Titanium Oxide Coatings Deposited on MnZn Ferrite by a Molten Salt Reaction
Abstract
:1. Introduction
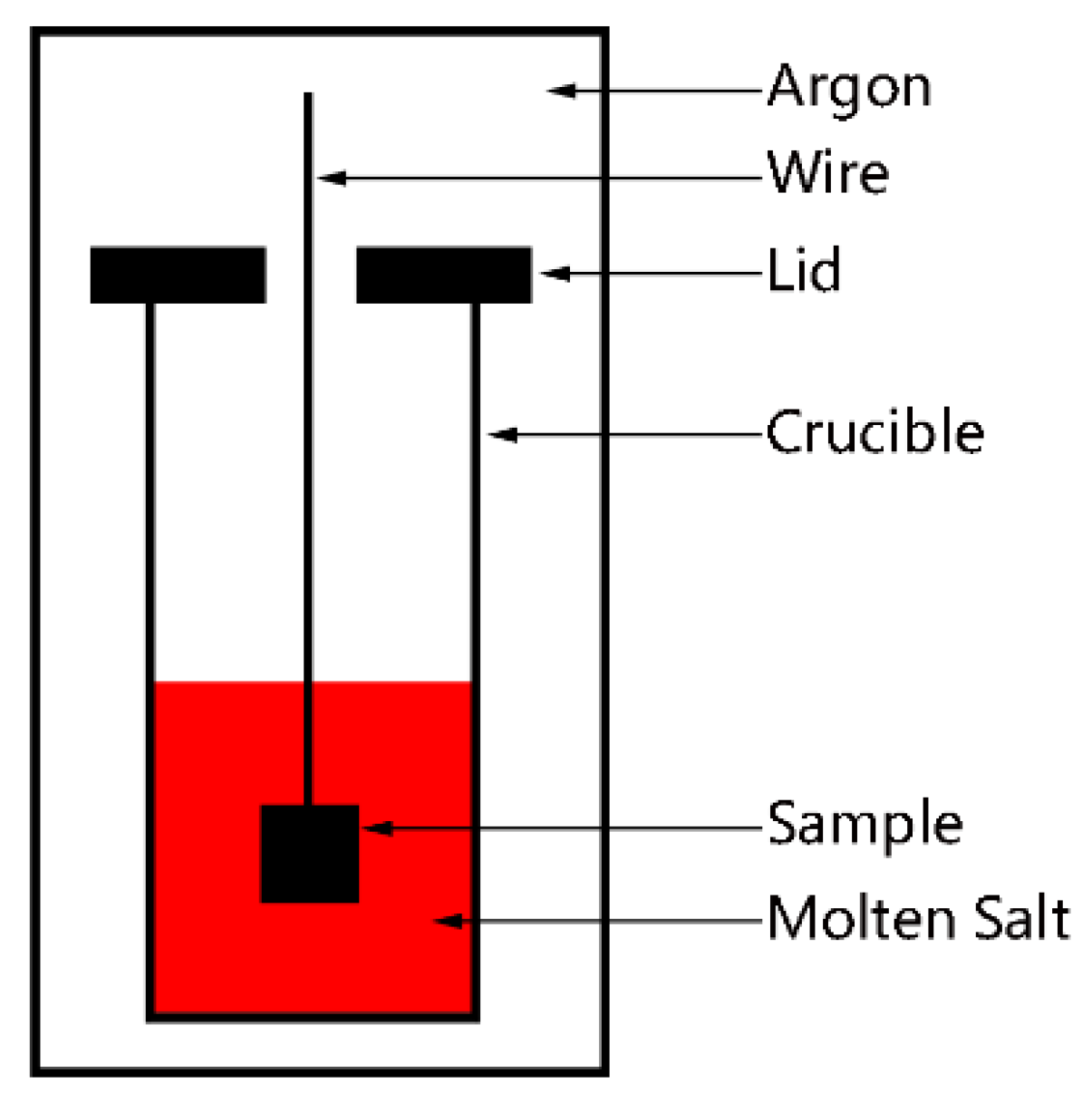
2. Experiment
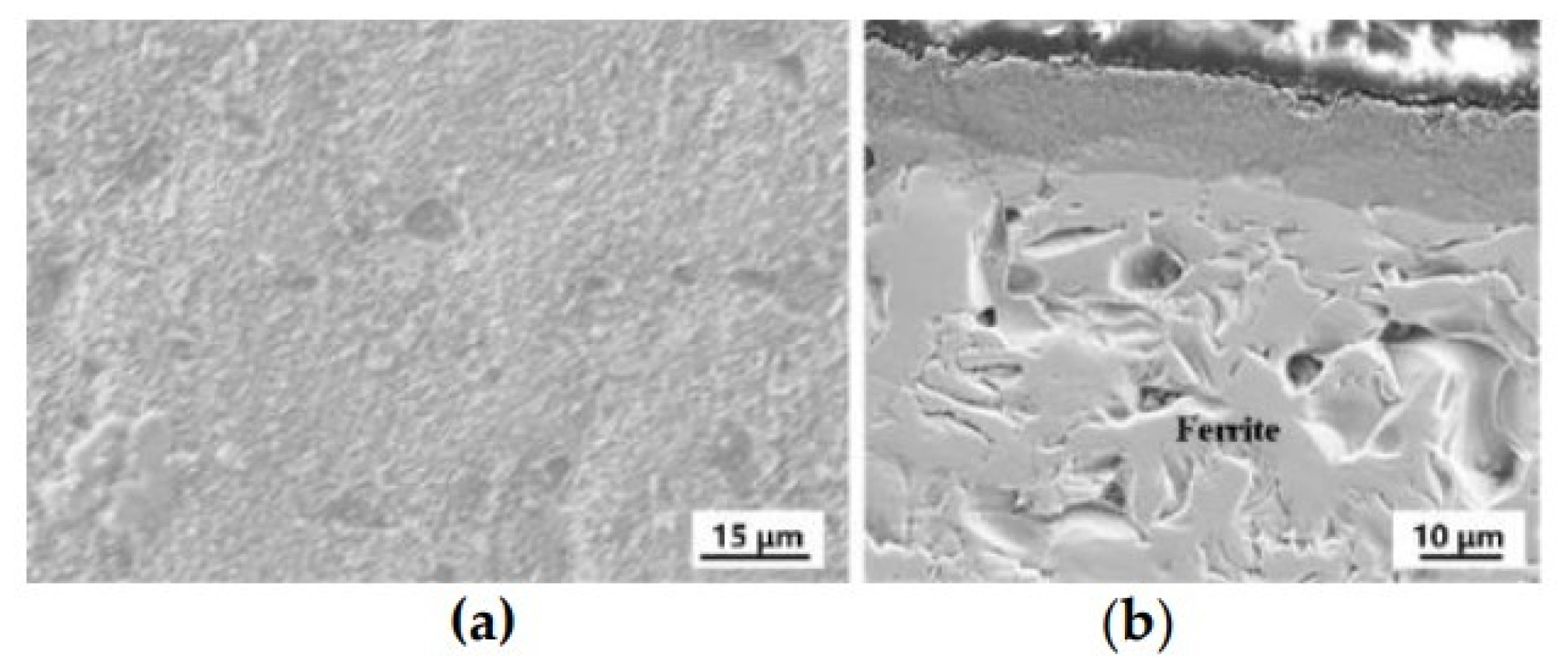
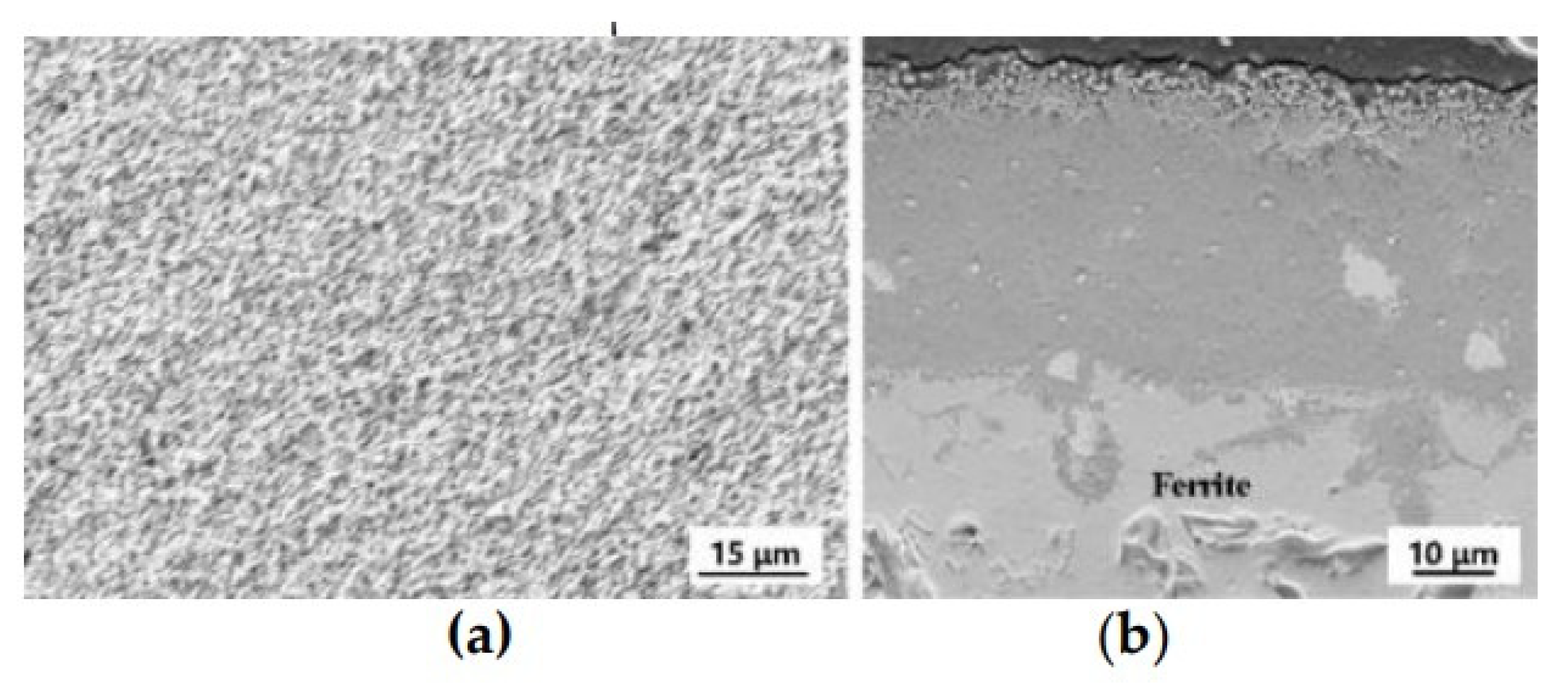
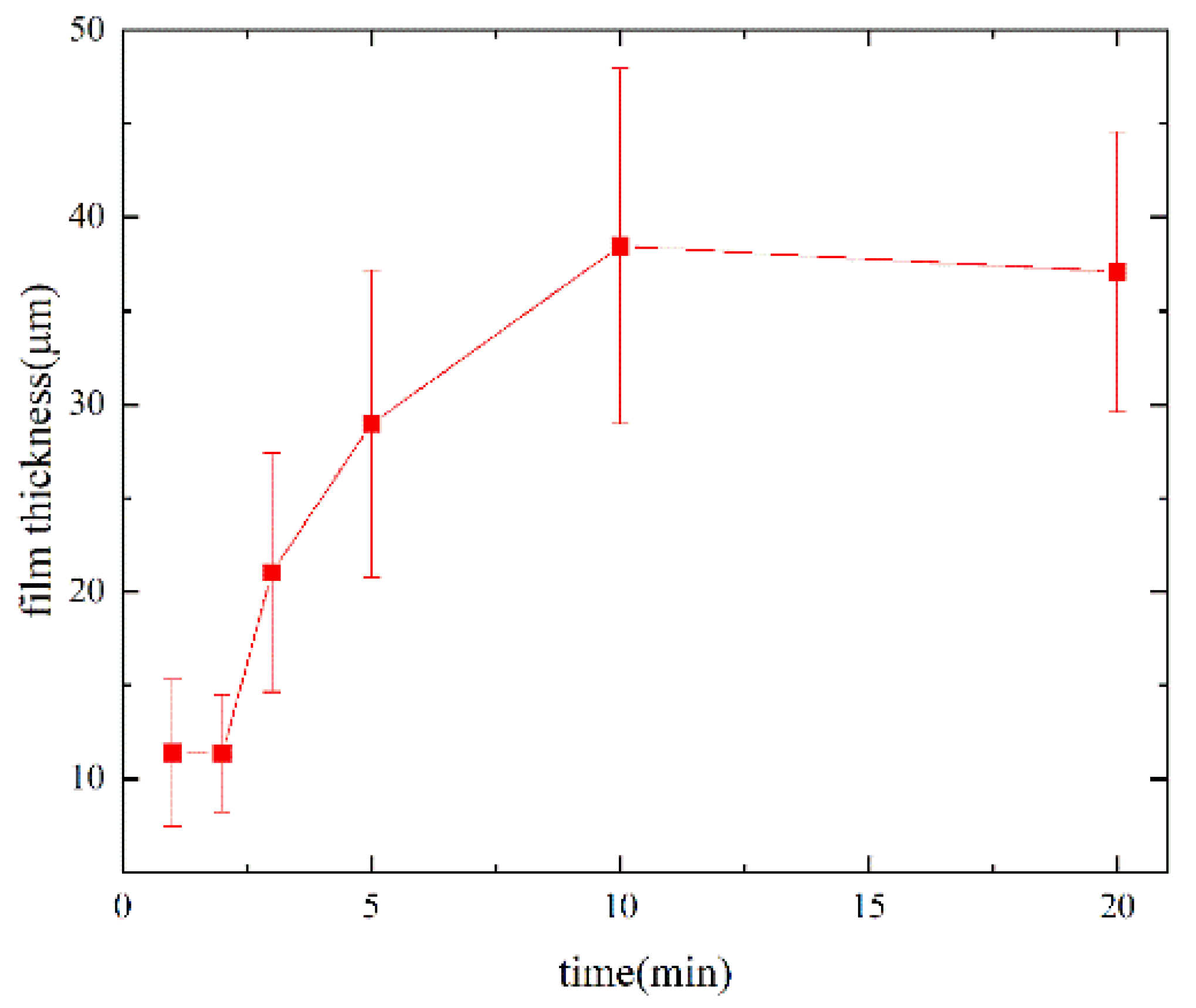
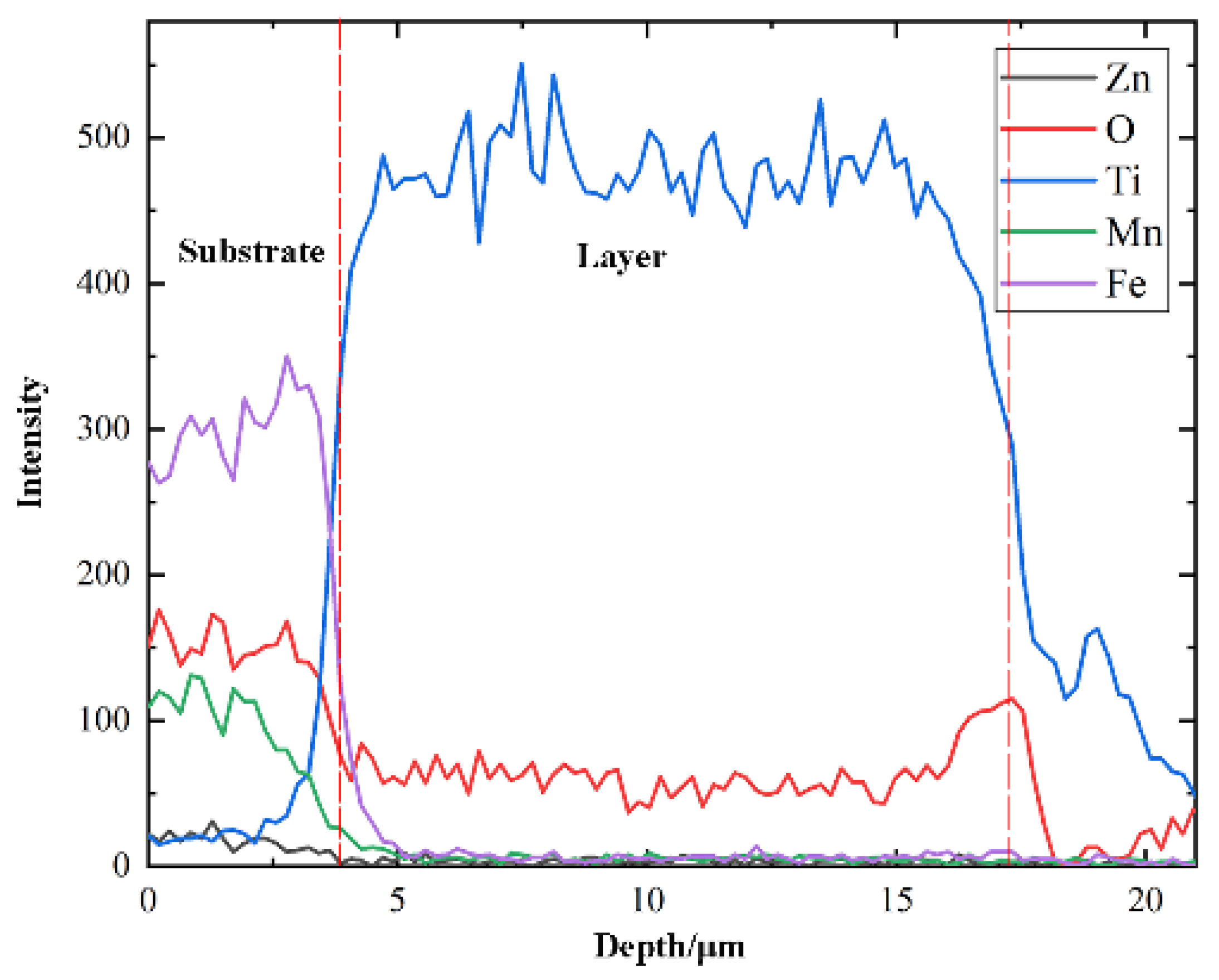
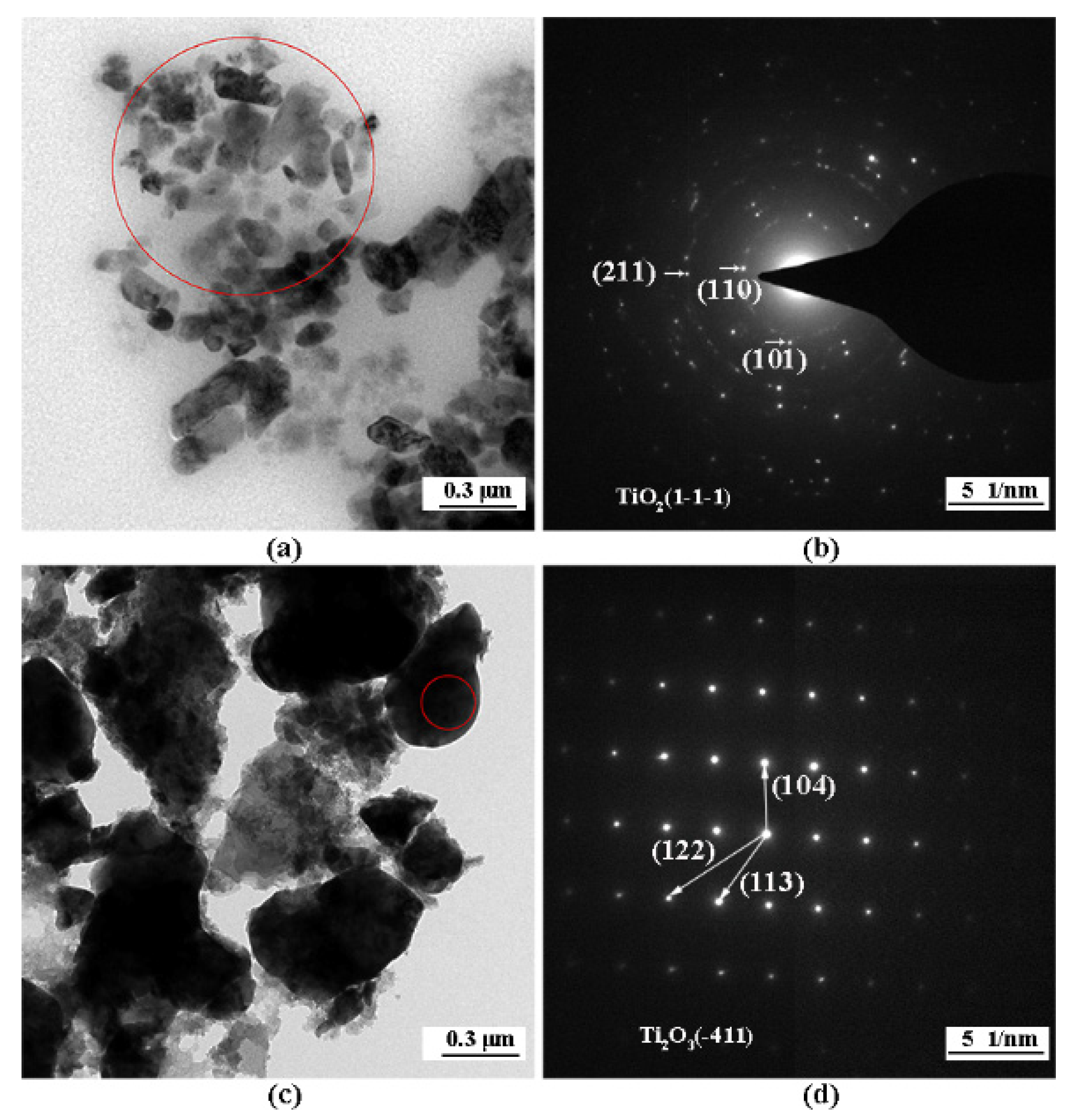
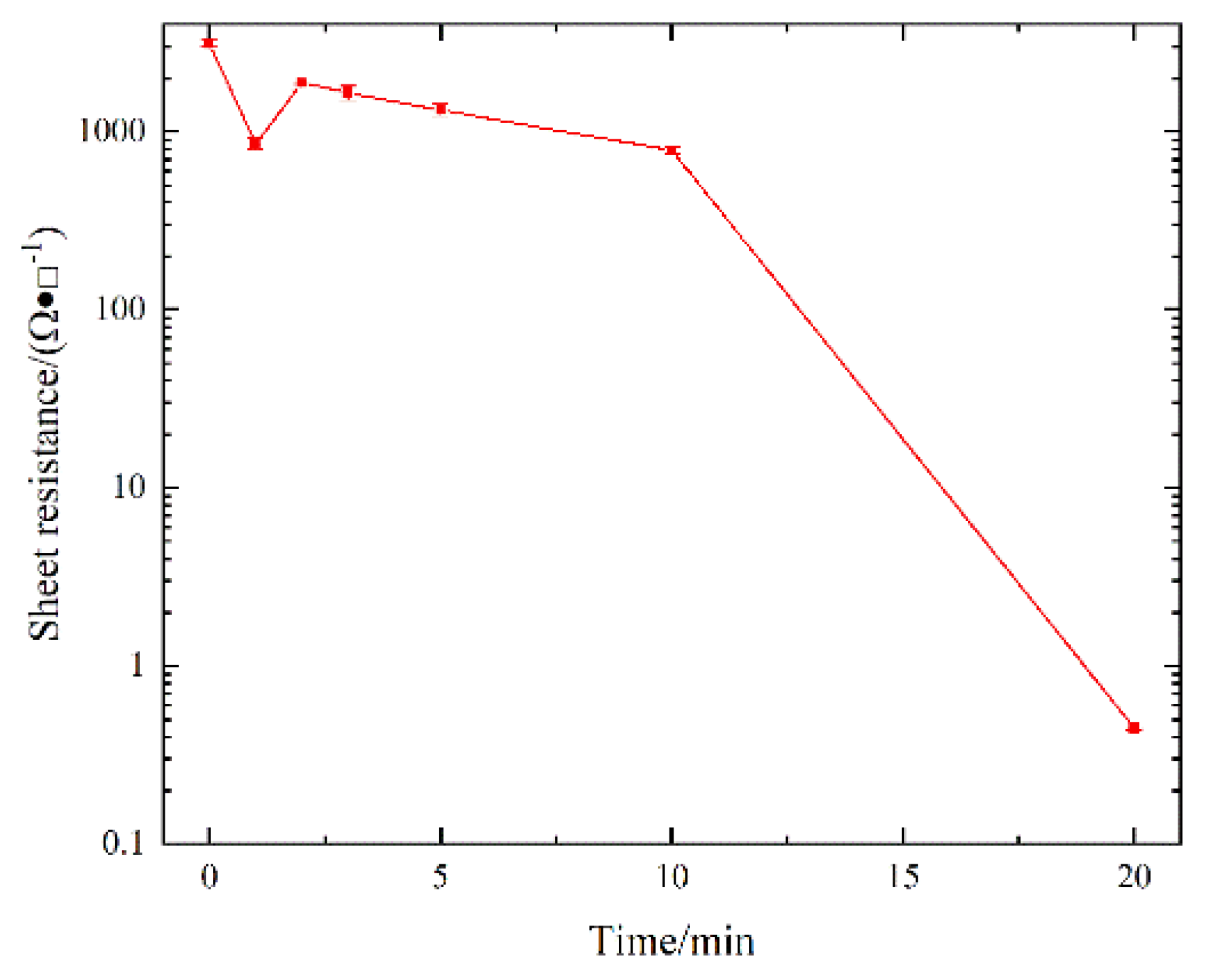
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugimoto, M. The Past, Present, and Future of Ferrites. J. Am. Ceram. Soc. 2004, 82, 269–280. [Google Scholar] [CrossRef]
- Skołyszewska, B.; Tokarz, W.; Przybylski, K.; Kakol, Z. Preparation and Magnetic Properties of MgZn and MnZn Ferrites. Phys. C Supercond. 2003, 387, 290–294. [Google Scholar] [CrossRef]
- Reza, M.G.; Daee, S.S.; Hossein Mohseni, H.; Torkian, S.; Ghasemi, M.; Ameriannejad, M.; Hoseinizade, M.; Pirnia, M.; Pourjafar, D.; Pourmahdavi, M. Structure and Magnetic Properties of Mn-Zn Ferrite Synthesized by Glycine-Nitrate Auto-Combustion Process. AMR 2011, 409, 520–525. [Google Scholar] [CrossRef]
- Thakur, P.; Chahar, D.; Taneja, S.; Bhalla, N.; Thakur, A. A review on MnZn ferrites: Synthesis, characterization and applications. Ceram. Int. 2020, 46, 15740–15763. [Google Scholar] [CrossRef] [PubMed]
- Houbi, A.; Aldashevich, Z.A.; Atassi, Y.; Telmanovna, Z.B.; Saule, M.; Kubanych, K. Microwave absorbing properties of ferrites and their composites: A review. J. Magn. Magn. Mater. 2021, 529, 167839. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Trukhanov, A.; Sadaqat, A.; Korkmaz, A.D.; Algarou, N.; Aydın, H.; Baykal, A.; Toprak, M.S. Review on functional bi-component nanocomposites based on hard/soft ferrites: Structural, magnetic, electrical and microwave absorption properties. Nano-Struct. Nano-Objects 2021, 26, 100728. [Google Scholar] [CrossRef]
- Zaquine, I.; Benazizi, H.; Mage, J.C. Ferrite thin films for microwave applications. J. Appl. Phys. 1988, 64, 5822–5824. [Google Scholar] [CrossRef]
- Lim Ju, D.; Rong, E.P.J.; Riko, I.M.; Sharif, A.; Zhang, L.J.; Long, L.F.; Lip, G.C.; Zhong, C.; Rhee, D.; Woo, M.; et al. Study of Thin Film Metallization Adhesion in Ceramic Multichip Module. In Proceedings of the 2012 IEEE 14th Electronics Packaging Technology Conference (EPTC), Singapore, 5–7 December 2012. [Google Scholar]
- Hasuyama, H.; Shima, Y.; Hayashi, N.; Sakamoto, I.; Baba, K.; Motoyama, M. Adhesion enhancement of metallized thin films on alumina ceramics by ion beam mixing. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1993, 80–81, 1304–1307. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, D.; Zhao, C.; Zhu, M.; Gao, Y.; Chen, Y.; Liang, X.; Chen, H.; Wang, J.; Wei, Y.; et al. Nonreciprocal Isolating Bandpass Filter With Enhanced Isolation Using Metallized Ferrite. IEEE Trans. Microw. Theory Tech. 2020, 68, 5307–5316. [Google Scholar] [CrossRef]
- Konishi, Y. Novel dielectric waveguide components-microwave applications of new ceramic materials. Proc. IEEE 1991, 79, 726–740. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, J.-W.; Kim, H.-J.; Yun, Y.-H.; Nam, S.-M. Silver Metallization for Micro-wave Device Using Aerosol Deposition. Ceram. Int. 2012, 38, S201–S204. [Google Scholar] [CrossRef]
- Equbal, A.; Sood, A.K. Metallization on FDM Parts Using the Chemical Deposition Technique. Coatings 2014, 4, 574–586. [Google Scholar] [CrossRef] [Green Version]
- De Juan, S.; Gordo, E.; Jiménez-Morales, A.; Sirois, F. Study on the Growth and Properties of Electrolessly Deposited Thin Copper Coatings on Epoxy-Based CFRP. Coatings 2020, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.; Su, H.; Lin, C.; Ko, T.; Chen, C.; Huang, J.; Chou, S.; Peng, C.; Hsieh, C.; Tsai, M.; et al. Direct plating of Cu on ALD TaN for 45 nm-node Cu BEOL metallization. In Proceedings of the IEEE International Electron Devices Meeting, San Francisco, CA, USA, 13–15 December 2004. [Google Scholar]
- Testov, O.A.; Komlev, A.E.; Gareev, K.G.; Khmelnitskiy, I.K.; Luchinin, V.V.; Sevost’Yanov, E.N.; Testov, I.O. Providing a Specified Level of Electromagnetic Shielding with Nickel Thin Films Formed by DC Magnetron Sputtering. Coatings 2021, 11, 1455. [Google Scholar] [CrossRef]
- Hu, Y.; Rasadujjaman, M.; Wang, Y.; Zhang, J.; Yan, J.; Baklanov, M.R. Study on the Electrical, Structural, Chemical and Optical Properties of PVD Ta(N) Films Deposited with Different N2 Flow Rates. Coatings 2021, 11, 937. [Google Scholar] [CrossRef]
- Behboudi, F.; Kakroudi, M.G.; Vafa, N.P.; Faraji, M.; Milani, S.S. Molten salt synthesis of in-situ TiC coating on graphite flakes. Ceram. Int. 2021, 47, 8161–8168. [Google Scholar] [CrossRef]
- Daoush, W.M.; Park, H.S.; Hong, S.H. Fabrication of TiN/cBN and TiC/diamond coated particles by titanium deposition process. Trans. Nonferrous Met. Soc. China 2014, 24, 3562–3570. [Google Scholar] [CrossRef]
- Dubrovskiy, A.; Okunev, M.; Makarova, O.; Kuznetsov, S. Superconducting Niobium Coatings Deposited on Spherical Substrates in Molten Salts. Coatings 2018, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Stern, K.H. Metallurgical and Ceramic Protective Coatings; Springer Science & Business Media: London, UK, 1996. [Google Scholar]
- Wei, P.; Qiliang, H.; Jian, C.; Juan, C.; Huang, Y. Mechanism of titanium deposition on Al2O3 ceramic surface by molten salt reaction. Mater. Lett. 1997, 31, 317–320. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, K.; Li, Y.; Liu, Y.; Zhu, M.; Zhong, A.; Cai, X.; Fan, P.; Weizhong, L. Employing TiO2 Buffer Layer to Improve VO2 Film Phase Transition Performance and Infrared Solar Energy Modulation Ability. J. Alloys Compd. 2016, 684, 719–725. [Google Scholar] [CrossRef]
- Stryhalski, J.; Diego, A.D.; Rebouta, L.M.; César Sagás, J.; José Tavares, C.; Fontana, L.C. Nb-Doped Ti2O3 Films Deposited through Grid-Assisted Magnetron Sputtering on Glass Substrate: Electrical and Optical Analysis. Mat. Res. 2019, 22. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Pulikkotil, J. Electronic phase transition and transport properties of Ti2O3. J. Alloys Compd. 2016, 658, 430–434. [Google Scholar] [CrossRef]
- Li, J.; Wei, P.; Qiliang, H.; Chen, J.; Zhang, Z. Mechanism of titanium deposition on AlN surface by molten salt reaction. Mater. Lett. 2003, 57, 1369–1373. [Google Scholar] [CrossRef]
- Wei, P.; Li, J.; Chen, J. Titanium Metallization of Si3N4 Ceramics by Molten Salt Reaction: Coating Microstructure and Brazing Property. Thin Solid Film. 2002, 422, 126–129. [Google Scholar] [CrossRef]
- Polovov, I.B.; Abramov, A.V.; Rebrin, O.I.; Volkovich, V.A.; Denisov, E.; Griffiths, T.R.; Iain May, I.; Kinoshita, H. Corrosion of Stainless Steel in NaCl-KCl Based Melts. ECS Trans. 2010, 33, 321. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cui, E.; Wang, C.; Yan, L.; Zhang, W.; Yu, G. Titanium Oxide Coatings Deposited on MnZn Ferrite by a Molten Salt Reaction. Coatings 2022, 12, 298. https://doi.org/10.3390/coatings12030298
Wang H, Cui E, Wang C, Yan L, Zhang W, Yu G. Titanium Oxide Coatings Deposited on MnZn Ferrite by a Molten Salt Reaction. Coatings. 2022; 12(3):298. https://doi.org/10.3390/coatings12030298
Chicago/Turabian StyleWang, Hongyang, Erchuang Cui, Chengbin Wang, Long Yan, Wei Zhang, and Guojun Yu. 2022. "Titanium Oxide Coatings Deposited on MnZn Ferrite by a Molten Salt Reaction" Coatings 12, no. 3: 298. https://doi.org/10.3390/coatings12030298




