thin film analogs of Mg(Zn, Cu, Al)2. J. Electrochem. Soc. 2001, 148, B348–B356. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Chen, K.H.; Peng, G.S.; Jia, L.; Dong, P.X. Effect of heat treatment on strength, exfoliation corrosion and electrochemical behavior of 7085 aluminum alloy. Mater. Des. 2012, 35, 93–98. [Google Scholar] [CrossRef]
- Li, J.F.; Zheng, Z.Q.; Jiang, N.; Tan, C.Y. Localized corrosion mechanism of 2×××−series Al alloy containing S(Al2CuMg) and θ’(Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1. Mater. Chem. Phys. 2005, 91, 325–329. [Google Scholar] [CrossRef]
- Ali, N.B.; Tanguy, D.; Estevez, R. Effects of microstructure on hydrogen-induced cracking in aluminum alloys. Scr. Mater. 2011, 65, 210–213. [Google Scholar] [CrossRef]
- Song, R.G.; Dietzel, W.; Zhang, B.J.; Liu, W.J.; Tseng, M.K.; Atrens, A. Stress corrosion cracking and hydrogen embrittlement of an Al-Zn-Mg-Cu alloy. Acta Mater. 2004, 52, 4727–4743. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Ye, L. Influence of aging treatments on the strength and localized corrosion resistance of aged Al–Zn–Mg–Cu alloy. J. Alloys Compd. 2020, 846, 156223. [Google Scholar] [CrossRef]
- Christodoulou, L.; Flower, H.M. Hydrogen embrittlement and trapping in Al-Zn-Mg alloys. Acta Metall. 1980, 28, 481–487. [Google Scholar] [CrossRef]
- Shi, Y.J.; Pan, Q.L.; Li, M.J.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behaviour of Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Ramgopal, T.; Gouma, P.I.; Frankel, G.S. Role of grain-boundary precipitates and solute-depleted zone on the intergranular corrosion of aluminum alloy 7150. Corrosion 2002, 58, 687–697. [Google Scholar] [CrossRef]
- Zeng, F.L.; Wei, Z.L.; Li, J.F.; Li, C.X.; Tan, X.; Zhang, Z.; Zheng, Z.Q. Corrosion mechanism associated with Mg2Si and Si particles in Al-Mg-Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Li, B.; Pan, Q.L.; Chen, C.P.; Yin, Z.M. Effect of aging time on precipitation behavior, mechanical and corrosion properties of a novel Al-Zn-Mg-Sc-Zr alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 2263–2275. [Google Scholar] [CrossRef]
- Li, H.; Zhao, P.P.; Wang, Z.X. The intergranular corrosion susceptibility of a heavily overaged Al-Mg-Si-Cu alloy. Corros. Sci. 2016, 107, 113–122. [Google Scholar] [CrossRef]
- Brunner, J.G.; Birbilis, N.; Ralston, K.D. Impact of ultrafinegrained microstructure on the corrosion of aluminium alloy AA2024. Corros. Sci. 2012, 57, 209–214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).