Fluid Films as Models for Understanding the Impact of Inhaled Particles in Lung Surfactant Layers
Abstract
:1. Introduction
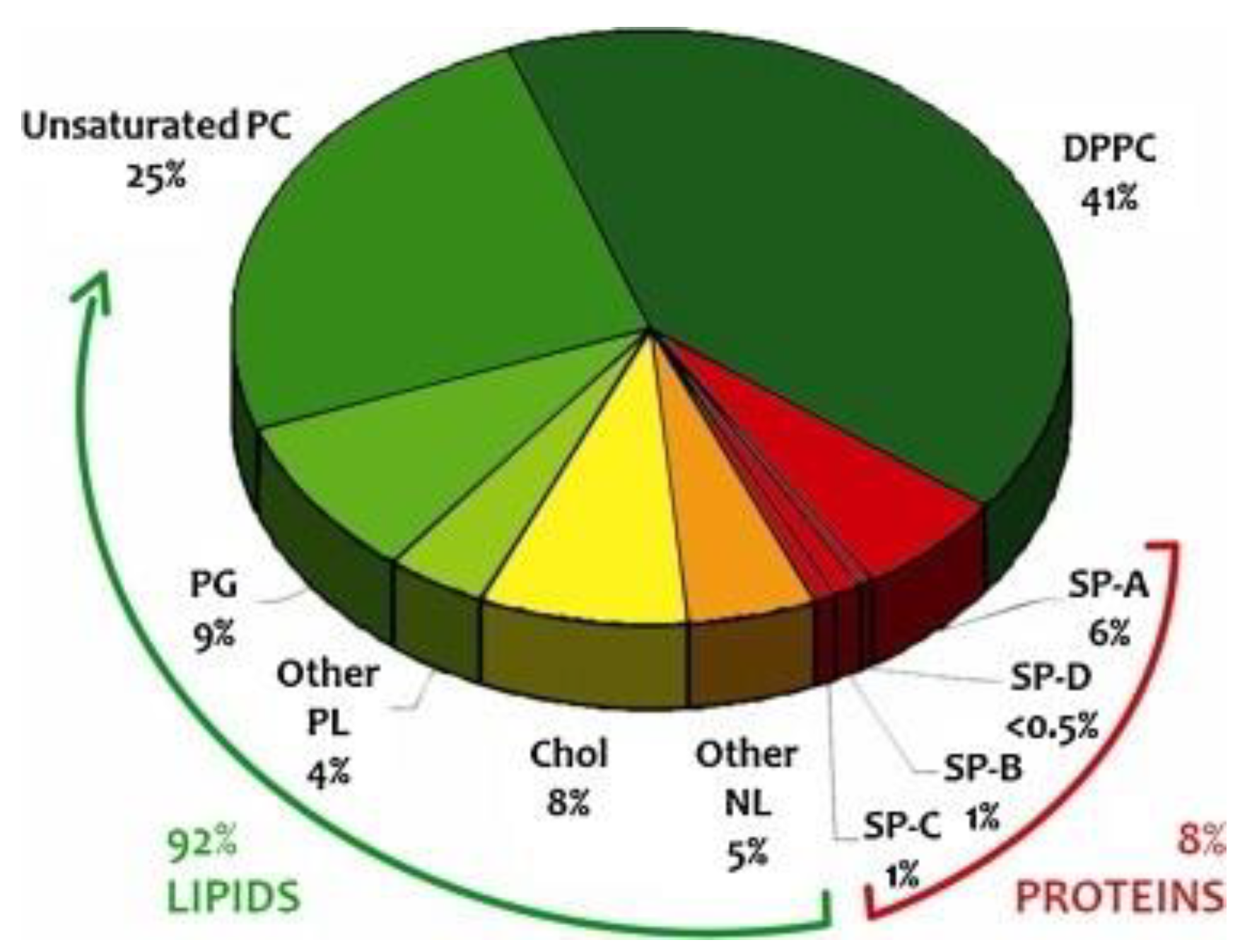
2. Lung Surfactant from Chemistry to a Functional Layer
Lung Surfactant Interfacial Activity: A Key Aspect for the Respiratory Function
3. Impact of Inhaled Particle Deposition in Lung Surfactant Film
4. Surface Science Approaches for Evaluating the Interaction of Particles with the Lung Surfactant Film: Tools and Models
4.1. Experimental Tools
4.2. Chemistry of Lung Surfactant Models
5. Evaluating the Interaction of Lung Surfactant and Particles: Methodological Approaches
6. How Do the Particle Physico-Chemical Properties Affect Their Interaction with LS Layers?
6.1. Interaction of Particles and Lung Surfactant Films: A Matter of Size
6.2. Role of Particle Surface Charge and Wettability on Their Interactions with Lung Surfactant Films
6.3. Impact of Particle Shape on the Interactions with LS Layers
6.4. Does the Particle Chemistry Matter in Their Interactions with Lung Surfactant Films?
7. Some Experimental Results of the Interaction of Particles with Interfacial Lung Surfactant Models
8. Beyond Experiments: Exploiting Computational Tools for Elucidating the Interaction of Lung Surfactant with Inhaled Particles
9. What Can We Learn Using Model Lung Surfactant Films?
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- WHO. How Air Pollution Is Destroying Our Health. Available online: https://www.who.int/airpollution/news-and-events/how-air-pollution-is-destroying-our-health (accessed on 16 August 2021).
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air Pollution and Cardiovascular Disease. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Pavese, G.; Alados-Arboledas, L.; Cao, J.; Satheesh, S.K. Carbonaceous Particles in the Atmosphere: Experimental and Modelling Issues. Adv. Meteorol. 2014, 2014, 529850. [Google Scholar] [CrossRef]
- Aili, A.; Xu, H.; Kasim, T.; Abulikemu, A. Origin and Transport Pathway of Dust Storm and Its Contribution to Particulate Air Pollution in Northeast Edge of Taklimakan Desert, China. Atmosphere 2021, 12, 113. [Google Scholar] [CrossRef]
- Garcia-Mouton, C.; Hidalgo, A.; Cruz, A.; Pérez-Gil, J. The Lord of the Lungs: The essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 2019, 144, 230–243. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef]
- Chio, C.-P.; Liao, C.-M. Assessment of atmospheric ultrafine carbon particle-induced human health risk based on surface area dosimetry. Atmos. Environ. 2008, 42, 8575–8584. [Google Scholar] [CrossRef]
- Fan, L.; Liu, S. Respirable nano-particulate generations and their pathogenesis in mining workplaces: A review. Int. J. Coal Sci. Technol. 2021, 8, 179–198. [Google Scholar] [CrossRef]
- Serra, D.S.; Araujo, R.S.; Oliveira, M.L.M.; Cavalcante, F.S.A.; Leal-Cardoso, J.H. Lung injury caused by occupational exposure to particles from the industrial combustion of cashew nut shells: A mice model. Arch. Environ. Occup. Health 2021, 76, 1–11. [Google Scholar] [CrossRef]
- Ghorani-Azam, A.; Riahi-Zanjani, B.; Balali-Mood, M. Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med. Sci. 2016, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased Particulate Air Pollution and the Triggering of Myocardial Infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-T.; Liao, C.-Y.; Kuo, C.-Y.; Kuo, H.-W. The Effects of PM2.5 from Asian Dust Storms on Emergency Room Visits for Cardiovascular and Respiratory Diseases. Int. J. Environ. Res. Public Health 2017, 14, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, M.; Guntern, J.; Lockyer, N.P.; Jones, F.H.; Hutton, B.M.; Lippmann, M.; Tetley, T.D. Urban PM2.5 surface chemistry and interactions with bronchoalveolar lavage fluid. Inhal. Toxicol. 2004, 16 (Suppl. S1), 115–129. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, N.; Srimuruganandam, B. Investigation of on-road fine particulate matter exposure concentration and its inhalation dosage levels in an urban area. Build. Environ. 2021, 198, 107914. [Google Scholar] [CrossRef]
- Borghi, F.; Spinazzè, A.; Mandaglio, S.; Fanti, G.; Campagnolo, D.; Rovelli, S.; Keller, M.; Cattaneo, A.; Cavallo, D.M. Estimation of the Inhaled Dose of Pollutants in Different Micro-Environments: A Systematic Review of the Literature. Toxics 2021, 9, 140. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Q.; Geng, Y.; Li, Y.; Huang, J.; Tian, S.; Ning, P. Interfacial interaction between benzo[a]pyrene and pulmonary surfactant: Adverse effects on lung health. Environ. Pollut. 2021, 287, 117669. [Google Scholar] [CrossRef]
- Hu, G.; Jiao, B.; Shi, X.; Valle, R.P.; Fan, Q.; Zuo, Y.Y. Physicochemical Properties of Nanoparticles Regulate Translocation across Pulmonary Surfactant Monolayer and Formation of Lipoprotein Corona. ACS Nano 2013, 7, 10525–10533. [Google Scholar] [CrossRef]
- Muthusamy, S.; Peng, C.; Ng, J.C. Effects of binary mixtures of benzo[a]pyrene, arsenic, cadmium, and lead on oxidative stress and toxicity in HepG2 cells. Chemosphere 2016, 165, 41–51. [Google Scholar] [CrossRef]
- Widziewicz, K.; Rogula-Kozłowska, W.; Loska, K.; Kociszewska, K.; Majewski, G. Health Risk Impacts of Exposure to Airborne Metals and Benzo(a)Pyrene during Episodes of High PM10 Concentrations in Poland. Biomed. Environ. Sci. 2018, 31, 23–36. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, E.; Pérez-Gil, J. Structure-function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta 2014, 1838, 1568–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnowski, T.; Podgórski, A. Assessment of the Pulmonary Toxicity of Inhaled Gases and Particles with Physicochemical Methods. Int. J. Occup. Saf. Ergon. 1999, 5, 431–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, E.; Pérez-Gil, J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem. Phys. Lipids 2015, 185, 153–175. [Google Scholar] [CrossRef] [PubMed]
- De Souza Carvalho, C.; Daum, N.; Lehr, C.M. Carrier interactions with the biological barriers of the lung: Advanced in vitro models and challenges for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2014, 75, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, Y.; Takahashi, M.; Nishitani, C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007, 9, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Griese, M. Pulmonary surfactant in health and human lung diseases: State of the art. Eur. Respir. J. 1999, 13, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R. Inhaled aerosols: Their role in COVID-19 transmission, including biophysical interactions in the lungs. Curr. Opin. Colloid Interface Sci. 2021, 54, 101451. [Google Scholar] [CrossRef]
- Rezaei, M.; Netz, R.R. Airborne virus transmission via respiratory droplets: Effects of droplet evaporation and sedimentation. Curr. Opin. Colloid Interface Sci. 2021, 55, 101471. [Google Scholar] [CrossRef]
- Veldhuizen, R.A.W.; Zuo, Y.Y.; Petersen, N.O.; Lewis, J.F.; Possmayer, F. The COVID-19 pandemic: A target for surfactant therapy? Exp. Rev. Respir. Med. 2021, 15, 597–608. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Uspal, W.E.; Wei, T. Airborne Transmission of COVID-19: Aerosol Dispersion, Lung Deposition, and Virus-Receptor Interactions. ACS Nano 2020, 14, 16502–16524. [Google Scholar] [CrossRef]
- Da Silva, E.; Vogel, U.; Hougaard, K.S.; Pérez-Gil, J.; Zuo, Y.Y.; Sørli, J.B. An adverse outcome pathway for lung surfactant function inhibition leading to decreased lung function. Curr. Res. Toxicol. 2021, 2, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, J. Dynamic dilational properties of composite surfaces. Colloids Surf. 1992, 65, 139–149. [Google Scholar] [CrossRef]
- Ling, X.; Mayer, A.; Yang, X.; Bournival, G.; Ata, S. Motion of Particles in a Monolayer Induced by Coalescing of a Bubble with a Planar Air-Water Interface. Langmuir 2021, 37, 3648–3661. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Bykov, A.G. Dilational rheology of monolayers of nano- and micropaticles at the liquid-fluid interfaces. Curr. Opin. Colloid Interface Sci. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Maestro, A. Tailoring the interfacial assembly of colloidal particles by engineering the mechanical properties of the interface. Curr. Opin. Colloid Interface Sci. 2019, 39, 232–250. [Google Scholar] [CrossRef]
- Guzmán, E.; Abelenda-Núñez, I.; Maestro, A.; Ortega, F.; Santamaria, A.; Rubio, R.G. Particle-laden fluid/fluid interfaces: Physico-chemical foundations. J. Phys. Cond. Matter 2021, 33, 333001. [Google Scholar] [CrossRef]
- Maestro, A.; Santini, E.; Guzmán, E. Physico-chemical foundations of particle-laden fluid interfaces. Eur. Phys. J. E 2018, 41, 97. [Google Scholar] [CrossRef]
- Sohail, M.; Guo, W.; Li, Z.; Xu, H.; Zhao, F.; Chen, D.; Fu, F. Nanocarrier-based Drug Delivery System for Cancer Therapeutics: A Review of the Last Decade. Curr. Med. Chem. 2021, 28, 3753–3772. [Google Scholar] [CrossRef]
- Radivojev, S.; Luschin-Ebengreuth, G.; Pinto, J.T.; Laggner, P.; Cavecchi, A.; Cesari, N.; Cella, M.; Melli, F.; Paudel, A.; Fröhlich, E. Impact of simulated lung fluid components on the solubility of inhaled drugs and predicted in vivo performance. Int. J. Pharm. 2021, 606, 120893. [Google Scholar] [CrossRef]
- Kirkpatrick, C.J.; Bonfield, W. NanoBioInterface: A multidisciplinary challenge. J. R. Soc. Interface 2010, 7, S1–S4. [Google Scholar] [CrossRef] [Green Version]
- Harishchandra, R.K.; Saleem, M.; Galla, H.-J. Nanoparticle interaction with model lung surfactant monolayers. J. R. Soc. Interface 2010, 7, S15–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhury, A.; Debnath, K.; Bu, W.; Jana, N.R.; Basu, J.K. Penetration and preferential binding of charged nanoparticles to mixed lipid monolayers: Interplay of lipid packing and charge density. Soft Matter 2021, 17, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, R.; Bianchi, V.; Macci, C.; Peruzzi, E.; Chiellini, C.; Petroni, G.; Masciandaro, G. Assessment of pollution impact on biological activity and structure of seabed bacterial communities in the Port of Livorno (Italy). Sci. Total Environ. 2012, 426, 56–64. [Google Scholar] [CrossRef]
- Wan, F.; Nylander, T.; Foged, C.; Yang, M.; Baldursdottir, S.G.; Nielsen, H.M. Qualitative and quantitative analysis of the biophysical interaction of inhaled nanoparticles with pulmonary surfactant by using quartz crystal microbalance with dissipation monitoring. J. Colloid Interface Sci. 2019, 545, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Rahman, L.; Nikota, J.; Poulsen, S.S.; Ding, Y.; Jackson, P.; Wallin, H.; Schmid, O.; Vogel, U.; Williams, A. Ranking of nanomaterial potency to induce pathway perturbations associated with lung responses. NanoImpact 2019, 14, 100158. [Google Scholar] [CrossRef]
- Kadoya, C.; Ogami, A.; Morimoto, Y.; Myojo, T.; Oyabu, T.; Nishi, K.; Yamamoto, M.; Todoroki, M.; Tanaka, I. Analysis of Bronchoalveolar Lavage Fluid Adhering to Lung Surfactant-Experiment on Intratracheal Instillation of Nickel Oxide with Different Diameters. Ind. Health 2012, 50, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Kendall, M. Fine airborne urban particles (PM2.5) sequester lung surfactant and amino acids from human lung lavage. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L1053–L1058. [Google Scholar] [CrossRef] [Green Version]
- Clifton, L.A.; Campbell, R.A.; Sebastiani, F.; Campos-Terán, J.; Gonzalez-Martinez, J.F.; Björklund, S.; Sotres, J.; Cárdenas, M. Design and use of model membranes to study biomolecular interactions using complementary surface-sensitive techniques. Adv. Colloid Interface Sci. 2020, 277, 102118. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The Evolution of the Protein Corona around Nanoparticles: A Test Study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Stefaniu, C.; Brezesinski, G.; Möhwald, H. Langmuir monolayers as models to study processes at membrane surfaces. Adv. Colloid Interface Sci. 2014, 208, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, A.; Sosnowski, T.R.; Gradoń, L. Deactivation of the Pulmonary Surfactant Dynamics by Toxic Aerosols and Gases. J. Aerosol Med. 2001, 14, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.M.; Fiegel, J. Low concentrations of negatively charged sub-micron particles alter the microstructure of DPPC at the air–water interface. Colloids Surf. A 2012, 415, 320–327. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Koliński, M.; Gradoń, L. Alteration of Surface Properties of Dipalmitoyl Phosphatidylcholine by Benzo[a]pyrene: A Model of Pulmonary Effects of Diesel Exhaust Inhalation. J. Biomed. Nanotechnol. 2012, 8, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, M.V.; Harishchandra, R.K.; Koshkina, O.; Maskos, M.; Galla, H.-J. Size Influences the Effect of Hydrophobic Nanoparticles on Lung Surfactant Model Systems. Biophys. J. 2014, 106, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnowski, T.R.; Kubski, P.; Wojciechowski, K. New experimental model of pulmonary surfactant for biophysical studies. Colloids Surf. A 2017, 519, 27–33. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E. Lung surfactant-particles at fluid interfaces for toxicity assessments. Curr. Opin. Colloid Interface Sci. 2019, 39, 24–39. [Google Scholar] [CrossRef]
- Ariga, K. Don’t Forget Langmuir-Blodgett Films 2020: Interfacial Nanoarchitectonics with Molecules, Materials, and Living Objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef]
- Bertsch, P.; Bergfreund, J.; Windhab, E.J.; Fischer, P. Physiological fluid interfaces: Functional microenvironments, drug delivery targets, and first line of defense. Acta Biomater. 2021, 130, 32–53. [Google Scholar] [CrossRef]
- Rubio, R.G.; Guzmán, E.; Ortega, F.; Liggieri, L. Monolayers of Cholesterol and Cholesteryl Stearate at the Water/Vapor Interface: A Physico-Chemical Study of Components of the Meibum Layer. Colloids Interfaces 2021, 5, 30. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Evaluation of the impact of carbonaceous particles in the mechanical performance of lipid Langmuir monolayers. Colloids Surf. A 2022, 634, 127974. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Evaluating the impact of hydrophobic silicon dioxide in the interfacial properties of lung surfactant films. Environ. Sci. Technol. 2022, 56. [Google Scholar] [CrossRef] [PubMed]
- Arick, D.Q.; Choi, Y.H.; Kim, H.C.; Won, Y.-Y. Effects of nanoparticles on the mechanical functioning of the lung. Adv. Colloid Interface Sci. 2015, 225, 218–228. [Google Scholar] [CrossRef]
- Sosnowski, T.R. Particles on the lung surface—Physicochemical and hydrodynamic effects. Curr. Opin. Colloid Interface Sci. 2018, 36, 1–9. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zeng, H. Interactions of particulate matter and pulmonary surfactant: Implications for human health. Adv. Colloid Interface Sci. 2020, 284, 102244. [Google Scholar] [CrossRef]
- Ravera, F.; Miller, R.; Zuo, Y.Y.; Noskov, B.A.; Bykov, A.G.; Kovalchuk, V.I.; Loglio, G.; Javadi, A.; Liggieri, L. Methods and models to investigate the physicochemical functionality of pulmonary surfactant. Curr. Opin. Colloid Interface Sci. 2021, 55, 101467. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, Y.E.; Zhao, X.; Loo, J.S.C.; Zuo, Y.Y. Adverse Biophysical Effects of Hydroxyapatite Nanoparticles on Natural Pulmonary Surfactant. ACS Nano 2011, 5, 6410–6416. [Google Scholar] [CrossRef] [Green Version]
- Valle, R.P.; Wu, T.; Zuo, Y.Y. Biophysical Influence of Airborne Carbon Nanomaterials on Natural Pulmonary Surfactant. ACS Nano 2015, 9, 5413–5421. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W. Lung surfactant: Function and composition in the context of development and respiratory physiology. Ann. Anat. 2016, 208, 146–150. [Google Scholar] [CrossRef]
- Castillo-Sánchez, J.C.; Cruz, A.; Pérez-Gil, J. Structural hallmarks of lung surfactant: Lipid-protein interactions, membrane structure and future challenges. Arch. Biochem. Biophys. 2021, 703, 108850. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.J.; Postle, A.D.; Orgeig, S.; Possmayer, F.; Bernhard, W.; Panda, A.K.; Jürgens, K.D.; Milsom, W.K.; Nag, K.; Daniels, C.B. Dipalmitoylphosphatidylcholine is not the major surfactant phospholipid species in all mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1426–R1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goerke, J. Pulmonary surfactant: Functions and molecular composition. Biochim. Biophys. Acta 1998, 1408, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Mottaghian, J.; Gebert, A.; Rau, G.A.; von der Hardt, H.; Poets, C.F. Commercial versus Native Surfactants. Am. J. Respir. Crit. Care Med. 2000, 162, 1524–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pynn, C.J.; Henderson, N.G.; Clark, H.; Koster, G.; Bernhard, W.; Postle, A.D. Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J. Lipid Res. 2011, 52, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Gebert, A.; Vieten, G.; Rau, G.A.; Hohlfeld, J.M.; Postle, A.D.; Freihorst, J. Pulmonary surfactant in birds: Coping with surface tension in a tubular lung. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R327–R337. [Google Scholar] [CrossRef]
- Bernhard, W.; Hoffmann, S.; Dombrowsky, H.; Rau, G.A.; Kamlage, A.; Kappler, M.; Haitsma, J.J.; Freihorst, J.; von der Hardt, H.; Poets, C.F. Phosphatidylcholine Molecular Species in Lung Surfactant. Am. J. Respir. Cell Mol. Biol. 2001, 25, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Postle, A.D.; Rau, G.A.; Freihorst, J. Pulmonary and gastric surfactants. A comparison of the effect of surface requirements on function and phospholipid composition. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 173–182. [Google Scholar] [CrossRef]
- Rau, G.A.; Vieten, G.; Haitsma, J.J.; Freihorst, J.; Poets, C.; Ure, B.M.; Bernhard, W. Surfactant in newborn compared with adolescent pigs: Adaptation to neonatal respiration. Am. J. Respir. Cell Mol. Biol. 2004, 30, 694–701. [Google Scholar] [CrossRef]
- Bernardino de la Serna, J.; Perez-Gil, J.; Simonsen, A.C.; Bagatolli, L.A. Cholesterol rules: Direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem. 2004, 279, 40715–40722. [Google Scholar] [CrossRef] [Green Version]
- Orgeig, S.; Daniels, C.B.; Johnston, S.D.; Sullivan, L.C. The pattern of surfactant cholesterol during vertebrate evolution and development: Does ontogeny recapitulate phylogeny? Reprod. Fertil. Dev. 2003, 15, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Sollfrank, L.; Paulsen, F.; Bräuer, L.; Schicht, M. Recombinant expression of surfactant protein H (SFTA3) in Escherichia coli. Ann. Anat. 2016, 208, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Haagsman, H.P.; Diemel, R.V. Surfactant-associated proteins: Functions and structural variation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 91–108. [Google Scholar] [CrossRef]
- Zasadzinski, J.A.; Ding, J.; Warriner, H.E.; Bringezu, F.; Waring, A.J. The physics and physiology of lung surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 506–513. [Google Scholar] [CrossRef]
- Liekkinen, J.; Enkavi, G.; Javanainen, M.; Olmeda, B.; Pérez-Gil, J.; Vattulainen, I. Pulmonary Surfactant Lipid Reorganization Induced by the Adsorption of the Oligomeric Surfactant Protein B Complex. J. Mol. Biol. 2020, 432, 3251–3268. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calle, M.; Prieto, M.; Olmeda, B.; Fedorov, A.; Loura, L.M.S.; Pérez-Gil, J. Pulmonary surfactant protein SP-B nanorings induce the multilamellar organization of surfactant complexes. Biochim. Biophys. Acta 2020, 1862, 183216. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.; Cutz, E.; Viero, S.; Moore, A.M.; Nogee, L. Ultrastructure of Lamellar Bodies in Congenital Surfactant Deficiency. Ultrastruct. Pathol. 2005, 29, 503–509. [Google Scholar] [CrossRef]
- Lukovic, D.; Cruz, A.; Gonzalez-Horta, A.; Almlen, A.; Curstedt, T.; Mingarro, I.; Pérez-Gil, J. Interfacial Behavior of Recombinant Forms of Human Pulmonary Surfactant Protein SP-C. Langmuir 2012, 28, 7811–7825. [Google Scholar] [CrossRef]
- Roldan, N.; Nyholm, T.K.M.; Slotte, J.P.; Pérez-Gil, J.; García-Álvarez, B. Effect of Lung Surfactant Protein SP-C and SP-C-Promoted Membrane Fragmentation on Cholesterol Dynamics. Biophys. J. 2016, 111, 1703–1713. [Google Scholar] [CrossRef] [Green Version]
- Roldan, N.; Pérez-Gil, J.; Morrow, M.R.; García-Álvarez, B. Divide & Conquer: Surfactant Protein SP-C and Cholesterol Modulate Phase Segregation in Lung Surfactant. Biophys. J. 2017, 113, 847–859. [Google Scholar] [CrossRef]
- Pérez-Gil, J. Structure of pulmonary surfactant membranes and films: The role of proteins and lipid–protein interactions. Biochim. Biophys. Acta 2008, 1778, 1676–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef] [Green Version]
- Perkins, W.R.; Dause, R.B.; Parente, R.A.; Minchey, S.R.; Neuman, K.C.; Gruner, S.M.; Taraschi, T.F.; Janoff, A.S. Role of Lipid Polymorphism in Pulmonary Surfactant. Science 1996, 273, 330–332. [Google Scholar] [CrossRef]
- Chavarha, M.; Khoojinian, H.; Schulwitz, L.E.; Biswas, S.C.; Rananavare, S.B.; Hall, S.B. Hydrophobic Surfactant Proteins Induce a Phosphatidylethanolamine to Form Cubic Phases. Biophys. J. 2010, 98, 1549–1557. [Google Scholar] [CrossRef] [Green Version]
- Chavarha, M.; Loney, R.W.; Kumar, K.; Rananavare, S.B.; Hall, S.B. Differential Effects of the Hydrophobic Surfactant Proteins on the Formation of Inverse Bicontinuous Cubic Phases. Langmuir 2012, 28, 16596–16604. [Google Scholar] [CrossRef] [Green Version]
- Chavarha, M.; Loney, R.W.; Rananavare, S.B.; Hall, S.B. An Anionic Phospholipid Enables the Hydrophobic Surfactant Proteins to Alter Spontaneous Curvature. Biophys. J. 2013, 104, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Hobi, N.; Giolai, M.; Olmeda, B.; Miklavc, P.; Felder, E.; Walther, P.; Dietl, P.; Frick, M.; Pérez-Gil, J.; Haller, T. A small key unlocks a heavy door: The essential function of the small hydrophobic proteins SP-B and SP-C to trigger adsorption of pulmonary surfactant lamellar bodies. Biochim. Biophys. Acta 2016, 1863, 2124–2134. [Google Scholar] [CrossRef]
- Olmeda, B.; García-Álvarez, B.; Gómez, M.J.; Martínez-Calle, M.; Cruz, A.; Pérez-Gil, J. A model for the structure and mechanism of action of pulmonary surfactant protein B. FASEB J. 2015, 29, 4236–4247. [Google Scholar] [CrossRef]
- Parra, E.; Moleiro, L.H.; López-Montero, I.; Cruz, A.; Monroy, F.; Pérez-Gil, J. A combined action of pulmonary surfactant proteins SP-B and SP-C modulates permeability and dynamics of phospholipid membranes. Biochem. J. 2011, 438, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Possmayer, F.; Keating, N.; Zuo, Y.; Petersen, N.; Veldhuizen, R. Pulmonary Surfactant Reduces Surface Tension to Low Values near Zero through a Modified Squeeze-Out Mechanism. Biophys. J. 2012, 102, 414a. [Google Scholar] [CrossRef] [Green Version]
- Keating, E.; Zuo, Y.Y.; Tadayyon, S.M.; Petersen, N.O.; Possmayer, F.; Veldhuizen, R.A. A modified squeeze-out mechanism for generating high surface pressures with pulmonary surfactant. Biochim. Biophys. Acta 2012, 1818, 1225–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Gil, J.; Keough, K.M. Interfacial properties of surfactant proteins. Biochim. Biophys. Acta 1998, 1408, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Bernardino de la Serna, J.; Vargas, R.; Picardi, V.; Cruz, A.; Arranz, R.; Valpuesta, J.M.; Mateu, L.; Pérez-Gil, J. Segregated ordered lipid phases and protein-promoted membrane cohesivity are required for pulmonary surfactant films to stabilize and protect the respiratory surface. Faraday Discuss. 2013, 161, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Llamas, S.; Ortega, F.; Rubio, R.G. Equilibration of a Polycation–Anionic Surfactant Mixture at the Water/Vapor Interface. Langmuir 2018, 34, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Schürch, D.; Ospina, O.L.; Cruz, A.; Pérez-Gil, J. Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophys. J. 2010, 99, 3290–3299. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.A.; Tummino, A.; Noskov, B.A.; Varga, I. Polyelectrolyte/surfactant films spread from neutral aggregates. Soft Matter 2016, 12, 5304–5312. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, A.J.; Slater, J.M.; Arnold, T.; Campbell, R.A.; Thompson, K.C. Changes to DPPC Domain Structure in the Presence of Carbon Nanoparticles. Langmuir 2017, 33, 10374–10384. [Google Scholar] [CrossRef] [Green Version]
- Tummino, A.; Toscano, J.; Sebastiani, F.; Noskov, B.A.; Varga, I.; Campbell, R.A. Effects of Aggregate Charge and Subphase Ionic Strength on the Properties of Spread Polyelectrolyte/Surfactant Films at the Air/Water Interface under Static and Dynamic Conditions. Langmuir 2018, 34, 2312–2323. [Google Scholar] [CrossRef]
- Cañadas, O.; Olmeda, B.; Alonso, A.; Pérez-Gil, J. Lipid–Protein and Protein–Protein Interactions in the Pulmonary Surfactant System and Their Role in Lung Homeostasis. Int. J. Mol. Sci. 2020, 21, 3708. [Google Scholar] [CrossRef]
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat. 2017, 209, 78–92. [Google Scholar] [CrossRef]
- Martínez-Calle, M.; Olmeda, B.; Dietl, P.; Frick, M.; Pérez-Gil, J. Pulmonary surfactant protein SP-B promotes exocytosis of lamellar bodies in alveolar type II cells. FASEB J. 2018, 32, 4600–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.K.; Strayer, D.S. Surfactant Protein a Regulates Pulmonary Surfactant Secretion via Activation of Phosphatidylinositol 3-Kinase in Type II Alveolar Cells. Exp. Cell Res. 2000, 255, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Durham, P.; Hickey, A.J. The role of particle physico-chemical properties in pulmonary drug delivery for tuberculosis therapy. J. Microencapsul. 2014, 31, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Tatur, S.; Badia, A. Influence of Hydrophobic Alkylated Gold Nanoparticles on the Phase Behavior of Monolayers of DPPC and Clinical Lung Surfactant. Langmuir 2012, 28, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.M.; Fiegel, J. Interaction of Dipalmitoyl Phosphatidylcholine Monolayers with a Particle-Laden Subphase. J. Phys. Chem. B 2013, 117, 12124–12134. [Google Scholar] [CrossRef]
- Guzmán, E.; Ferrari, M.; Santini, E.; Liggieri, L.; Ravera, F. Effect of silica nanoparticles on the interfacial properties of a canonical lipid mixture. Colloids Surf. B 2015, 136, 971–980. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Zabiegaj, D.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of Carbon Black Particles and Dipalmitoylphosphatidylcholine at Water/Air Interface: Thermodynamics and Rheology. J. Phys. Chem. C 2015, 119, 26937–26947. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Effect of the Incorporation of Nanosized Titanium Dioxide on the Interfacial Properties of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers. Langmuir 2017, 33, 10715–10725. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Ruppert, C.; Schmehl, T.; Guenther, A.; Betz, T.; Bakowsky, U.; Seeger, W.; Kissel, T.; Gessler, T. Biophysical investigation of pulmonary surfactant surface properties upon contact with polymeric nanoparticles in vitro. Nanomedicine 2011, 7, 341–350. [Google Scholar] [CrossRef]
- Santamaria, A.; Batchu, K.C.; Matsarskaia, O.; Prévost, S.F.; Russo, D.; Natali, F.; Seydel, T.; Hoffmann, I.; Laux, V.; Haertlein, M.; et al. Strikingly Different Roles of SARS-CoV-2 Fusion Peptides Uncovered by Neutron Scattering. J. Am. Chem. Soc. 2022, 144. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Y.; Liu, H.; Chen, Y.; Shang, Y.; Liu, H. The effect of chitin nanoparticles on surface behavior of DPPC/DPPG Langmuir monolayers. J. Colloid Interface Sci. 2018, 519, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Wexler, A.S. Size-dependent deposition of particles in the human lung at steady-state breathing. J. Aerosol Sci. 2008, 39, 266–276. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Lynch, I.; Elder, A. Disposition of nanoparticles as a function of their interactions with biomolecules. In Nanomaterials: Risks and Benefits; NATO Science for Peace and Security Series C: Environmental Security; Linkov, I., Steevens, J., Eds.; Springer: Dordrecht, Germany, 2009; pp. 31–41. [Google Scholar] [CrossRef]
- Stachowicz-Kuśnierz, A.; Cwiklik, L.; Korchowiec, J.; Rogalska, E.; Korchowiec, B. The impact of lipid oxidation on the functioning of a lung surfactant model. Phys. Chem. Chem. Phys. 2018, 20, 24968–24978. [Google Scholar] [CrossRef] [PubMed]
- Long, D.L.; Hite, R.D.; Grier, B.L.; Suckling, B.N.; Safta, A.M.; Morris, P.E.; Waite, B.M.; Seeds, M.C. Secretory Phospholipase A2-Mediated Depletion of Phosphatidylglycerol in Early Acute Respiratory Distress Syndrome. Am. J. Med. Sci. 2012, 343, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of Particles with Langmuir Monolayers of 1,2-Dipalmitoyl-Sn-Glycero-3-Phosphocholine: A Matter of Chemistry? Coatings 2020, 10, 469. [Google Scholar] [CrossRef]
- Muñoz-López, R.; Guzmán, E.; Velázquez, M.M.; Fernández-Peña, L.; Merchán, M.D.; Maestro, A.; Ortega, F.; Rubio, G.R. Influence of Carbon Nanosheets on the Behavior of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers. Processes 2020, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Gradon, L.; Podgorski, A. Hydrodynamical model of pulmonary clearance. Chem. Eng. Sci. 1989, 44, 741–749. [Google Scholar] [CrossRef]
- Gradon, L.; Podgórski, A.; Sosnowski, T.R. Experimental and theoretical investigations of transport properties of DPPC monolayer. J. Aerosol Med. 1996, 9, 357–367. [Google Scholar] [CrossRef]
- Wrobel, S.; Clements, J.A. Bubbles, babies and biology: The story of surfactant—Second breath: A medical mystery solved. FASEB J. 2004, 18, 1624e. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interfacial Properties of Mixed DPPC–Hydrophobic Fumed Silica Nanoparticle Layers. J. Phys. Chem. C 2015, 119, 21024–21034. [Google Scholar] [CrossRef]
- Orsi, D.; Rimoldi, T.; Guzmán, E.; Liggieri, L.; Ravera, F.; Ruta, B.; Cristofolini, L. Hydrophobic Silica Nanoparticles Induce Gel Phases in Phospholipid Monolayers. Langmuir 2016, 32, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Notter, R.H.; Wang, Z. Pulmonary surfactant: Physical chemistry, physiology, and replacement. Rev. Chem. Eng. 1997, 13, 1–118. [Google Scholar] [CrossRef]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Influence of silica nanoparticles on phase behavior and structural properties of DPPC—Palmitic acid Langmuir monolayers. Colloids Surf. A 2012, 413, 280–287. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Changes in the Activity of the Pulmonary Surfactant after Contact with Bentonite Nanoclay Particles. Chem. Eng. Trans. 2012, 26, 531–536. [Google Scholar] [CrossRef]
- Kramek-Romanowska, K.; Odziomek, M.; Sosnowski, T.R. Dynamic tensiometry studies on interactions of novel therapeutic inhalable powders with model pulmonary surfactant at the air-water interface. Colloids Surf. A 2015, 480, 149–158. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Contact angle of micro- and nanoparticles at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 355–367. [Google Scholar] [CrossRef]
- Ariki, S.; Nishitani, C.; Kuroki, Y. Diverse functions of pulmonary collectins in host defense of the lung. J. Biomed. Biotechnol. 2012, 2012, 532071. [Google Scholar] [CrossRef] [Green Version]
- Raesch, S.S.; Tenzer, S.; Storck, W.; Rurainski, A.; Selzer, D.; Ruge, C.A.; Perez-Gil, J.; Schaefer, U.F.; Lehr, C.-M. Proteomic and Lipidomic Analysis of Nanoparticle Corona upon Contact with Lung Surfactant Reveals Differences in Protein, but Not Lipid Composition. ACS Nano 2015, 9, 11872–11885. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Pulmonary surfactant and nanocarriers: Toxicity versus combined nanomedical applications. Biochim. Biophys. Acta 2017, 1859, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Schüer, J.J.; Arndt, A.; Wölk, C.; Pinnapireddy, S.R.; Bakowsky, U. Establishment of a Synthetic In Vitro Lung Surfactant Model for Particle Interaction Studies on a Langmuir Film Balance. Langmuir 2020, 36, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Barrow, R.E.; Hills, B.A. A critical assessment of the Wilhelmy method in studying lung surfactants. J. Physiol. 1979, 295, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.J.; Guzmán, E.; Martínez-Pedrero, F.; Ritacco, H.; Rubio, R.G.; Ortega, F.; Starov, V.M.; Miller, R. Particle laden fluid interfaces: Dynamics and interfacial rheology. Adv. Colloid Interface Sci. 2014, 206, 303–319. [Google Scholar] [CrossRef]
- Bykov, A.G.; Loglio, G.; Ravera, F.; Liggieri, L.; Miller, R.; Noskov, B.A. Dilational surface elasticity of spread monolayers of pulmonary lipids in a broad range of surface pressure. Colloids Surf. A 2018, 541, 137–144. [Google Scholar] [CrossRef]
- Ulman, A. An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-Assembly; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Rubinger, C.P.L.; Moreira, R.L.; Cury, L.A.; Fontes, G.N.; Neves, B.R.A.; Meneguzzi, A.; Ferreira, C.A. Langmuir–Blodgett and Langmuir–Schaefer films of poly(5-amino-1-naphthol) conjugated polymer. Appl. Surf. Sci. 2006, 253, 543–548. [Google Scholar] [CrossRef]
- Hui, S.W.; Viswanathan, R.; Zasadzinski, J.A.; Israelachvili, J.N. The structure and stability of phospholipid bilayers by atomic force microscopy. Biophys. J. 1995, 68, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.; Alig, T.; Yoon, J.; Bringezu, F.; Warriner, H.; Zasadzinski, J.A. More Than a Monolayer: Relating Lung Surfactant Structure and Mechanics to Composition. Biophys. J. 2004, 87, 4188–4202. [Google Scholar] [CrossRef] [Green Version]
- Enhorning, G. Pulmonary surfactant function studied with the pulsating bubble surfactometer (PBS) and the capillary surfactometer (CS). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 221–226. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Li, D.; Acosta, E.; Cox, P.N.; Neumann, A.W. Effect of Surfactant on Interfacial Gas Transfer Studied by Axisymmetric Drop Shape Analysis−Captive Bubble (ADSA-CB). Langmuir 2005, 21, 5446–5452. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, M.; Luo, Y.; Deng, L.; Hu, Z.; Song, Y. Determination of rheology and surface tension of airway surface liquid: A review of clinical relevance and measurement techniques. Respir. Res. 2019, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Echaide, M.; Autilio, C.; López-Rodríguez, E.; Cruz, A.; Pérez-Gil, J. In Vitro Functional and Structural Characterization of a Synthetic Clinical Pulmonary Surfactant with Enhanced Resistance to Inhibition. Sci. Rep. 2020, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Ding, M.; Li, D.; Neumann, A.W. Further development of Axisymmetric Drop Shape Analysis-captive bubble for pulmonary surfactant related studies. Biochim. Biophys. Acta 2004, 1675, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Autilio, C.; Pérez-Gil, J. Understanding the principle biophysics concepts of pulmonary surfactant in health and disease. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F443–F451. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.S.; Zhao, L.; Smith, R.; Possmayer, F.; Petersen, N.O. Metal Nanoparticle Pollutants Interfere with Pulmonary Surfactant Function In Vitro. Biophys. J. 2008, 94, 855–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondej, D.; Sosnowski, T. Physicochemical mechanisms of mineral nanoparticles effects on pulmonary gas/liquid interface studied in model systems. Physicochem. Probl. Miner. Process. 2014, 50, 57–69. [Google Scholar] [CrossRef]
- Dobrowolska, K.; JabBczzDska, K.; Kondej, D.; Sosnowski, T.R. Interactions of insoluble micro- and nanoparticles with the air-liquid interface of the model pulmonary fluids. Physicochem. Probl. Miner. Process. 2017, 54, 151–162. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Interactions of Carbon Nanotubes and Carbon Nanohorns with a Model Membrane Layer and Lung Surfactant In Vitro. J. Nanomater. 2019, 2019, 9457683. [Google Scholar] [CrossRef] [Green Version]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte–surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef]
- Notter, R.H.; Taubold, R.; Mavis, R.D. Hysteresis in Saturated Phospholipid Films and its Potential Relevance for Lung Surfactant Function In Vivo. Exp. Lung Res. 1982, 3, 109–127. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Koliński, M.; Gradón, L. Interactions of benzo[a]pyrene and diesel exhaust particulate matter with the lung surfactant system. Ann. Occup. Hyg. 2011, 55, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Influence of silica nanoparticles on dilational rheology of DPPC–palmitic acid Langmuir monolayers. Soft Matter 2012, 8, 3938–3948. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Interfacial rheology for the assessment of potential health effects of inhaled carbon nanomaterials at variable breathing conditions. Sci. Rep. 2020, 10, 14044. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Tsuji, T.; Jha, R.; Zuo, Y.Y. Determining the surface dilational rheology of surfactant and protein films with a droplet waveform generator. J. Colloid Interface Sci. 2019, 537, 547–553. [Google Scholar] [CrossRef]
- Sørli, J.B.; Låg, M.; Ekeren, L.; Perez-Gil, J.; Haug, L.S.; Da Silva, E.; Matrod, M.N.; Gützkow, K.B.; Lindeman, B. Per- and polyfluoroalkyl substances (PFASs) modify lung surfactant function and pro-inflammatory responses in human bronchial epithelial cells. Toxicol. In Vitro 2020, 62, 104656. [Google Scholar] [CrossRef]
- Liekkinen, J.; de Santos Moreno, B.; Paananen, R.O.; Vattulainen, I.; Monticelli, L.; Bernardino de la Serna, J.; Javanainen, M. Understanding the Functional Properties of Lipid Heterogeneity in Pulmonary Surfactant Monolayers at the Atomistic Level. Front. Cell Dev. Biol. 2020, 8, 581016. [Google Scholar] [CrossRef]
- Arroyo, R.; Martín-González, A.; Echaide, M.; Jain, A.; Brondyk, W.H.; Rosenbaum, J.; Moreno-Herrero, F.; Pérez-Gil, J. Supramolecular Assembly of Human Pulmonary Surfactant Protein SP-D. J. Mol. Biol. 2018, 430, 1495–1509. [Google Scholar] [CrossRef]
- Finot, E.; Markey, L.; Hane, F.; Amrein, M.; Leonenko, Z. Combined atomic force microscopy and spectroscopic ellipsometry applied to the analysis of lipid-protein thin films. Colloids Surf. B 2013, 104, 289–293. [Google Scholar] [CrossRef]
- Przybyla, R.J.; Wright, J.; Parthiban, R.; Nazemidashtarjandi, S.; Kaya, S.; Farnoud, A.M. Electronic cigarette vapor alters the lateral structure but not tensiometric properties of calf lung surfactant. Respir. Res. 2017, 18, 193. [Google Scholar] [CrossRef]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. DPPC–DOPC Langmuir monolayers modified by hydrophilic silica nanoparticles: Phase behaviour, structure and rheology. Colloids Surf. A 2012, 413, 174–183. [Google Scholar] [CrossRef]
- Arora, S.; Kappl, M.; Haghi, M.; Young, P.M.; Traini, D.; Jain, S. An investigation of surface properties, local elastic modulus and interaction with simulated pulmonary surfactant of surface modified inhalable voriconazole dry powders using atomic force microscopy. RSC Adv. 2016, 6, 25789–25798. [Google Scholar] [CrossRef] [Green Version]
- Carrascosa-Tejedor, J.; Santamaria, A.; Pereira, D.; Maestro, A. Structure of DPPC Monolayers at the Air/Buffer Interface: A Neutron Reflectometry and Ellipsometry Study. Coatings 2020, 10, 507. [Google Scholar] [CrossRef]
- Lee, K.Y.C.; Majewski, J.; Kuhl, T.L.; Howes, P.B.; Kjaer, K.; Lipp, M.M.; Waring, A.J.; Zasadzinski, J.A.; Smith, G.S. Synchrotron X-Ray Study of Lung Surfactant-Specific Protein SP-B in Lipid Monolayers. Biophys. J. 2001, 81, 572–585. [Google Scholar] [CrossRef]
- Liu, X.; Counil, C.; Shi, D.; Mendoza-Ortega, E.E.; Vela-Gonzalez, A.V.; Maestro, A.; Campbell, R.A.; Krafft, M.P. First quantitative assessment of the adsorption of a fluorocarbon gas on phospholipid monolayers at the air/water interface. J. Colloid Interface Sci. 2021, 593, 1–10. [Google Scholar] [CrossRef]
- Orsi, D.; Guzmán, E.; Liggieri, L.; Ravera, F.; Ruta, B.; Chushkin, Y.; Rimoldi, T.; Cristofolini, L. 2D dynamical arrest transition in a mixed nanoparticle-phospholipid layer studied in real and momentum spaces. Sci. Rep. 2015, 5, 17930. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, E.; Orsi, D.; Cristofolini, L.; Liggieri, L.; Ravera, F. Two-Dimensional DPPC Based Emulsion-like Structures Stabilized by Silica Nanoparticles. Langmuir 2014, 30, 11504–11512. [Google Scholar] [CrossRef]
- Mandal, P.; Bhatta, F.; Kooijman, E.E.; Allender, D.W.; Mann, E.K. Combined Brewster Angle and Fluorescence Microscopy of DMPC/D-Cholesterol Mixed Langmuir Monolayers. Biophys. J. 2012, 102, 96a–97a. [Google Scholar] [CrossRef] [Green Version]
- Mandal, P.; Noutsi, P.; Chaieb, S. Cholesterol Depletion from a Ceramide/Cholesterol Mixed Monolayer: A Brewster Angle Microscope Study. Sci. Rep. 2016, 6, 26907. [Google Scholar] [CrossRef] [Green Version]
- Mendelsohn, R.; Mao, G.; Flach, C.R. Infrared reflection–absorption spectroscopy: Principles and applications to lipid–protein interaction in Langmuir films. Biochim. Biophys. Acta 2010, 1798, 788–800. [Google Scholar] [CrossRef] [Green Version]
- Can, S.Z.; Chang, C.F.; Walker, R.A. Spontaneous formation of DPPC monolayers at aqueous/vapor interfaces and the impact of charged surfactants. Biochim. Biophys. Acta 2008, 1778, 2368–2377. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.C.; Chapman, D. Monolayer characteristics of saturated 1,2-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim. Biophys. Acta 1968, 163, 301–313. [Google Scholar] [CrossRef]
- Klopfer, K.J.; Vanderlick, T.K. Isotherms of Dipalmitoylphosphatidylcholine (DPPC) Monolayers: Features Revealed and Features Obscured. J. Colloid Interface Sci. 1996, 182, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Arriaga, L.R.; López-Montero, I.; Ignés-Mullol, J.; Monroy, F. Domain-Growth Kinetic Origin of Nonhorizontal Phase Coexistence Plateaux in Langmuir Monolayers: Compression Rigidity of a Raft-Like Lipid Distribution. J. Phys. Chem. B 2010, 114, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Hifeda, Y.F.; Rayfield, G.W. Evidence for first-order phase transitions in lipid and fatty acid monolayers. Langmuir 1992, 8, 197–200. [Google Scholar] [CrossRef]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Mixed DPPC–cholesterol Langmuir monolayers in presence of hydrophilic silica nanoparticles. Colloids Surf. B 2013, 105, 284–293. [Google Scholar] [CrossRef]
- Behyan, S.; Borozenko, O.; Khan, A.; Faral, M.; Badia, A.; DeWolf, C. Nanoparticle-induced structural changes in lung surfactant membranes: An X-ray scattering study. Environ. Sci. Nano 2018, 5, 1218–1230. [Google Scholar] [CrossRef]
- Stachowicz-Kuśnierz, A.; Trojan, S.; Cwiklik, L.; Korchowiec, B.; Korchowiec, J. Modeling Lung Surfactant Interactions with Benzo[a]pyrene. Chem. Eur. J. 2017, 23, 5307–5316. [Google Scholar] [CrossRef]
- Rose, D.; Rendell, J.; Lee, D.; Nag, K.; Booth, V. Molecular dynamics simulations of lung surfactant lipid monolayers. Biophys. Chem. 2008, 138, 67–77. [Google Scholar] [CrossRef]
- Klenz, U.; Saleem, M.; Meyer, M.C.; Galla, H.-J. Influence of Lipid Saturation Grade and Headgroup Charge: A Refined Lung Surfactant Adsorption Model. Biophys. J. 2008, 95, 609–799. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Li, Y.; Chai, X.; Zhang, L.; Xu, L.; Huang, J.; Ning, P.; Tian, S. Interaction of nano carbon particles and anthracene with pulmonary surfactant: The potential hazards of inhaled nanoparticles. Chemosphere 2019, 215, 746–752. [Google Scholar] [CrossRef]
- Suri, L.N.M.; McCaig, L.; Picardi, M.V.; Ospina, O.L.; Veldhuizen, R.A.W.; Staples, J.F.; Possmayer, F.; Yao, L.-J.; Perez-Gil, J.; Orgeig, S. Adaptation to low body temperature influences pulmonary surfactant composition thereby increasing fluidity while maintaining appropriately ordered membrane structure and surface activity. Biochim. Biophys. Acta 2012, 1818, 1581–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, F.J.; Gordon, L.M.; Waring, A.J. Advances in synthetic lung surfactant protein technology. Exp. Rev. Med. Respir. 2019, 13, 499–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, B. Lung surfactant for replacement therapy. Clin. Physiol. 1983, 3, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Egan, E.A.; Notter, R.H.; Kwong, M.S.; Shapiro, D.L. Natural and artificial lung surfactant replacement therapy in premature lambs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 875–883. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Dembinski, R.; Maedler, K. Lung Surfactant for Pulmonary Barrier Restoration in Patients With COVID-19 Pneumonia. Front. Med. 2020, 7, 254. [Google Scholar] [CrossRef]
- Farnoud, A.M.; Fiegel, J. Calf Lung Surfactant Recovers Surface Functionality After Exposure to Aerosols Containing Polymeric Particles. J. Aerosol Med. Pulm. Drug Deliv. 2015, 29, 10–23. [Google Scholar] [CrossRef]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Effect of Hydrophilic and Hydrophobic Nanoparticles on the Surface Pressure Response of DPPC Monolayers. J. Phys. Chem. C 2011, 115, 21715–21722. [Google Scholar] [CrossRef]
- Baoukina, S.; Rozmanov, D.; Mendez-Villuendas, E.; Tieleman, D.P. The Mechanism of Collapse of Heterogeneous Lipid Monolayers. Biophys. J. 2014, 107, 1136–1145. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.D.; Lee, J.; Shin, K. Collapsed States of Langmuir Monolayers. J. Oleo Sci. 2016, 65, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-C.; Kodama, A.T.; Boatwright, T.; Dennin, M. Particle Size Effects on Collapse in Monolayers. Langmuir 2012, 28, 13976–13983. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takei, T.; Aiba, T.; Masuda, K.; Kiuchi, A.; Fujiwara, T. Development of synthetic lung surfactants. J. Lipid Res. 1986, 27, 475–485. [Google Scholar] [CrossRef]
- Casals, C.; Cañadas, O. Role of lipid ordered/disordered phase coexistence in pulmonary surfactant function. Biochim. Biophys. Acta 2012, 1818, 2550–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel Diez, M.; Buckley, A.; Tetley, T.D.; Smith, R. The method of depositing CeO2 nanoparticles onto a DPPC monolayer affects surface tension behaviour. NanoImpact 2019, 16, 100186. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Elandaloussi, I.; Ghazvini, S.; Berkland, C.J.; Dhar, P. Effect of Lipid Headgroup Charge and pH on the Stability and Membrane Insertion Potential of Calcium Condensed Gene Complexes. Langmuir 2015, 31, 4232–4245. [Google Scholar] [CrossRef] [Green Version]
- Mousseau, F.; Berret, J.F. The role of surface charge in the interaction of nanoparticles with model pulmonary surfactants. Soft Matter 2018, 14, 5764–5774. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, Y.; Ren, Q.; Zhang, L.G.; Liu, S.; Zuo, Y.Y. Biophysical Assessment of Pulmonary Surfactant Predicts the Lung Toxicity of Nanomaterials. Small Methods 2018, 2, 1700367. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Jabłczyńska, K.; Odziomek, M.; Schlage, W.K.; Kuczaj, A.K. Physicochemical studies of direct interactions between lung surfactant and components of electronic cigarettes liquid mixtures. Inhal. Toxicol. 2018, 30, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Taneva, S.G.; Stewart, J.; Taylor, L.; Keough, K.M.W. Method of purification affects some interfacial properties of pulmonary surfactant proteins B and C and their mixtures with dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta 1998, 1370, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Beck-Broichsitter, M.; Ruppert, C.; Schmehl, T.; Günther, A.; Seeger, W. Biophysical inhibition of synthetic vs. naturally-derived pulmonary surfactant preparations by polymeric nanoparticles. Biochim. Biophys. Acta 2014, 1838, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Sachan, A.K.; Harishchandra, R.K.; Bantz, C.; Maskos, M.; Reichelt, R.; Galla, H.-J. High-Resolution Investigation of Nanoparticle Interaction with a Model Pulmonary Surfactant Monolayer. ACS Nano 2012, 6, 1677–1687. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, L.; Bai, T.; Guo, Z. Interaction Between Hydrophobic Au Nanoparticles and Pulmonary Surfactant (DPPC) Monolayers. J. Biomed. Nanotechnol. 2018, 14, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.S.; Patchin, E.S.; Silva, R.M.; Uyeminami, D.L.; Sharmah, A.; Guo, T.; Das, G.K.; Brown, J.M.; Shannahan, J.; Gordon, T.; et al. Influence of Particle Size on Persistence and Clearance of Aerosolized Silver Nanoparticles in the Rat Lung. Toxicol. Sci. 2015, 144, 366–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yang, Y.; Xu, B.; Wang, S.; Li, B.; Ma, J.; Gao, J.; Zuo, Y.Y.; Liu, S. Mesoporous carbon nanomaterials induced pulmonary surfactant inhibition, cytotoxicity, inflammation and lung fibrosis. J. Environ. Sci. 2017, 62, 100–114. [Google Scholar] [CrossRef]
- Curtis, E.M.; Bahrami, A.H.; Weikl, T.R.; Hall, C.K. Modeling nanoparticle wrapping or translocation in bilayer membranes. Nanoscale 2015, 7, 14505–14514. [Google Scholar] [CrossRef] [Green Version]
- Ku, T.; Gill, S.; Löbenberg, R.; Azarmi, S.; Roa, W.; Prenner, E.J. Size Dependent Interactions of Nanoparticles with Lung Surfactant Model Systems and the Significant Impact on Surface Potential. J. Nanosc. Nanotechnol. 2008, 8, 2971–2978. [Google Scholar] [CrossRef]
- Kodama, A.T.; Kuo, C.-C.; Boatwright, T.; Dennin, M. Investigating the Effect of Particle Size on Pulmonary Surfactant Phase Behavior. Biophys. J. 2014, 107, 1573–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Schleh, C.; Hohlfeld, J.M. Interaction of nanoparticles with the pulmonary surfactant system. Inhal. Toxicol. 2009, 21, 97–103. [Google Scholar] [CrossRef]
- Kim, J.; Chankeshwara, S.V.; Thielbeer, F.; Jeong, J.; Donaldson, K.; Bradley, M.; Cho, W.-S. Surface charge determines the lung inflammogenicity: A study with polystyrene nanoparticles. Nanotoxicology 2016, 10, 94–101. [Google Scholar] [CrossRef]
- Hao, C.; Li, J.; Mu, W.; Zhu, L.; Yang, J.; Liu, H.; Li, B.; Chen, S.; Sun, R. Adsorption behavior of magnetite nanoparticles into the DPPC model membranes. Appl. Surf. Sci. 2016, 362, 121–125. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 1–10. [Google Scholar]
- Chen, P.; Zhang, Z.; Gu, N.; Ji, M. Effect of the surface charge density of nanoparticles on their translocation across pulmonary surfactant monolayer: A molecular dynamics simulation. Mol. Simul. 2018, 44, 85–93. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M. Biophysical Activity of Impaired Lung Surfactant upon Exposure to Polymer Nanoparticles. Langmuir 2016, 32, 10422–10429. [Google Scholar] [CrossRef] [PubMed]
- Valle, R.P.; Huang, C.L.; Loo, J.S.C.; Zuo, Y.Y. Increasing Hydrophobicity of Nanoparticles Intensifies Lung Surfactant Film Inhibition and Particle Retention. ACS Sustain. Chem. Eng. 2014, 2, 1574–1580. [Google Scholar] [CrossRef]
- Konduru, N.V.; Damiani, F.; Stoilova-McPhie, S.; Tresback, J.S.; Pyrgiotakis, G.; Donaghey, T.C.; Demokritou, P.; Brain, J.D.; Molina, R.M. Nanoparticle Wettability Influences Nanoparticle–Phospholipid Interactions. Langmuir 2018, 34, 6454–6461. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Effect of clay nanoparticles on model lung surfactant: A potential marker of hazard from nanoaerosol inhalation. Environ. Sci. Poll. Res. 2016, 23, 4660–4669. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Deng, L.; Ren, H.; Zhang, X.; Huang, F.; Yue, T. Transport of nanoparticles across pulmonary surfactant monolayer: A molecular dynamics study. Phys. Chem. Chem. Phys. 2017, 19, 17568–17576. [Google Scholar] [CrossRef]
- Sacanna, S.; Pine, D.J. Shape-anisotropic colloids: Building blocks for complex assemblies. Curr. Opin. Colloid Interface Sci. 2011, 16, 96–105. [Google Scholar] [CrossRef]
- Yang, K.; Ma, Y.-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010, 5, 579–583. [Google Scholar] [CrossRef]
- Lin, X.; Zuo, Y.Y.; Gu, N. Shape affects the interactions of nanoparticles with pulmonary surfactant. Sci. China Mater. 2015, 58, 28–37. [Google Scholar] [CrossRef]
- Aramrak, S.; Flury, M.; Harsh, J.B.; Zollars, R.L.; Davis, H.P. Does Colloid Shape Affect Detachment of Colloids by a Moving Air-Water Interface? Langmuir 2013, 29, 5770–5780. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck-Broichsitter, M.; Ruppert, C.; Schmehl, T.; Günther, A.; Seeger, W. Biophysical inhibition of pulmonary surfactant function by polymeric nanoparticles: Role of surfactant protein B and C. Acta Biomater. 2014, 10, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.I.; Luo, Z.; Deplazes, E.; Saha, S.C. Shape matters—The interaction of gold nanoparticles with model lung surfactant monolayers. J. R. Soc. Interface 2021, 18, 20210402. [Google Scholar] [CrossRef] [PubMed]
- Stenger, P.C.; Alonso, C.; Zasadzinski, J.A.; Waring, A.J.; Jung, C.-L.; Pinkerton, K.E. Environmental tobacco smoke effects on lung surfactant film organization. Biochim. Biophys. Acta 2009, 1788, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.; Autilio, C.; Hougaard, K.S.; Baun, A.; Cruz, A.; Perez-Gil, J.; Sørli, J.B. Molecular and biophysical basis for the disruption of lung surfactant function by chemicals. Biochim. Biophys. Acta 2021, 1863, 183499. [Google Scholar] [CrossRef]
- Kanno, S.; Hirano, S.; Kato, H.; Fukuta, M.; Mukai, T.; Aoki, Y. Benzalkonium chloride and cetylpyridinium chloride induce apoptosis in human lung epithelial cells and alter surface activity of pulmonary surfactant monolayers. Chem. Biol. Interact. 2020, 317, 108962. [Google Scholar] [CrossRef]
- Nisoh, N.; Karttunen, M.; Monticelli, L.; Wong-Ekkabut, J. Lipid monolayer disruption caused by aggregated carbon nanoparticles. RSC Adv. 2015, 5, 11676–11685. [Google Scholar] [CrossRef]
- Choe, S.; Chang, R.; Jeon, J.; Violi, A. Molecular Dynamics Simulation Study of a Pulmonary Surfactant Film Interacting with a Carbonaceous Nanoparticle. Biophys. J. 2008, 95, 4102–4114. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Stein, A.J.; Kruger, A.; Leblanc, R.M. Head Groups of Lipids Govern the Interaction and Orientation between Graphene Oxide and Lipids. J. Phys. Chem. C 2013, 117, 16150–16158. [Google Scholar] [CrossRef]
- Kanishtha, T.; Banerjee, R.; Venkataraman, C. Effect of particle emissions from biofuel combustion on surface activity of model and therapeutic pulmonary surfactants. Environ. Toxicol. Pharmacol. 2006, 22, 325–333. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Li, S.; Zhang, X.; Feng, H.; Dai, Y.; Zhao, J.; Yue, T. Molecular modeling of nanoplastic transformations in alveolar fluid and impacts on the lung surfactant film. J. Hazard. Mater. 2022, 427, 127872. [Google Scholar] [CrossRef] [PubMed]









| Commercial Name | Origin | Producer |
|---|---|---|
| Infasurf | lavage of calf lung fluid | ONY Biotech Inc., Amherst, NY, USA |
| Curosurf | lavage of porcine lung fluid | Chiesi Farmaceutici S.p.A, Parma, Italy |
| Survanta | lavage of bovine lung fluid | AbbVie Inc., North Chicago, IL, USA |
| BLES | lavage of bovine lung fluid | BLES Biochemicals Inc., London, ON, Canada |
| Alveofact | lavage of bovine lung fluid | Lyomark Pharma, Oberhaching, Germany |
| Venticute | synthetic | Byk Gulden Pharmaceuticals, Konstanz, Germany |
| Surfaxin | synthetic | Discovery Laboratory Inc., Warrington, PA, USA |
| Exosurf | synthetic | GlaxoSmithKline, Brentford, UK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E. Fluid Films as Models for Understanding the Impact of Inhaled Particles in Lung Surfactant Layers. Coatings 2022, 12, 277. https://doi.org/10.3390/coatings12020277
Guzmán E. Fluid Films as Models for Understanding the Impact of Inhaled Particles in Lung Surfactant Layers. Coatings. 2022; 12(2):277. https://doi.org/10.3390/coatings12020277
Chicago/Turabian StyleGuzmán, Eduardo. 2022. "Fluid Films as Models for Understanding the Impact of Inhaled Particles in Lung Surfactant Layers" Coatings 12, no. 2: 277. https://doi.org/10.3390/coatings12020277
APA StyleGuzmán, E. (2022). Fluid Films as Models for Understanding the Impact of Inhaled Particles in Lung Surfactant Layers. Coatings, 12(2), 277. https://doi.org/10.3390/coatings12020277






