Hydrogen Inhibition as Explosion Prevention in Wet Metal Dust Removal Systems
Abstract
:1. Introduction
- Analysis of the inhibitory effect and the optimum concentration of sodium citrate with regards to 1.5 g of aluminum powder.
- Experimental confirmation (by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS)) of the inhibition film formation on the Al particles surface.
- Investigation of the inhibition mechanism (with the help of Fourier transform infrared (FTIR) spectroscopy) of the reaction between Al and water by sodium citrate.
2. Experimental Section
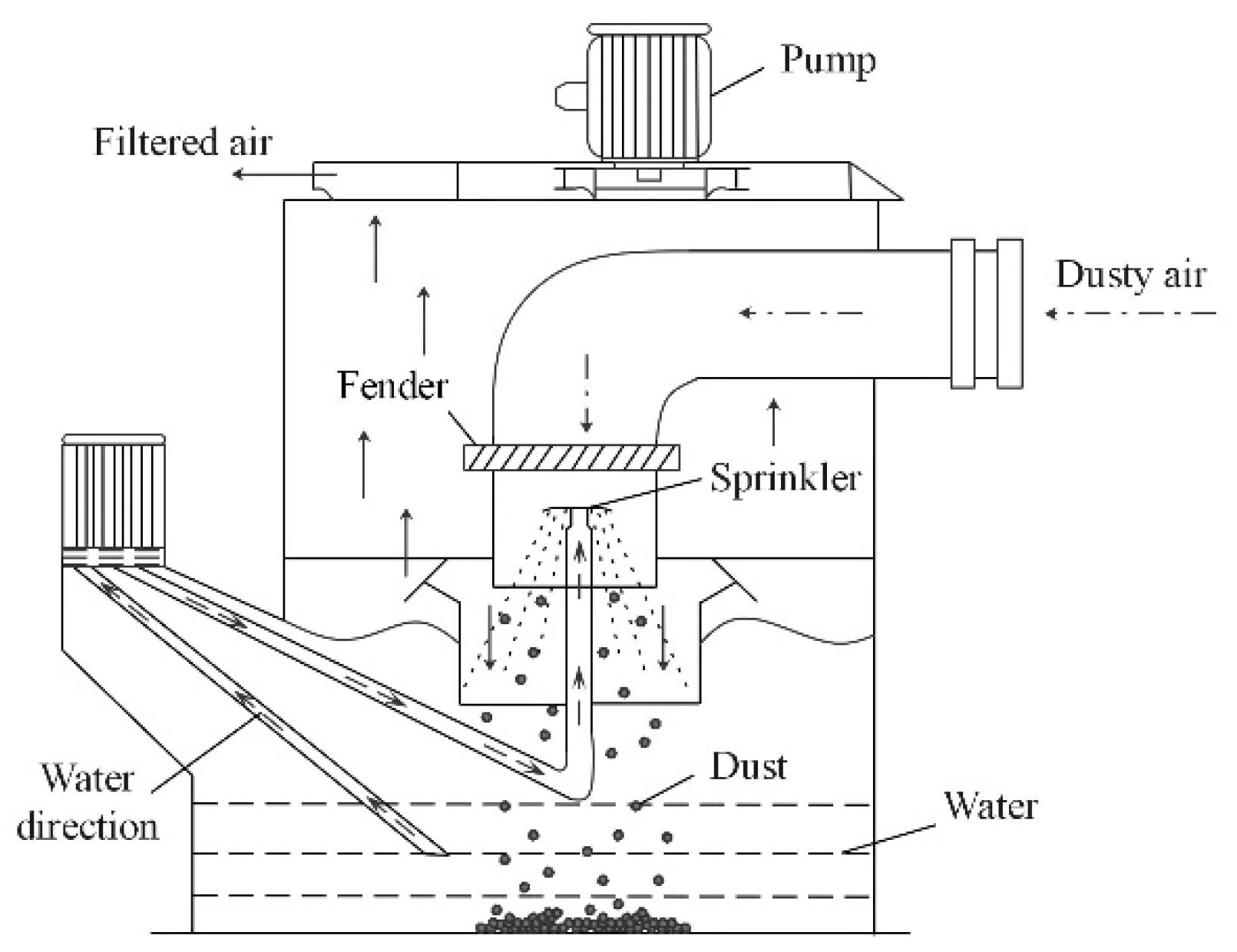
2.1. Setup
2.2. Materials
2.3. Characterization
3. Results and Discussion
3.1. Influence of Sodium Citrate Content
3.1.1. Results of Hydrogen Inhibition Tests
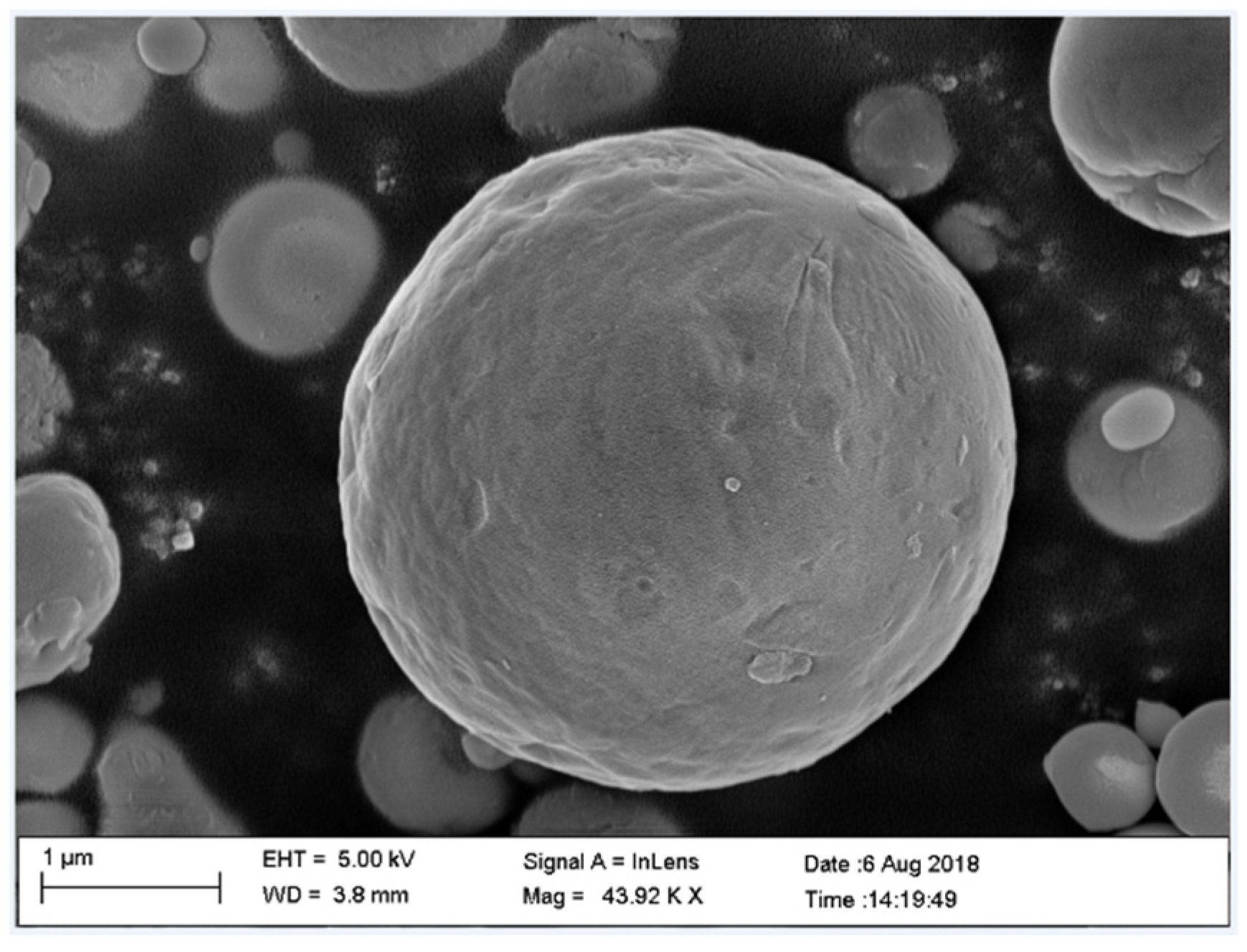
3.1.2. SEM and EDS Results
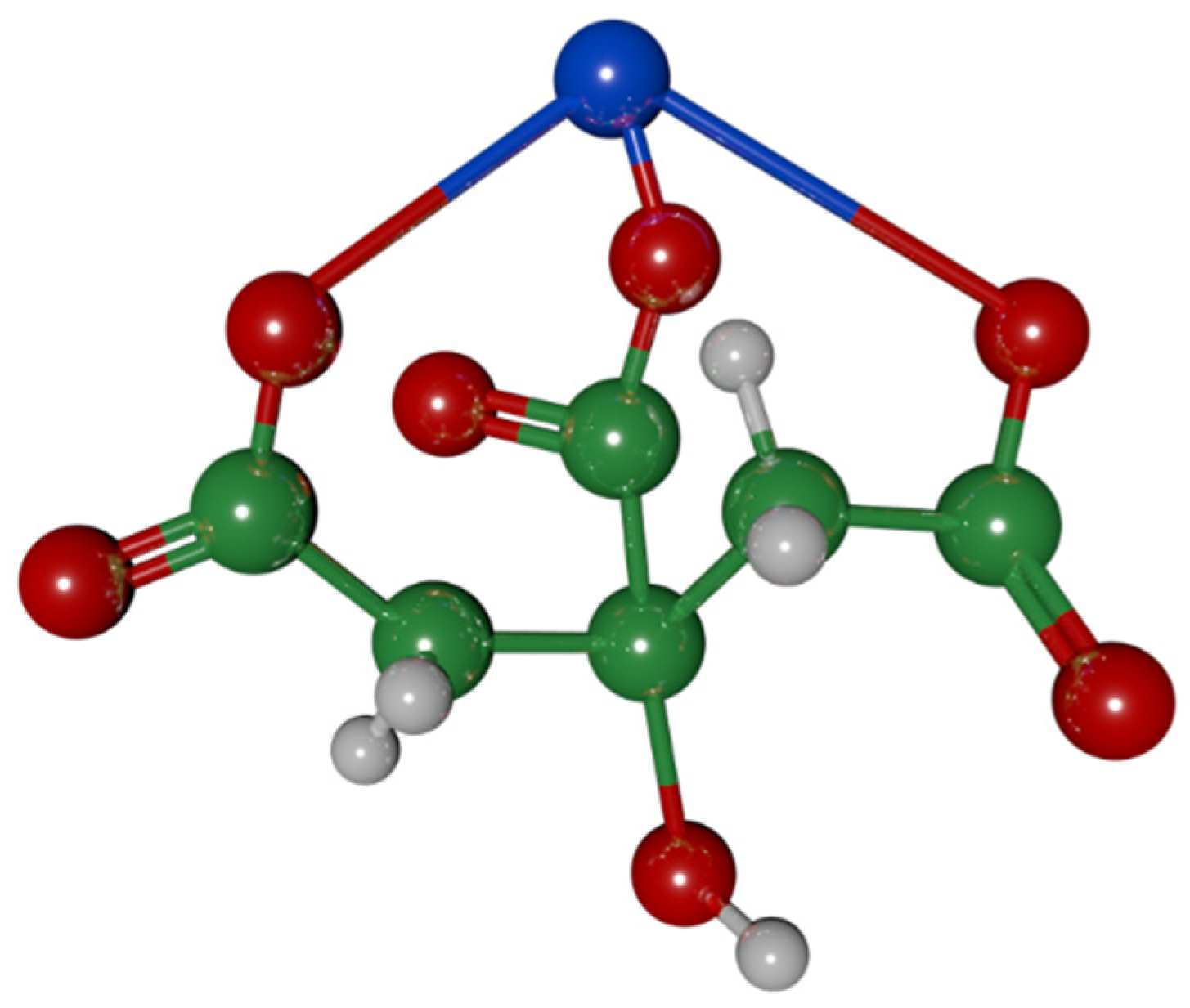
3.1.3. Type of Protective Coating
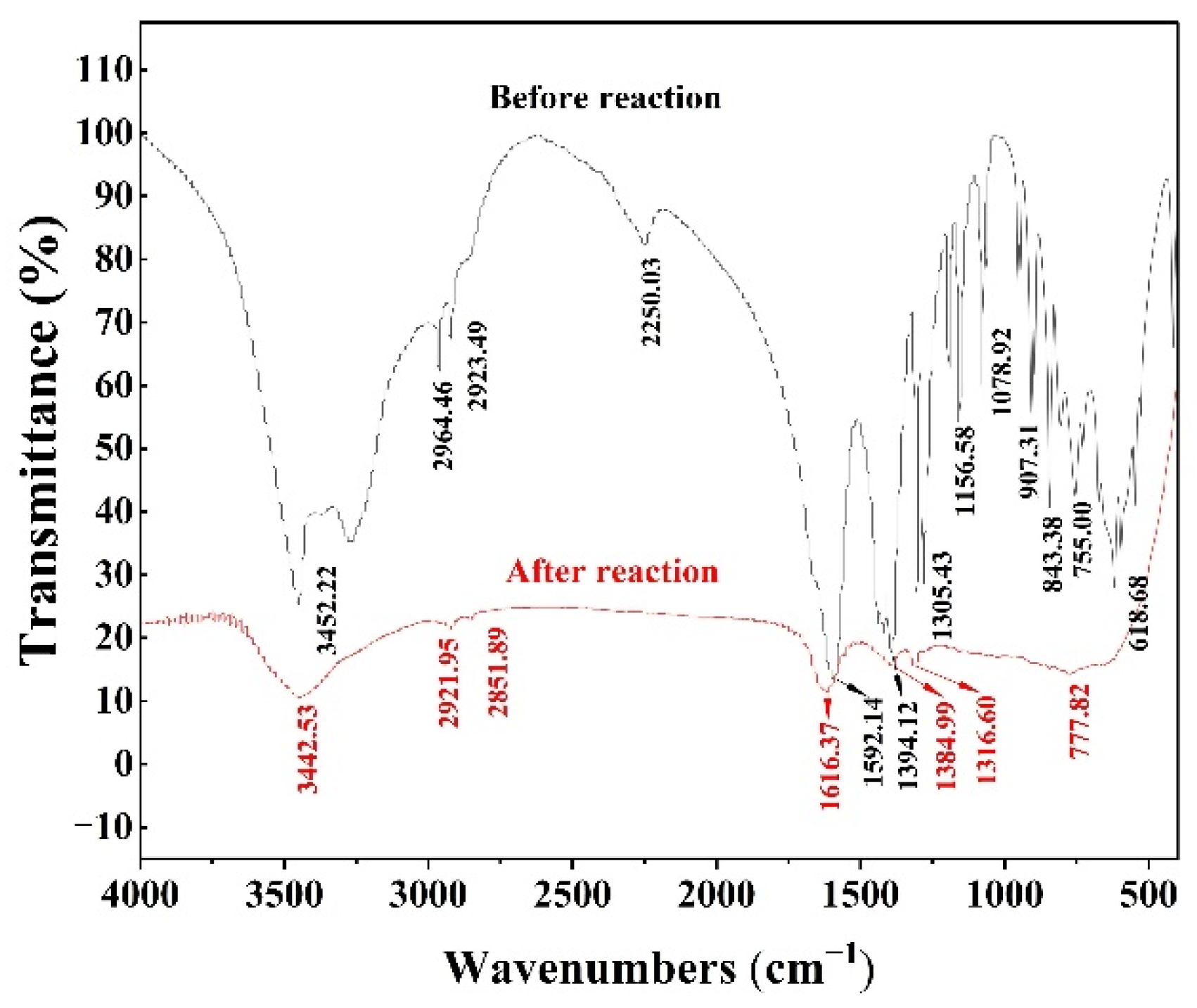
3.1.4. FTIR Data
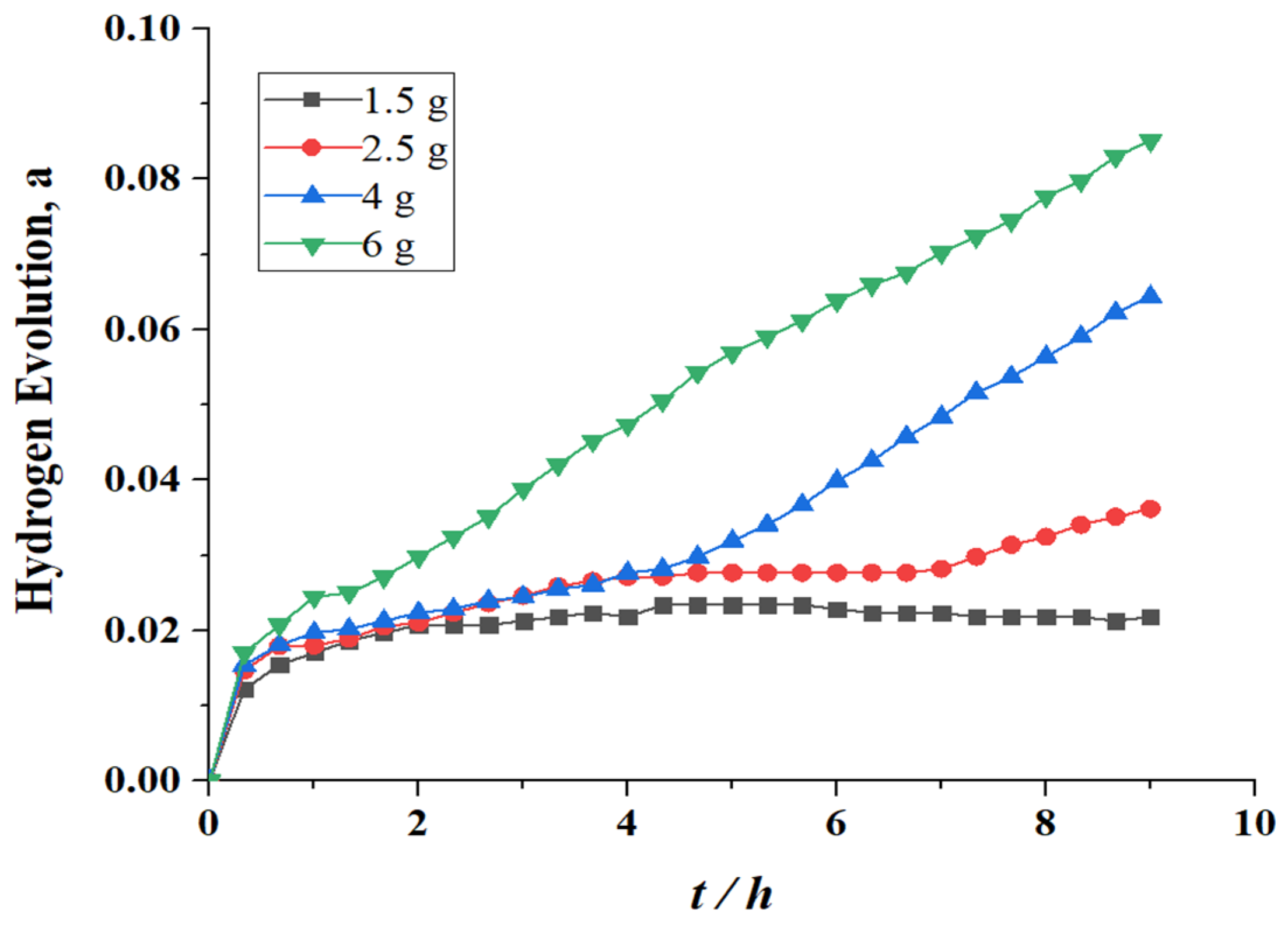
3.2. Influence of Al Particles Content
3.3. Economic Feasibility Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Yang, H.-X.; Yuan, C.-M.; Eckhoff, R. A catastrophic aluminium-alloy dust explosion in China. J. Loss Prev. Process. Ind. 2016, 39, 121–130. [Google Scholar] [CrossRef]
- Zhong, S.-J.; Miao, N.; Liu, H.-Y. Analysis and protection of dust explosion accident in aluminum magnesium polishing process. Mod. Occup. Saf. 2014, 10, 26–29. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Xu, K.-L.; Wang, B.; Zhang, J.-J. Hydrogen inhibition in a wet aluminum dust collection system using dichromate solution. RSC Adv. 2017, 7, 47867–47876. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-T.; Xu, K.-L.; Li, L. Inhibition of the reaction between aluminium dust and water based on the HIM. RSC Adv. 2017, 7, 33327–33334. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-T.; Xu, K.-L.; Wang, B.B.; Wang, Q.-S. Hydrogen inhibition by using Cr(NO3)3·9H2O in the wet dust removal system for the treatment of aluminum dust. Int. J. Hydrogen Energy 2018, 43, 2514–2523. [Google Scholar] [CrossRef]
- Xu, K.-L.; Wang, Y.-T.; Shen, R.-Q.; Wang, Q.-S. Inhibition of hydrogen production reactions in the wet dust removal system using CeCl3solutions. Int. J. Hydrogen Energy 2018, 43, 14859–14865. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, K.-L.; Wang, Y.-T.; Shen, R.-Q.; Wang, Q.-S. Study of hydrogen explosion control measures by using l-phenylalanine for aluminum wet dust removal systems. RSC Adv. 2018, 8, 41308–41316. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Xu, K.-L.; Shen, R.-Q.; Wang, Q.-S.; Wang, Y.-T. Hydrogen inhibition in wet dust removal systems by using calcium lignosulfonate (CLS). Int. J. Hydrogen Energy 2019, 44, 25091–25100. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, K.-L.; Wang, Y.-T.; Wang, Q.-S. Hydrogen inhibition method for preventing hydrogen explosion accident in wet dust removal systems. Int. J. Hydrogen Energy 2019, 44, 17195–17201. [Google Scholar] [CrossRef]
- Wang, B.; Xu, K.-L.; Wang, Y.-T. Using sodium D-gluconate to suppress hydrogen production in wet aluminium waste dust collection system. J. Hazard. Mater. 2020, 397, 122780. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Xu, K.-L.; Zheng, X.; Yao, X.-W.; Wang, Y.-T.; Ge, J. Study of a Hydrogen Inhibition Method with Sodium Tungstate for Wet Aluminum Dust Removal Systems. Coatings 2020, 10, 431. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; Gu, Y.; Song, X.; Zhao, J. Carcinogenicity of chromium and chemoprevention: A brief update. Onco. Targets Ther. 2017, 10, 4056–4079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, B.; Klager, W.; Kubitzki, G. Metal Chelates of citric zcid as corrosion inhibitors for Zinc pigment. Corros. Sci. 1997, 39, 1481–1485. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Asghari, E.; Mohammadi, M. Effects of solution hydrodynamics on corrosion inhibition of steel by citric acid in cooling water. J. Mater. Eng. Perform. 2014, 23, 2992–3000. [Google Scholar] [CrossRef]
- Muller, B. Citric acid as corrosion inhibitor for aluminium pigment. Corros. Sci. 2004, 46, 159–167. [Google Scholar] [CrossRef]
- Li, G.-Y.; Zhang, M.-M.; Wang, W. Study on corrosion inhibition performances of inhibitors on 7075 aluminum alloy. Plat. Finish. 2015, 37, 34–37. [Google Scholar] [CrossRef]
- Zhong, K.-K.; Huang, Z.-G.; Han, X.-J.; Zhang, C.-M. Modification of activated carbon using sodium citrate and its effect on the adsorption of copper ions. New Carbon Mater. 2013, 28, 156–160. Available online: http://www.cnki.com.cn/Article/CJFDTotal-XTCL201302012.htm (accessed on 24 February 2022). [CrossRef]
- Liu, Y.-F.; He, C.-C.; Liu, S.-C.; Wang, X.-H.; Ren, H.-J.; Chen, Q.; Ren, Y. Research on the performance of new citric acid corrosion inhibitor. Appl. Chem. Ind. 2015, 44, 466–469. Available online: http://www.cnki.com.cn/Article/CJFDTotal-SXHG201503021.htm (accessed on 24 February 2022).
- Zhou, N. Study of Corrosion Inhibitors for AZ31 Magnesium in NaCl Solution. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, May 2014. [Google Scholar]
- Wysocka, J.; Krakowiak, S.; Ryl, J. Evaluation of citric acid corrosion inhibition efficiency and passivation kinetics for aluminium alloys in alkaline media by means of dynamic impedance monitoring. Electrochim. Acta 2017, 258, 1463–1475. [Google Scholar] [CrossRef]
- Li, W.-H.; He, Q.; Pei, C.-L.; Hou, B.-R. Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim. Acta 2001, 52, 6386–6394. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.-B.; Lv, C.; Peng, J.-H.; Li, S.-W.; Ma, A.-Y.; Chen, W.-H.; Xie, F. Role of sodium citrate in leaching of low-grade and multiphase zinc oxide ore in ammonia–Ammonium sulfate solution. Hydrometallurgy 2017, 169, 534–541. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Sun, X.-Y.; Pan, Y.-S. Preparation of CaCO3 under the presence of sodium citrate. J. Synth. Cryst. 2012, 41, 539–543. Available online: http://www.cnki.com.cn/Article/CJFDTotal-RGJT201202064.htm (accessed on 24 February 2022).
- Rao, S.-X.; Liu, R.-M.; Zhang, D.-Q.; Liu, Z.-Q.; Si, M.-Z. NIR-SERS studies of sodium citrate and chloride hematin based on NIR-SERS substrate. J. Light Scatt. 2011, 23, 114–119. Available online: http://www.cnki.com.cn/Article/CJFDTotal-GSSX201102005.htm (accessed on 24 February 2022).
- Wang, Y.-B.; Liu, F.-L.; Wang, X.; Yang, L.-Q.; Yuan, Z.-T.; Han, Y.-X. Effect of sodium citrate on the crysta1 shape of hemihydrate calcium sulfate whiskers. Ind. Miner. Process. 2010, 39, 8–10. [Google Scholar] [CrossRef]
- Soliwoda, K.-R.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Li, X. Synthesis of Aluminum Citrate and Kinetics of Crosslinking System between Aluminum Citrate and HPAM. Master’s Thesis, East China University of Science and Technology, Shanghai, China, May 2001. [Google Scholar]
- Hu, J.-J. Calculation research of hydrogen production amount in containment after LOCAL in PWR Nuclear Power Plants. Nucl. Power Eng. 2013, 34, 95–98. [Google Scholar] [CrossRef]




| Inhibitor Type | Inhibitor | Toxic? | Optimal Inhibitory Concentration (g·L−1) | References |
|---|---|---|---|---|
| Precipitation | K2Cr2O7 | Yes | 2.03 | [3] |
| Na2Cr2O7·2H2O | Yes | 2.01 | [3] | |
| Cr(NO3)3·9H2O | Yes | 5.32 | [5] | |
| CrK(SO4)2·12H2O | Yes | 0.0516 | [4] | |
| CeCl3 | Yes | 6.02 | [6] | |
| Na2O·nSiO2 | No | 2.1~3.25 | [9] | |
| Na2WO4 | No | 100 | [11] | |
| Adsorption | C9H11NO2 | No | 24.8 | [7] |
| C20H24CaO10S2 | No | 0.5 | [8] | |
| C6H11NaO7 | No | 0.4 | [10] |
| Concentration of Sodium Citrate Solution, g/L | wt.% | ||
|---|---|---|---|
| Al | O | Mg | |
| 0.005 | 58.2 | 38.8 | 4.0 |
| 0.4 | 95.1 | 1.1 | 3.9 |
| Position and Frequency, cm−1 | Functional Groups |
|---|---|
| 3452.22 | –COOH (OH stretching vibration of carboxylic acid) |
| 2923.49, 2964.46 | –CH2 (CH stretching vibration of methyl) |
| 2250.03 | Noise or baseline calibration |
| 1592.14 | –COOH (C=O stretching vibration of carboxylate) |
| 1394.12 | –CH2 (CH in-plane bending vibration) |
| 1305.43 | –COOH (C–O stretching vibration of carboxylate) |
| 1156.58 | C–C (reverse telescopic vibration) |
| 1078.92 | –OH (C–O stretching vibration) |
| 720~950 | –CH2 (CH out-of-plane bending vibration) |
| 618.68 | OH (out-of-plane bending vibration) |
| Equipment, Facilities or Chemicals | Economic Input/$ |
|---|---|
| Fan | 20,333 |
| Sodium citrate solution | 19 |
| Electrical components | 4006 |
| Energy | 13,333 |
| Total cost | 37,691 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Wang, H.; Hao, T.; Xu, K.; Wang, Y. Hydrogen Inhibition as Explosion Prevention in Wet Metal Dust Removal Systems. Coatings 2022, 12, 349. https://doi.org/10.3390/coatings12030349
Zheng X, Wang H, Hao T, Xu K, Wang Y. Hydrogen Inhibition as Explosion Prevention in Wet Metal Dust Removal Systems. Coatings. 2022; 12(3):349. https://doi.org/10.3390/coatings12030349
Chicago/Turabian StyleZheng, Xin, Huiyu Wang, Tengteng Hao, Kaili Xu, and Yantong Wang. 2022. "Hydrogen Inhibition as Explosion Prevention in Wet Metal Dust Removal Systems" Coatings 12, no. 3: 349. https://doi.org/10.3390/coatings12030349





