Abstract
Ni-Cr-Mo alloy coatings with different content of Cr element were produced on carbon structural steel by laser cladding. Coatings exhibited similar phase composition containing γ-Ni solid solution and similar microstructure composed of primary grains and eutectics with segregated Mo. Corrosion behavior of coatings in a sulfuric acid dew point corrosion environment were investigated through the combination of immersion test, electrochemical measurements, and surface analysis. Coatings exhibited similar passive behavior and passive film with a bilayer structure consisting of a compact inner layer and a porous outer layer. An increase in Cr resulted in a more protective passive film with thicker and more compacting inner layer. However, the corrosion resistance was firstly degraded as the content of Cr increased from 18 wt.% to 22 wt.%, and then improved as the content of Cr increased from 22 wt.% to 26 wt.%. This phenomenon was attributed to the competition effect between the increase in Cr and relative decrease in Ni.
1. Introduction
It is well known that Ni-Cr-Mo alloys, such as Hastelloy C22, C276, and Inconel 625, exhibit excellent corrosion resistance in both oxidizing and reducing environments. These alloys are considered as the optimal candidates for construction or lining materials of the flue gas desulfurization (FGD), back-end ductworks, reheaters, fans, and chimneys in power plants to overcome the severe acid dew point corrosion problems caused by the condensation of flue gas [1,2,3,4,5,6,7,8,9,10,11]. Meanwhile, researchers have conducted plenty of studies that investigate the acid dewpoint corrosion resistance and behavior of Ni-Cr-Mo alloys through laboratory and filed tests [1,2,5,8,12,13]. The excellent corrosion resistance is generally attributed to the protective oxide layer and stable passivation behavior in this aggressive environment. In-depth research has proceeded to investigate the passive behavior and passive film properties of Ni-Cr-Mo alloys [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Lloyd et al. confirmed that the passive film of Ni-Cr-Mo alloys in 1.0 M NaCl + 0.1 M H2SO4 solution consists of an inner Cr/Ni oxide layer and an outer Mo/W oxide layer [16,17,18]. Gary et al. demonstrated the corrosion and passive film properties of C22 alloy in different acid solutions [19]. Jakupi et al. related the impedance properties of passive film on C22 alloy in NaCl solution to the propertied of Cr2O3 barrier layer [20]. Zhang et al. observed a similarly layered structure of passive film on a C-2000 alloy in 5M NaCl solution, and claimed that the transpassive oxidation of Cr(III) to Cr(VI) in the barrier layer of the film and its subsequent dissolution leads to the loss of the Cr2O3-rich inner layer and the destruction of passivity [21,22]. Luo et al. confirmed that the passive film on Alloy 59 in sulfuric acid mainly consists of Cr2O3, Cr(OH)3 and Ni(OH)2 [23]. Li et al. specified the accumulation and dissolution of Cr and Mo in the passive film of Ni-Cr-Mo alloys in sulfuric solution along with the contribution of two elements on the passivity [29,30]. The Cr element is considered as the primary factor in enforcing passivity by lowering both the passivation potential and the passive dissolution current, and the presence of Mo and W in the outer regions of the passive film is thought to suppress transpassive dissolution and localized corrosion [16,17,18,27,28,29,30].
In spite of the excellent corrosion resistance and stable passivity of Ni-Cr-Mo alloys in aggressive environments, the persistently high cost of nickel and molybdenum raw materials restricts the extensive application of Ni-Cr-Mo alloys in FGD systems and other facilities [8,31,32]. Laser cladding is widely approved as an effective and cost-effective technology to obtain comparable surface properties to the same material in bulk [32,33,34,35,36]. Numerous investigations have been made studying the corrosion resistance of laser cladding Ni-Cr-Mo alloy coating in different aggressive environments [37,38,39,40]. In our previous work, the laser cladding C22 coating exhibited comparable corrosion resistance and unique intergranular corrosion in simulated sulfuric acid dew point corrosion environment comparing with a cast C22 alloy [13]. The different corrosion resistance and behavior of laser cladding coating is generally attributed to the dense oxide film, grain refinement and non-equilibrium segregation in grain boundary. However, the effect of alloying elements on the corrosion behavior of laser cladding coating have rarely been investigated, which is conceivably different from the casting or wrought alloys as a result of the unique microstructure and phase composition introduced by laser cladding technology.
The aim of the present study is to clarify the effect of the Cr element on the corrosion behavior of laser cladding Ni-Cr-Mo alloy coating in sulfuric acid dew point corrosion environment. On the basis of the content of the Ni, Cr and Mo elements in Hastelloy C22, Ni-Cr-Mo alloy coatings with different content of Cr element (18 wt.%, 22 wt.% and 26 wt.%) were prepared through coaxial laser cladding technology. The corrosion behavior of prepared Ni-Cr-Mo alloy coatings in 50 wt.% H2SO4 solution at 60 °C was investigated through the combination of immersion test, electrochemical measurements and surface analysis. The experimental results were discussed thoroughly, and the effect of the Cr element on corrosion behavior was revealed in detail.
2. Materials and Methods
2.1. Materials and Laser Cladding Process
The chemical compositions of Ni-Cr-Mo alloy powders with different content of Cr element used for laser cladding are listed in Table 1. The powders with a diameter in the range of 46–150 μm were offered by Beijing General Research Institute of Mining and Metallurgy (BGRIMM), Beijing, China. The substrate for the laser cladding process was Q235 carbon structural steel plate, of which the chemical composition is 0.08 wt.% Mn, 0.37 wt.% Si, 0.16 wt.% Si and Bal. Fe, with 10 mm thickness. The coaxial laser cladding process employed a high-power fiber laser (ZKZM-3000, zKzM Laser Technology Co., Ltd., Xi’an, China) and a self-designed coaxial powder feed nozzle. The high-power fiber laser produces a collimated laser beam with a maximum 3000 W power and a beam spot of 1.4 mm in diameter. The coaxial powder feed nozzle was designed to eject three powder streams from uniformly distributed directions around the laser beam, and the powder streams were formed by argon carrier gas with a flow rate of 5 L/min. A multi-track and multilayer cladding process with an overlap ratio of 40%, and a laser scan speed of 8 mm/s was adopted to prepare 5mm thick coating specimens. The schematic of the multi-track coaxial laser cladding process was shown in our previous work [13]. The laser cladding coating specimens prepared from Ni-Cr-Mo alloy powders containing 18 wt.%, 22 wt.%, and 26 wt.% Cr element were named of Cr18, Cr22, and Cr26, respectively. The chemical composition of three Ni-Cr-Mo alloy powders used for laser cladding is listed in Table 1.

Table 1.
Chemical compositions of three Ni-Cr-Mo alloy powders in wt.%.
The specimens for phase composition and microstructure analysis were cut from as-prepared coatings along with substrates with the upper-surface dimension of 10 mm × 10 mm by a wire cutting machine. The upper surface of coating was polished to 2000 grit Al2O3 paper and 1.5 µm diamond abrasive, then ultrasonically cleaned in distilled water and alcohol. The phase composition and microstructure of the prepared Ni-Cr-Mo alloy coating were analyzed by an X-ray diffractometer (Rigaku D/Max-2400, Tokyo, Japan) and a scanning electron microscope (FEI Quanta 200F, Eindhoven, The Netherlands) equipped with energy dispersive spectroscopy (EDS) (EDAX, Pleasanton, CA, USA), respectively. The upper surfaces of specimens for scanning electron microscope (SEM) observing are etched by the aqua regia and cleaned by distilled water in advance.
The immersion testing specimens were cut from as-prepared coatings without substrates, with a dimension of 20 mm × 10 mm × 3 mm by a wire cutting machine. A 2 mm-diameter hole was drilled on the corner of each specimen, and used to be hung by a PTFE wire in test solution. Prior to immersion testing, each specimen was polished with 1500 grit Al2O3 paper, then ultrasonically cleaned in distilled water and alcohol.
Electrochemical working electrodes were prepared identically to the specimens for phase composition and microstructure analysis. A connecting brass rod was attached to the back of the substrate for electric connection. After being abraded to 400 grit Al2O3 paper and then sequentially ultrasonically cleaned in distilled water and acetone, each working electrode was fixed in epoxy resin with an attached brass rod. The 10 mm × 10 mm-dimension exposed the surface of the working electrode and was abraded to 2000 grit Al2O3 paper and then polished with 1.5 µm diamond abrasive. Prior to electrochemical measurements, each prepared specimen was ultrasonically cleaned in distilled water and alcohol, then stored in glass drying vessel.
To simulate the sulfuric acid dew point corrosion environment in FDG systems and back-end ductworks [3,41,42,43,44], a 50 wt.% H2SO4 solution at 60 °C was adopted as the immersion solution and electrolyte solution. The solution was freshly prepared with deionized water and analytical grade 98 wt.% H2SO4.
2.2. Immersion Testing
The immersion testing designed according to ASTM G1-03 and ASTM G31-2012 was performed to study the corrosion resistance of the laser cladding Ni-Cr-Mo alloy coatings in the 50 wt.% H2SO4 solution [45,46]. The temperature of the testing solution was maintained at 60 °C through a water bath. Triplicate parallel specimens for each test were applied to reduce measurement errors and occasional exceptions. The duration of immersion testing in each condition consisted of six 12-h cycles up to 72 h in total. Specimens were taken out after each testing cycle to measure the mass. The process of removing corrosion products before each measurement adopted 15 vol.% HCl solution as a corrosion product remover solution, according to the chemical cleaning method specified in ASTM G1-03 and ASTM G31-2012 [45,46]. The corrosion rate was employed to evaluate the corrosion resistance, and the calculation formula is expressed as
where R is the corrosion rate in mm·a−1 (mm·year−1), M is the initial mass in g, M1 is the mass after one cycle in g, S is the initial area in cm2, T is the time in hours, and D is the density in g·cm−3 with 8.76 g·cm−3 for Cr18, 8.69 g·cm−3 for Cr22, and 8.63 g·cm−3 for Cr22, The densities of three specimens were calculated according to the proportion of three elements on the basis of 8.69 g·cm−3 for Hastelloy C22. The calculated corrosion rates are only used to compare the corrosion resistance between three specimens.
2.3. Surface Analysis
The surface morphologies and compositions of passive film on specimens after 72-h immersion testing were analyzed using SEM and XPS, respectively. A scanning electron microscope (FEI Quanta 200F, Eindhoven, The Netherlands) equipped with EDS (EDAX, Pleasanton, CA, USA) was applied to observe the surface morphology and elemental composition. Compositions of passive films and chemical states of alloying elements were analyzed through XPS measurements on an X-ray photoelectron spectrometer (K-Alpha, ThermoFisher, Waltham, MA, USA) with a monochromatic Al Kα (1486.6 eV). The X-ray gun was operated at 150 W (15 kV, 10 mA) and the photoelectron take-off angle was 90°. During the experiments, a base pressure was maintained at approximately 5 × 10−9 mbar. High-resolution spectra with an analyzed area of 400 µm2 were recorded at a pass energy of 50.0 eV with an energy step of 0.1 eV, whereas survey spectra were recorded at a pass energy of 150.0 eV with an energy step of 1 eV. The values of measured binding energies were corrected by referencing to C 1s (284.8 eV). A Gaussian–Lorentzian product function and a Shirley background subtraction were employed to fit the spectra by applying Avantage software (version 5.984).
2.4. Electrochemical Measurements
All electrochemical measurements were conducted with an electrochemical workstation (CHI660e, Shanghai, China) in a standard three–electrode cell, using a pure platinum sheet as the counter electrode and a mercurous sulfate electrode (MSE) with a Luggin capillary salt bridge as the reference electrode. The temperature of electrolyte solution during measurement was maintained at 60 °C through a water bath. Open circuit potential (OCP), potentiodynamic polarization curve and electrochemical impedance spectra (EIS) of each specimen was measured. Each measurement was repeated three times and the most representative measuring results were presented for discussion in the present study.
Prior to polarization and EIS measurement, each specimen went through open circuit potential measurement with 10-h duration to achieve a stabilized state and passive film. According to the measured stable OCPs for three materials, potentiodynamic polarization curves were recorded at a scan rate of 0.5 mV·s−1 with the potential ranges of −700 to 700 mVmse. EIS measurements were carried out in the frequency range of 105 to 10−2 Hz with an amplitude of 10 mV peak-to-peak and AC signals at the measured open circuit potential. For analysis of the impedance data, the ZSimpWin program (version 3.60) was used to numerically fit the measured impedance data and establish equivalent electric circuits (EEC).
3. Results and Discussion
3.1. Phase Composition and Microstructure
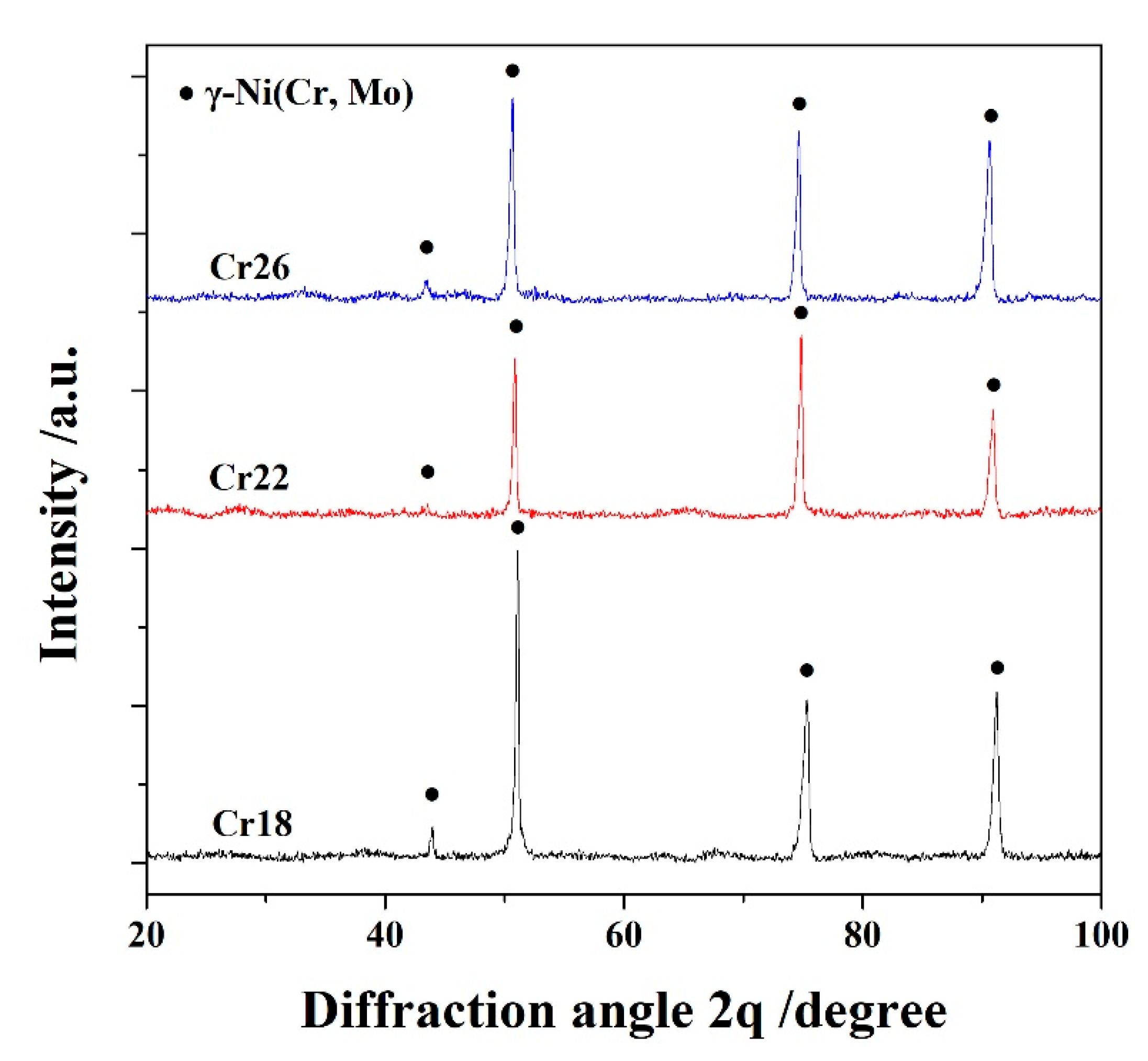
The X-ray diffraction (XRD) patterns of laser cladding Ni-Cr-Mo alloy coatings, named as Cr18, Cr22 and Cr26, are presented in Figure 1. It can be seen that all three specimens contain γ-Ni solid solution as the primary phase. The slight broadening and shift towards a small angle of diffraction peaks of γ-Ni solid solution are attributed to the grain refinement effect of laser cladding and the solution of Cr and Mo elements, respectively [13,37,39]. The absence of an Fe element in the XRD patterns confirms the suppression of diffusion from the substrate resulting from the rapid cooling rate of the laser cladding. It is concluded that the phase composition of the studied Ni-Cr-Mo alloy coatings is independent of the content of the Cr element.
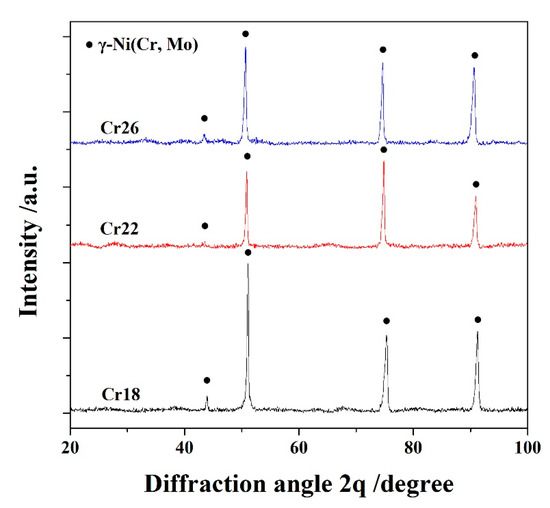
Figure 1.
XRD patterns of laser cladding Ni-Cr-Mo alloy coatings.
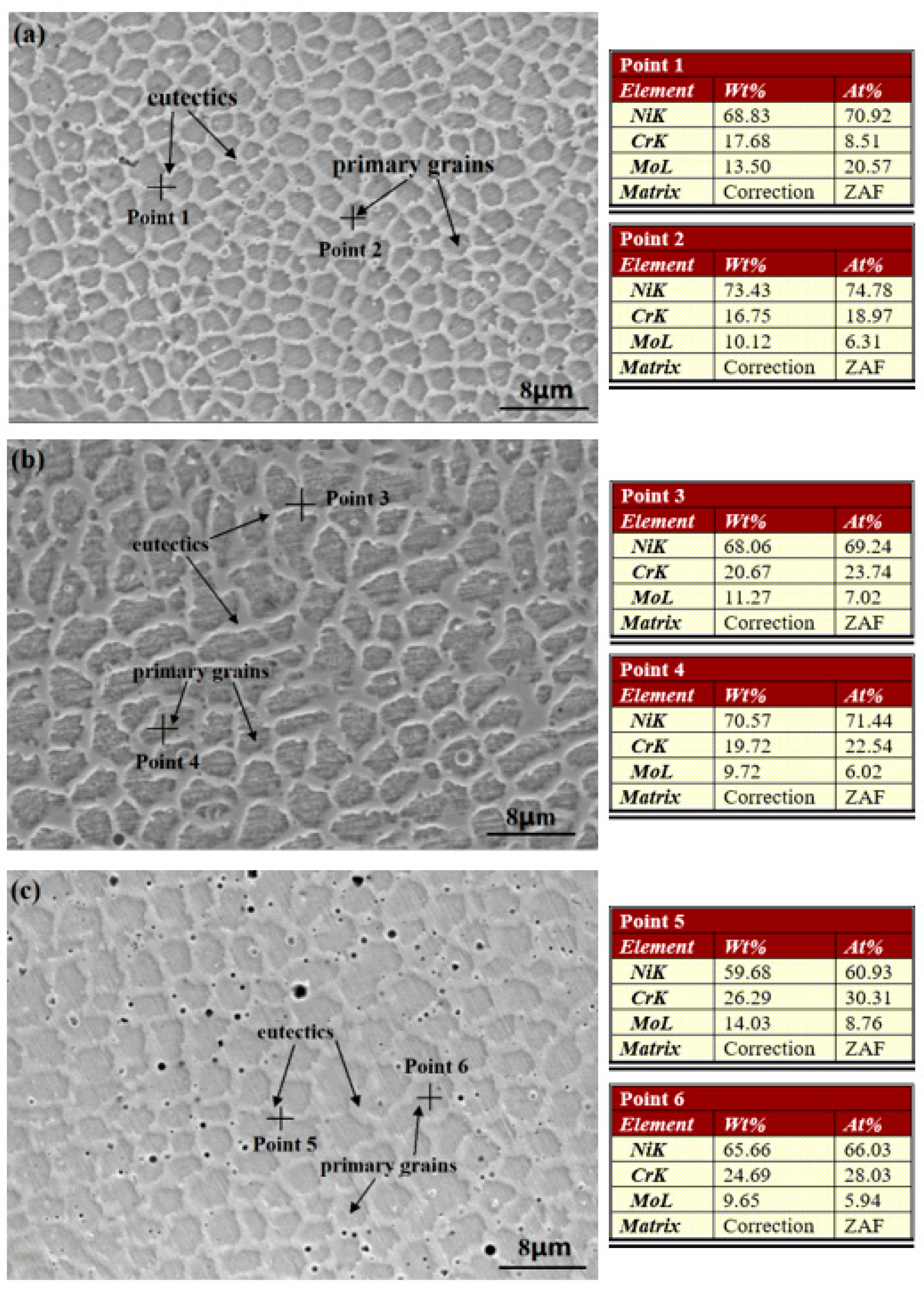
As observed on the microstructure of cross-section not presented here, the distribution of solidification type from the bottom to the top of three coatings is similar to the laser cladding C22 coating, studied in our previous work [13], which is mainly cellular, columnar, and equiaxed in turn. This distribution exhibits the typical solidification behavior of the laser cladding process reported and illustrated by other researchers [13,33,35,37,39]. Therefore, the solidification of laser cladding coatings is not discussed further in the present study. The SEM micrographs and the elemental content analyses of the upper surfaces of the as-prepared Cr18, Cr22, and Cr26 are presented in Figure 2. It could be seen that the microstructure of each specimen is mainly composed of gray primary grains and light eutectics forming on the grain boundaries. The black micropores distributing in the eutectic networks probably due to the shrinkage defects and ablation formed during the laser cladding process. The large constitutional supercooling and rapid cooling lead to the trapping of element diffusion, thus eutectics with different element compositions form on the grain boundaries and result in the microstructure with intercrystalline segregation [13,37,39]. As shown by the elemental content analyses of three specimens in Figure 2, the Mo element accumulates in the eutectics (Point 1 in Figure 2a, Point 3 in Figure 2b, and Point 5 in Figure 2c marked by crosses), while the Cr element nearly uniformly distributes in the primary grains (Point 2 in Figure 2a, Point 3 in Figure 2b, and Point 6 in Figure 2c marked by crosses) and eutectics. The difference in the content of the Cr element in three specimens is basically consistent with the content of the alloy powders. It is notable that the amount of black micropores in Cr26 is obviously bigger than that of the other two specimens. This phenomenon is attributed to relatively lower content in the Ni element performing as the host atoms of the solid solution. There is no considerable variation in the size of the primary grain and eutectics with the increase in content of the Cr element.
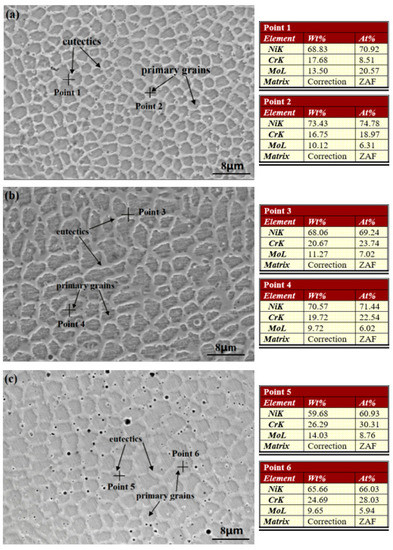
Figure 2.
Surface Morphologies and elemental content analysis of laser cladding Ni-Cr-Mo alloy coatings: Cr18 (a), Cr22 (b), and Cr26 (c).
3.2. Corrosion Rate
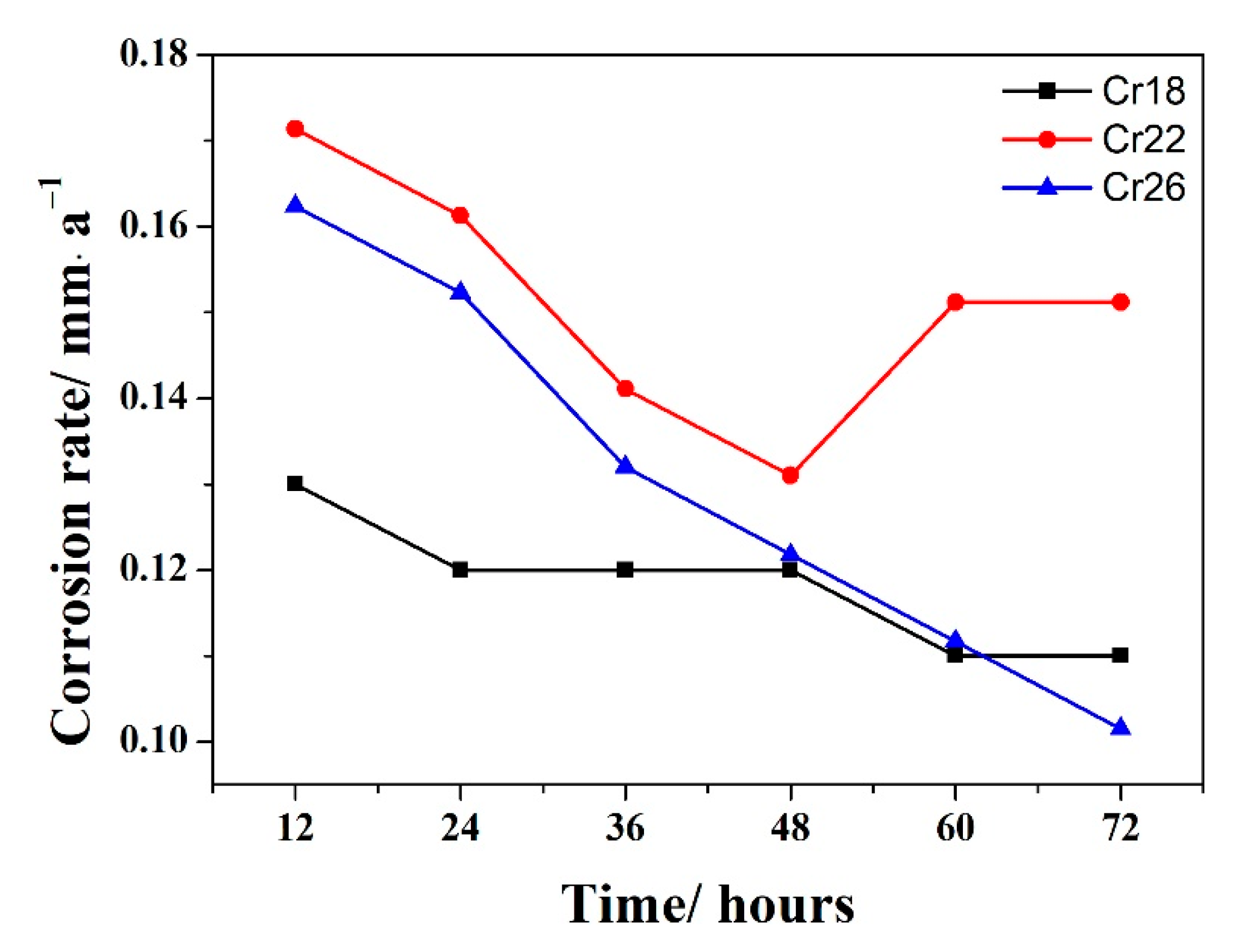
The corrosion rate versus time curves of Cr18, Cr22, and Cr26 in 50 wt.% H2SO4 solution at 60 °C are presented in Figure 3. It could be seen that the corrosion rates of three specimens locate at an extremely low scale in the region of 0.1–0.18 mm·a−1, exhibiting the good corrosion resistance in the studied environment. The Cr22 performs a slightly higher corrosion rate than the other two specimens during the whole immersion testing, while the Cr26 performs a slightly higher and lower corrosion rate than Cr18 in the early period and the ending of immersion testing, respectively. The higher corrosion rate of Cr26 in the early period is attributed it having more micropores than Cr18. The corrosion rates of all three specimens exhibit a similar decrease with time in the early period, although with a different variation trend in the late period. The corrosion rates of both Cr18 and Cr22 stabilize after 62 h, however, the latter one firstly increases and then stabilizes after the early decrease. The corrosion rate of Cr26 continues to decrease and reach a lower value than that of Cr18 by the end of the test.
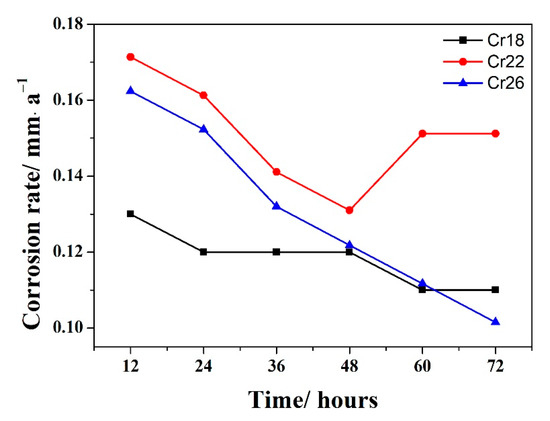
Figure 3.
Corrosion rate (R) versus time curve of laser cladding Ni-Cr-Mo alloy coatings in 50 wt.% H2SO4 solution at 60 °C.
The decrease in corrosion rate in the early stage is attributed to the dissolution of the air-formed oxide film and/or exposed base metal, and sequential formation of a productive passive film [13,47,48]. The stabilizing in the late period is related to the equilibrium between the dissolution and solution of passive film retarding the corrosion to a nearly constant rate [47]. Correspondingly, the continual decrease for Cr26 indicates that the passive film keeps formatting and/or thickening during the whole duration of the test. The sudden increase with Cr22 before stabilizing is thought to be due to the localized deterioration of the passive film and/or incomplete removal of corrosion products [12,49,50]. It is concluded that Cr22 exhibits a poorer corrosion resistance compared to the other two specimens, and that Cr26 exhibits a slightly better corrosion resistance compared to Cr18. The corrosion resistance of three specimens is dependent on the properties of forming the passive film, which will be discussed in the following sections.
3.3. Surface Morphology after Immersion Testing
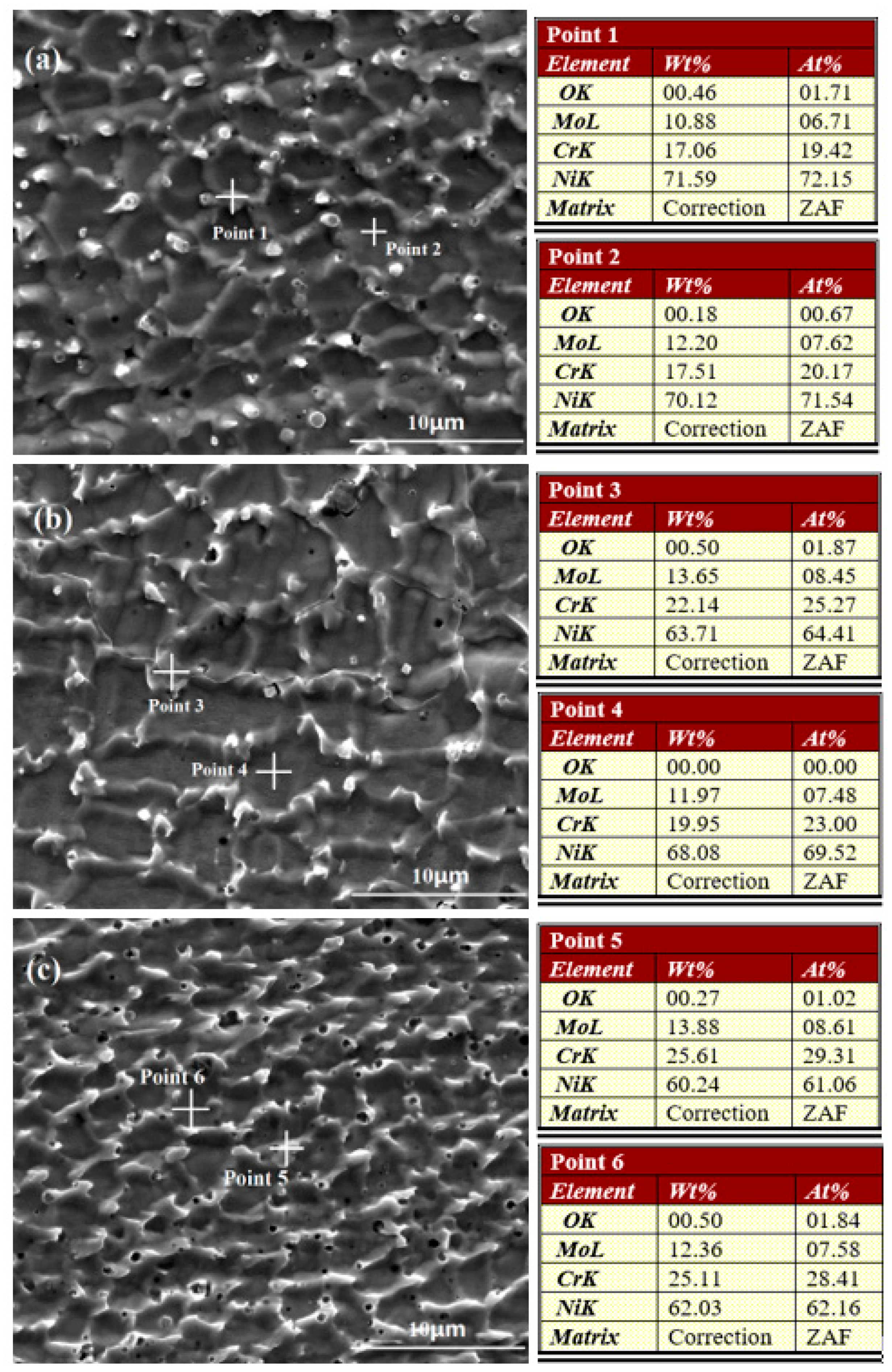
The surface morphologies and elemental content analyses of Cr18, Cr22, and Cr26 after 72 h immersion testing in 50 wt.% H2SO4 solution at 60 °C are presented in Figure 4. It could be seen that the corrosion of all the three specimens performs as the preferential dissolution of primary grains and the pitting corrosion initiates at the micropores. There is also dissolution of eutectics occurring in three specimens, and the dissolution gradually increasing from the interior to the edge of eutectics. In addition, the dissolution of eutectics increases as the content of Cr element increases, presenting as the most fragmentary eutectics networks on Cr26. Nevertheless, it is hard to compare the corrosion degree between three specimens through the morphologies.
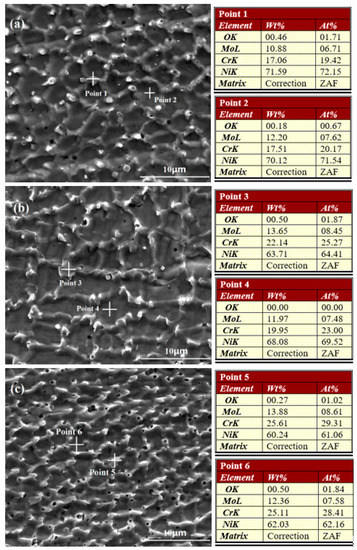
Figure 4.
Corrosion Morphologies and elemental content analysis of Ni-Cr-Mo alloy coatings after immersion testing: Cr18 (a), Cr22 (b), and Cr26 (c).
When comparing with the elemental content analyses of as-prepared specimens (Figure 2), it could be seen that the content of the Ni element in the primary grains of the three specimens (Point 2 in Figure 4a, Point 4 in Figure 4b, and Point 6 in Figure 4c marked by crosses) slightly decreases after immersion testing. Meanwhile, the content of each element in the eutectics of three specimens remains nearly unchanged, except a similar slight decrease in the content of the Ni element of individual eutectics like Point 4 in Figure 4b. This variation indicates the preferential depletion of the Ni element in primary grains performs as the main mass loss behavior. Moreover, the O element is detected in both the eutectics and primary grains of the three specimens, indicating that there is protective oxide film forming on the surface to restrain the corrosion. The observed preferential dissolution of primary grains is quite different with the intergranular corrosion performing as the preferential dissolution of eutectics reported by other researchers on the laser cladding C22 alloy coating [13,40]. It is speculated that the lack of other elements, just like Co, W, and Fe, in studied Ni-Cr-Mo alloy coatings results in the degradation of corrosion resistance of primary grain. The preferential dissolution of primary grains could be explained through the micro-scale corrosion cell system. A potential difference exists between the prime grains and the eutectics as a result of the compositional heterogeneity, which establishes micro-batteries between the prime grains and the eutectics and accelerates the dissolution of primary grain with lower potential [5,48,49].
3.4. Composition of Passive Film
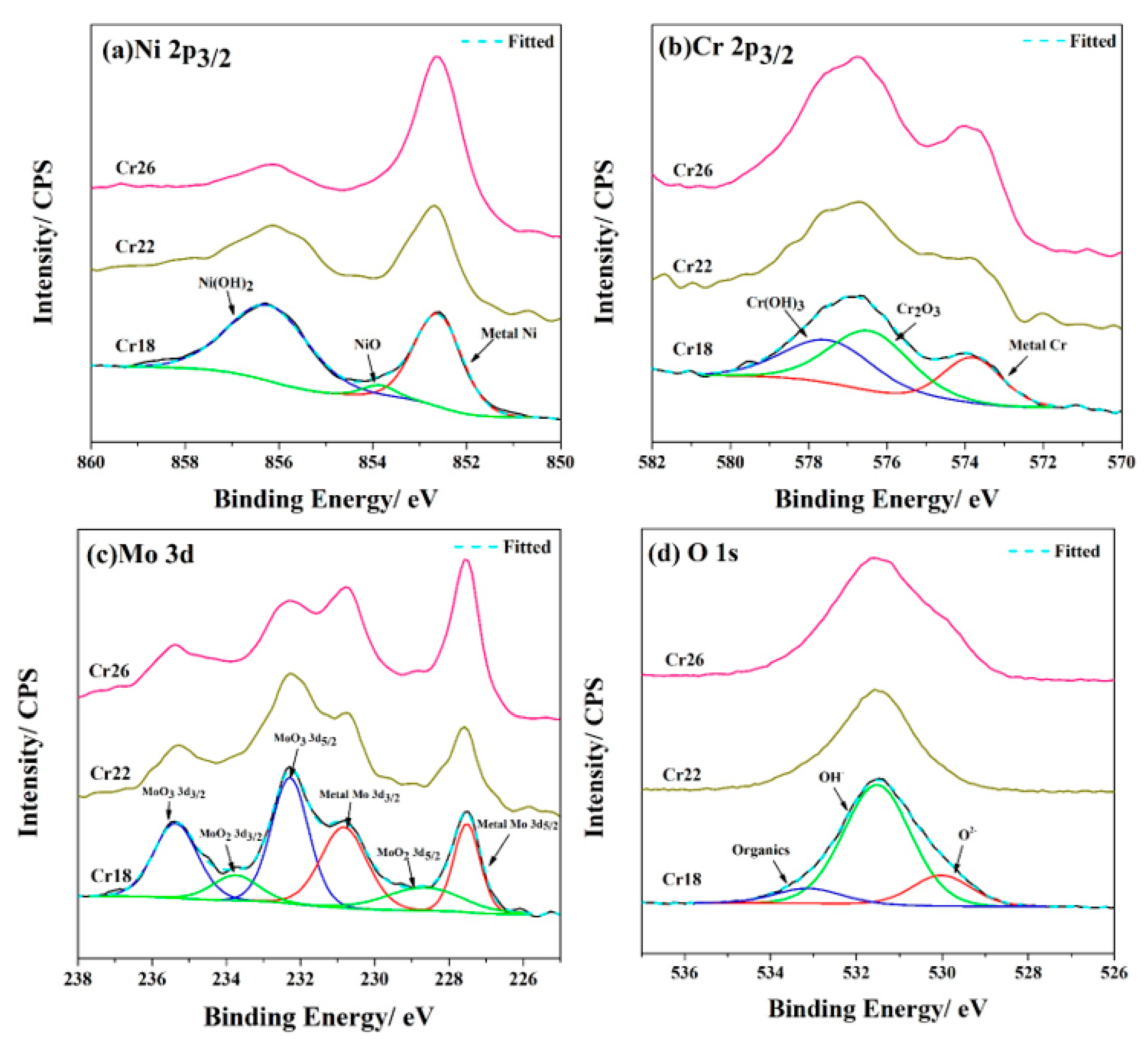
The passive films formed on the Cr18, Cr22, and Cr26 after 72h immersion testing in 50 wt.% H2SO4 solution at 60 °C were characterized through XPS. The analyses were performed according to the binding energy published in the NIST X-ray Photoelectron Spectroscopy Database. High-resolution spectra of Ni 2p3/2, Cr 2p3/2, Mo 3d, and O 1s were registered to determine the chemical state. The curve fits for the high-resolution spectra of three specimens are presented in Figure 5.
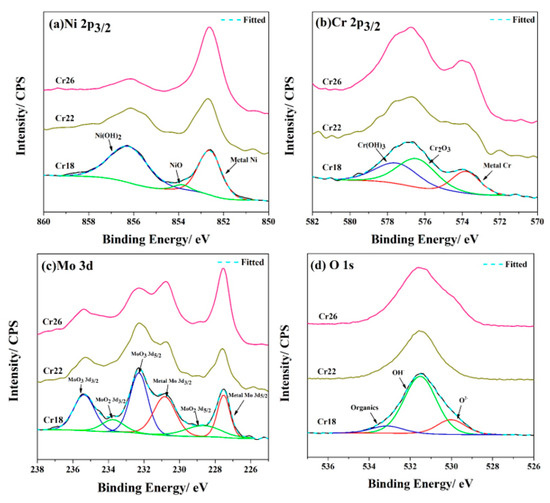
Figure 5.
High-resolution XPS profiles for Ni 2p3/2 (a), Cr 2p3/2 (b), Mo 3d (c), and O 1s (d) of the surface of Ni-Cr-Mo alloy coatings after immersion testing.
As Figure 5 shows, the passive films of three specimens possess similar composition consisting primarily of dense oxides and porous hydroxides of Ni, Cr, and Mo—specifically, NiO, Cr2O3, MoO2, MoO3, Ni(OH)2 and Cr(OH)3. Thereinto, Ni(OH)2, Cr2O3, and MoO3 were the primary oxidized species of Ni, Cr, and Mo elements, respectively. In addition, there were organics, OH−, and O2− as the primary species of the O element. The organics were introduced for C calibration during XPS detection. These observations are in agreement with the results reported by other researchers on Ni-Cr-Mo alloys, in which the passive film exhibited a bilayer structure consisting of a Cr2O3-dominated compact inner layer and a porous outer layer containing oxides of Mo and hydroxides of Ni and Cr [17,23,25,51,52,53,54,55]. However, the structure of passive film on the studied Ni-Cr-Mo alloy coatings needs to be confirmed through combining the results of EIS measurements. As the content of the Cr element in the coatings increases, the relative content of the Ni and Cr element, excluding the metallic state, in the passive film determined by the survey spectra of three specimens (not shown here) exhibits a drastic decrease and increase, respectively. It is speculated that the oxidized species of Cr replace those of Ni in the passive film. Meanwhile, there is an obvious decrease in relative content of Ni(OH)2, which implies the compacting of the passive film. The decrease in relative content of OH− supports this speculation. These monotonic variations in the passive film composition are not consistent with the variation in corrosion resistance presented by the result of immersion testing. This contradiction will be discussed in the following sections, in combination with the results of electrochemical measurements.
3.5. Open Circuit Potential (OCP)
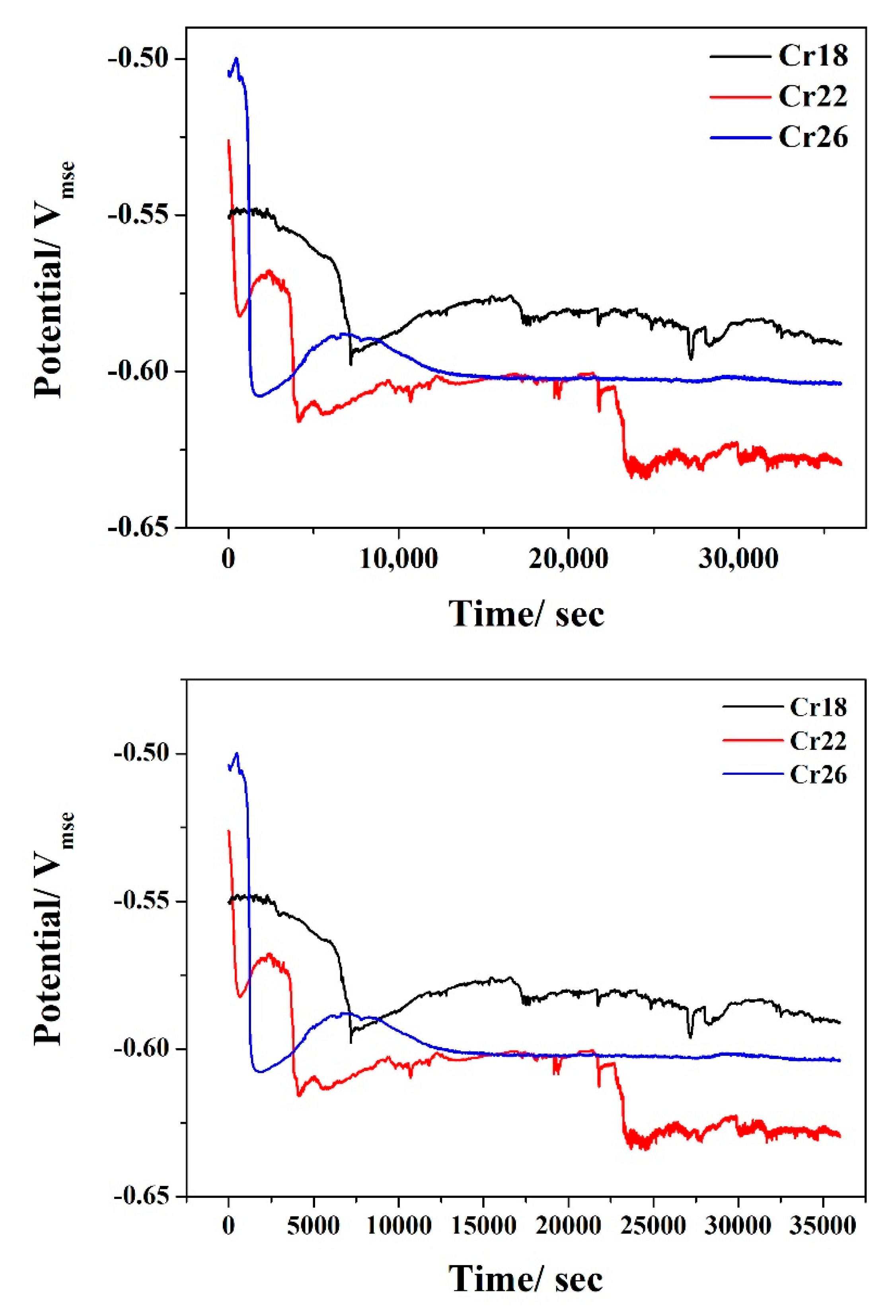
The OCP vs. time curves of Cr18, Cr22, and Cr26 in 50 wt.% H2SO4 solution at 60 °C are presented in Figure 6. It can be seen that there is nearly similar variation trends of OCP with time exhibited by three specimens. The negative shift in the early period indicates the degradation of air-formed oxide film and initiation of localized corrosion at micropores [40,56,57,58]. The highest extent of shifting on OCP of Cr26 is consistent with the highest number of micropores shown by the surface morphologies before and after immersion testing. Distinguishingly, a second negative shift occurs in the OCP of Cr22 after 22,500 s, which is attributed to the degradation of the formed passive film. The stabilization of the OCP after a slight positive shift in the late period indicates the formation of passive film and subsequent equilibrium between the dissolution and growth [40,52,59]. As the stable OCP values in the late period present, the Cr22 and the Cr18 display the highest and the lowest corrosion tendency, respectively. However, the difference is not remarkable in consideration of the approximate 50mV gap between the stable OCPs of Cr22 and Cr18.
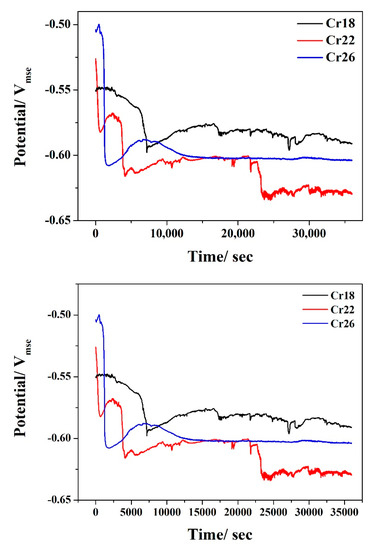
Figure 6.
Open circuit potential of Ni-Cr-Mo alloy coatings in 50 wt.% H2SO4 solution at 60 °C.
3.6. Potentiodynamic Polarization Measurement
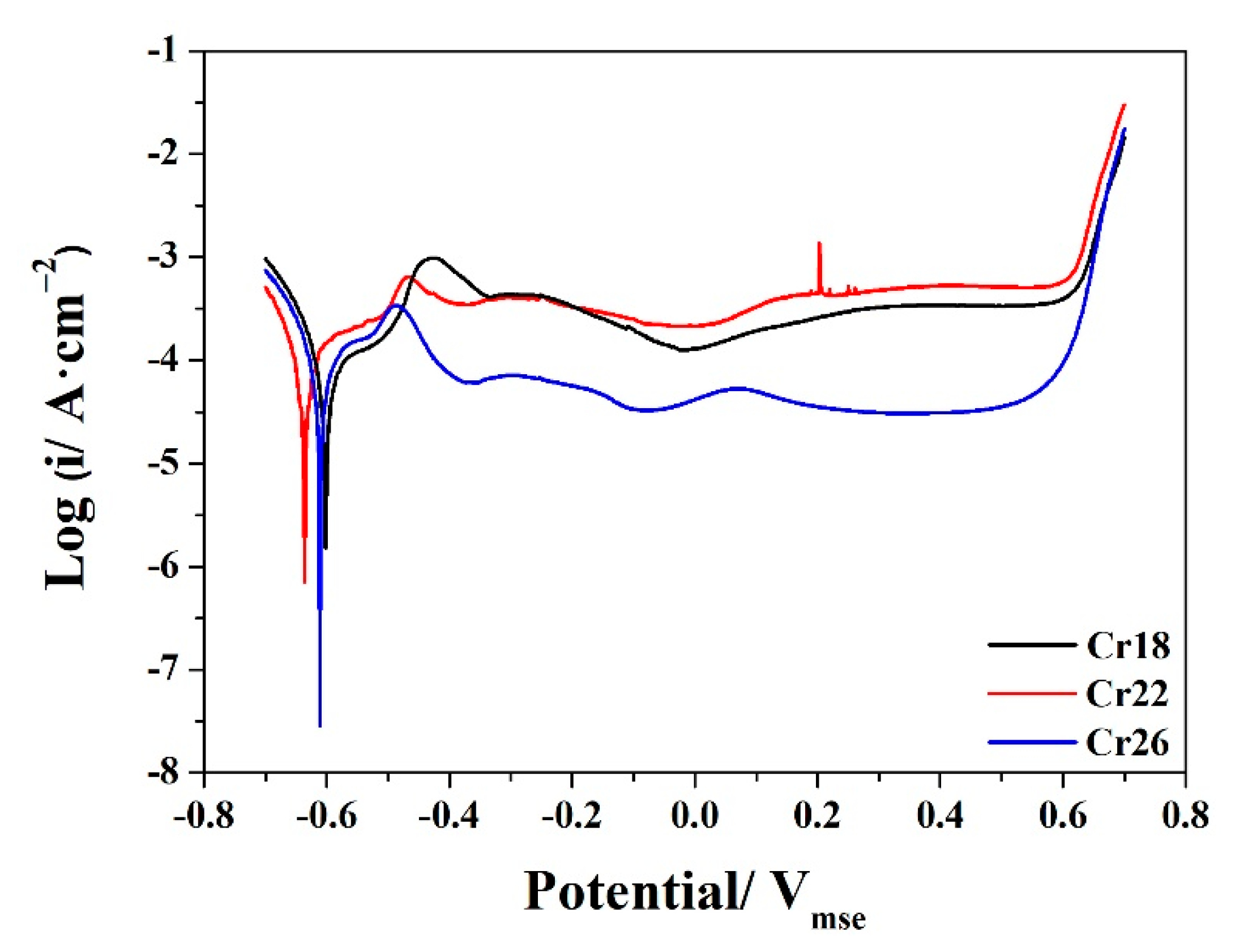
The potentiodynamic polarization curves of Cr18, Cr22, and Cr26 in 50 wt.% H2SO4 solution at 60 °C after OCP measurements are presented in Figure 7. All three specimens exhibit typical passivation behavior along with the active region, active–passive transition region, passive region and transpassive region, which is similar to that reported by other researchers for Ni-Cr-Mo alloys in sulfuric acid solutions [23,40,43,50]. The existence of active–passive transition and locating of stable OCP at an active region of each specimen indicate the spontaneous active behavior of studied Ni-Cr-Mo alloy coatings [12,60,61,62]. The corrosion potential of Cr18 and Cr26 locates at a similar value, which is slightly higher than that of Cr22. The current densities of Cr18 and Cr26 in the active region are nearly similar, although clearly lower than that of Cr22. The passive current density of Cr22 remains at a slightly higher value than that of Cr18, while that of Cr26 is much lower than that of the former two. Three specimens possess similar passive region and transpassive potential. The step in the active region and fluctuation in the passive region of each specimen indicates there is transformation on the passive film formed during OCP measurement, which is attributed to the bilayer structure.
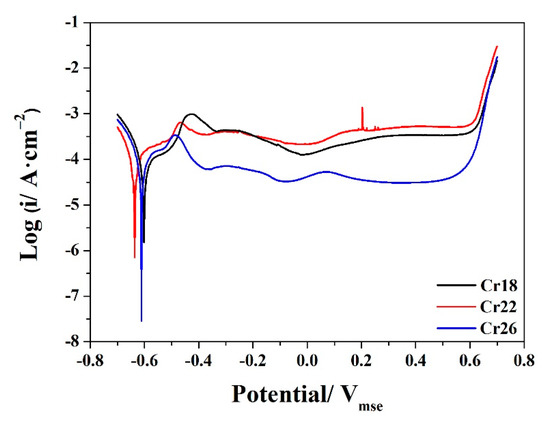
Figure 7.
Potentiodynamic polarization curves of Ni-Cr-Mo alloy coatings in 50 wt.% H2SO4 solution at 60 °C.
By combining the results of the OCP measurements and potentiodynamic polarization measurement, it is concluded that the increase in the content of the Cr element from 18 wt.% to 22 wt.% facilitates the corrosion tendency and corrosion reaction rate, whereas the increase from 22 wt.% to 26 wt.% suppresses the corrosion tendency and corrosion reaction rate. Meanwhile, the later variation in the Cr element clearly promotes the passive ability, presenting as the decrease in anodic current density due to the ennoblement of passive film and suppressed electrochemical reaction. It is speculated that the relative decrease in the content of the Ni element dominates the influence on corrosion behavior when the content of the Cr element increases from 18 wt.% to 22 wt.%, and the further increase in the content of Cr element dominates instead by promoting the passivity. The spontaneous active behavior of the three specimens in a studied environment leads to the competitive effect of the Ni element and Cr element on the variation in corrosion behavior. This phenomenon will be further specified by combining the results of EIS in the following section.
3.7. Electrochemical Impedance Spectroscopy (EIS)
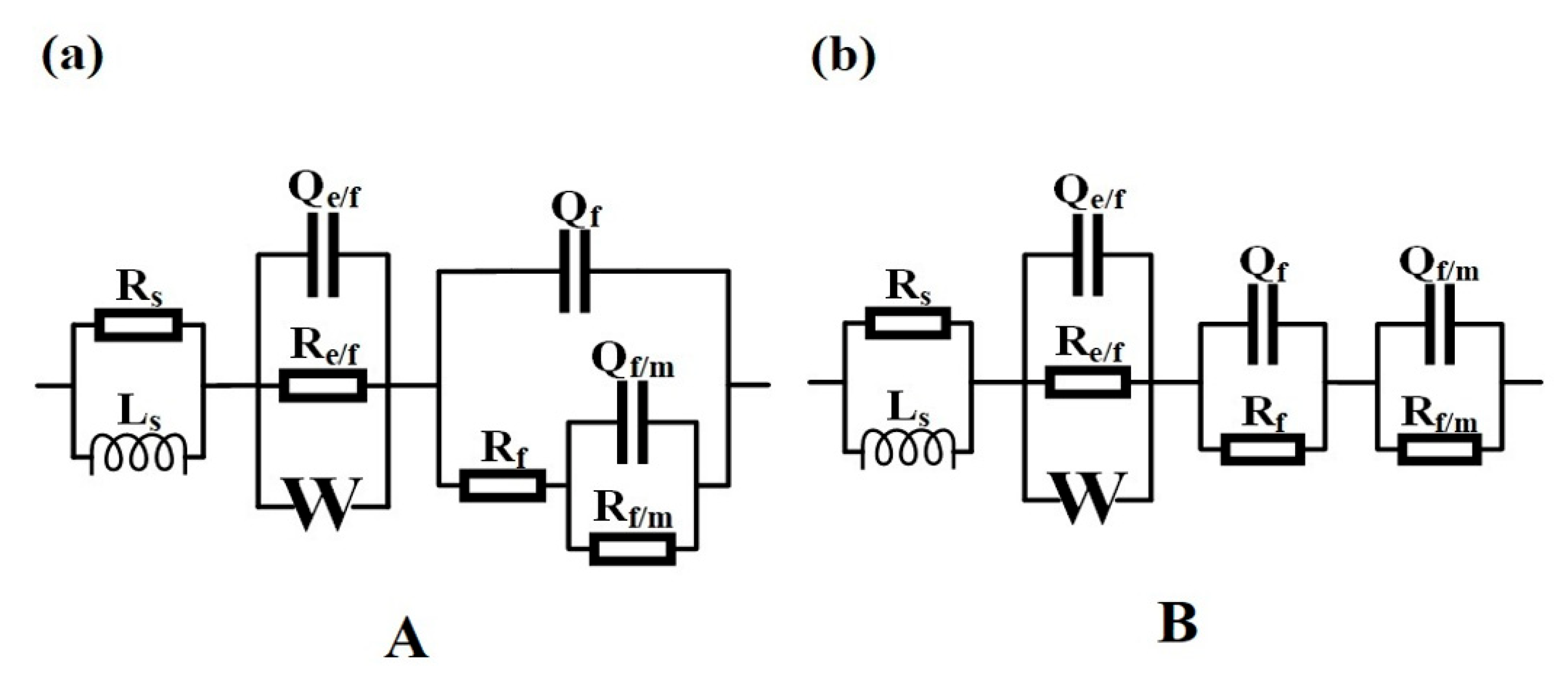
The measured EIS spectra of Cr18, Cr22, and Cr26 in 50 wt.% H2SO4 solution at 60 °C after OCP measurements are presented in Figure 8. There are two different equivalent electric circuits (EEC) used for analysis and fitting the EIS spectra, which are presented in Figure 9. An impedance response model consisting of three time constants is adopted to construct EECs in this study [20,24,59], expressed as:
where Ze/f (Re/fCe/f), Zf (RfCf), and Zf/m (Rf/mCf/m), represents the impedance for electrolyte/film interface, film, and film/metal interface. Considering the phase angle modulus is lower than 90° and the depression of the capacitive loop, a constant phase element CPE (Q) is used instead of the ideal capacitor C. This non-ideal behavior is attributed to various factors as surface heterogeneity, roughness, grain boundary, corrosion products adsorption and formation of porous layer [23,56]. Rs represents the solution resistance of electrolyte. Ls connected in parallel with Rs represents the inductive behavior in the high frequency region (4.5 × 104 Hz to 1 × 105 Hz), which is associated with the inductance of an electrochemical testing system.
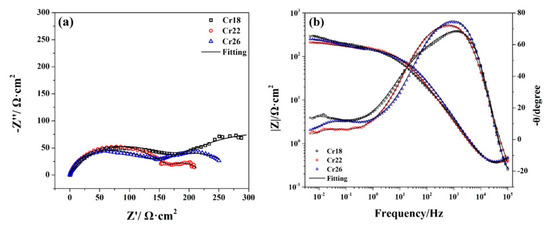
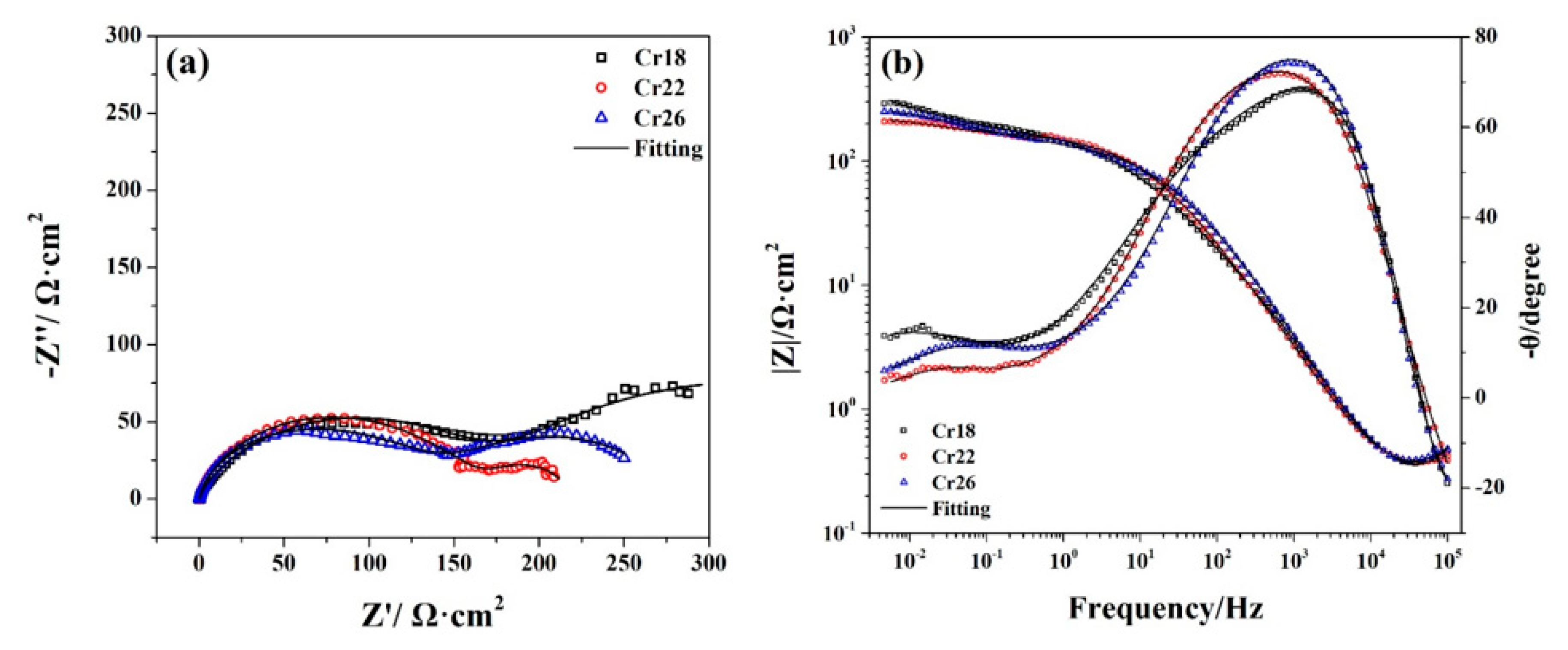
Figure 8.
Nyquist plots (a) and Bode plots (b) of Ni-Cr-Mo alloy coatings at OCP in 50 wt.% H2SO4 solution at 60 °C.
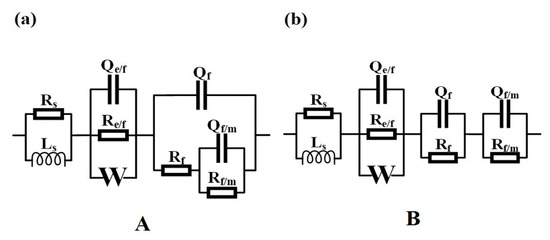
Figure 9.
Equivalent electric circuits (EEC) for the Ni-Cr-Mo alloy coatings at OCP in 50 wt.% H2SO4 solution at 60 °C: EEC A (a) and EEC B (b).
As Figure 8 shows, there is a similar depressed capacitive arc covering the high frequency region in the Nyquist plots of each specimen, which is associated with the capacitive response of the passive film. Meanwhile, there is a broad phase angle peak covering the corresponding high frequency region in the Bode plots of each specimen, especially the two shoulders presented by the phase angle peak of Cr18. This indicates the existence of two time constants associated with the bilayer structure of the passive film [56,61]. Thus, it can be confirmed that the passive film of the studied Ni-Cr-Mo alloy coatings obtains a bilayer structure consisting of a Cr2O3-dominated compact inner layer and a porous outer layer containing oxides of Mo and hydroxides of Ni and Cr. The Nyquist and Bode plots of three specimens present two slightly different shapes in the low frequency region. Whereas, there are two slightly different shapes presented by EIS spectra of three specimens in the low frequency regions. An incomplete capacitive arc with a larger diameter covers the low frequency region in the Nyquist plots of Cr18, while a depressed capacitive arc with a smaller diameter for Cr22 and Cr26. Meanwhile, there is a higher phase angle peak located at the lower frequency along with higher impedance modulus covering the low frequency region in the Bode plots of Cr18. This difference implies the transition of surface state and electrochemical behavior as the content of the Cr element in the coating increases from 18 wt.% to 22 wt.%.
Thus, two types of EEC are necessary to fit plots and reveal the changes in electrochemical behavior, as EEC A relating to the response of Cr18 and EEC B to Cr22 and Cr26 show in Figure 9. All the measured EIS spectra can be well fitted using the related EEC as shown in Figure 8. The similar series connection of Re/fCe/f and RfCf is related to the impedance response in high frequency region, indicating the effect of bilayer structure of the passive film on the electrochemical behavior. The Re/fCe/f represents the charge transfer process through the electrolyte/film interface in the defects of the porous outer layer, and the RfCf represents the electrochemical reaction within the inner layer [20,23,47,63,64,65]. A Warburg impedance (W) connected in parallel with Re/fCe/f is included to represent the reaction product diffusion through the electrolyte or porous outer layer, which was proposed by other researchers in studying the passive behavior of the Ni-Cr-Mo alloys [20,23,61]. No reasonable EEC fitting to measure the EIS spectra could be achieved without the inclusion of the Warburg element. The Rf/mCf/m represents the behavior at the metal/film interface related to the impedance response in the low frequency region, and the connection types between the Rf/mCf/m and other impedance elements in EEC A and EEC B are different. The series connection of Rf/mCf/m and Rf presented by EEC A indicates that the metal surface of Cr18 is exposed due to the incomplete passive film on Cr18, while the series connection of Rf/mCf/m and RfCf presented by EEC B indicates that the metal surface of Cr22 and Cr26 are overcovered by the passive film leading to the existence of metal/film interface [40,51,66]. This phenomenon suggests that the increase in the content of the Cr element improves the compactness of the passive film and localized corrosion resistance.
In considering the electrochemical behavior and the passive film structure of the three specimens, the fitted results of Re/fCe/f, RfCf and Rf/mCf/m are listed in Table 2. It could be seen that the resistance and capacitance of each RC element exhibit different variations with the increase in the content of the Cr element in the coating. The decrease in both Re/f and Qe/f indicates the change in the composition of the porous outer layer [63], which is consistent with the decrease in Ni(OH)2 presented by the XPS analysis. Meanwhile, the decrease in Re/f indicates the facilitation of the charge transfer progress through the electrolyte/film interface. The increase in Rf accompanying the decrease in Qf suggests that the inner layer becomes thicker and more protective, resulting from the more oxidized species of Cr in the passive film [20,23,65]. The drastic decrease in Rf/m and increase in Qf/m as the content of the Cr element increases from 18 wt.% to 22 wt.% resulted from the transformation of the state of metal/film interface. The metal surface of Cr18 is exposed to electrolytes due to the incomplete passive film, while the metal surface of Cr22 is overcovered by passive film. The decrease in nf/m from 0.9853 to 0.4173 synchronously confirms this transformation. According to the resistance of each RC element, it is speculated that the controlling behavior of the corrosion of Cr18 is the charge transfer process through the electrolyte/film interface in the defects of the porous outer layer and the electric double layer on the exposed metal surface, however, with Cr22 and Cr26, it is the combination of the charge transfer process and the electrochemical reaction of passive film.

Table 2.
EIS fitted results for Cr18, Cr22, and Cr26 in 50 wt.% H2SO4 solution at 60 °C.
By comprehensively studying the results of immersion testing, surface analysis and electrochemical measurements, it is confirmed that the Cr element performs a different effect on the corrosion behavior of laser cladding Ni-Cr-Mo coatings under different content of the Cr element in the coating. As the content of the Cr element increases from 18 wt.% to 22 wt.%, comparing between Cr18 and Cr22, there is a degradation in the corrosion resistance presented by the higher corrosion rate, more negative OCP and corrosion potential, and slightly higher passive current density of Cr22. It is attributed to the effect of relative decrease in the content of the Ni element, which results in the decrease in Ni(OH)2 in the porous outer layer of passive film leading to the facilitation of charge transfer progress. In spite of the increase in content of the Cr element leading to the thicker and more protective inner layer of the passive film, the consequent improvement in corrosion resistance is not a match for the degradation resulted from the decrease in the content of the Ni element. As the content of the Cr element increases from 22 wt.% to 26 wt.%, comparing between Cr22 and Cr26, there is an improvement in the corrosion resistance presented by the lower corrosion rate, more positive OCP and corrosion potential, and much lower passive current density of Cr22. This opposite variation in corrosion resistance is attributed to the predominant effect of increase in the content of the Cr element on the passive film properties. It is concluded that the increase in the content of the Cr element results in the more protective passive film of laser cladding Ni-Cr-Mo alloy coatings by thickening and compacting the inner layer. However, the presented corrosion resistance depends on the competition with the effects of the relative decrease in the content of the Ni element. In addition, it is speculated that there is critical content of the Cr element, beyond which the corrosion resistance of the laser cladding Ni-Cr-Mo alloy coatings exhibits considerable improvement.
4. Conclusions
Ni-Cr-Mo alloy coatings with different content of Cr element (18 wt.%, 22 wt.%, and 26 wt.%) were produced on Q235 steel by laser cladding. The phase composition, microstructure, and corrosion behavior in sulfuric acid dew point corrosion environment (50 wt.% H2SO4 at 60 °C) were investigated. The main conclusion obtained are presented below:
- (1)
- All the Ni-Cr-Mo alloy coatings with different content of Cr element exhibit similar phase composition and microstructure. The increase in Cr results in more black micropores in the eutectic networks;
- (2)
- The passive film forming on each Ni-Cr-Mo alloy coating exhibits a bilayer structure consisting of a Cr2O3-dominated compact inner layer and a porous outer layer containing oxides of Mo and hydroxides of Ni and Cr. The increase in Cr leads to the decrease in Ni(OH)2 and the replacing of oxidized species of Ni with those Cr;
- (3)
- The inner layer becomes thicker and more compact with the increase in Cr, leading to the more protective passive film;
- (4)
- The corrosion resistance of Ni-Cr-Mo alloy coating is degraded and then improved with an increase in Cr. This phenomenon is attributed to the competition effect between the increase in Cr and relative decrease in Ni.
Author Contributions
Conceptualization, C.Z. and Z.L.; methodology, C.Z. and Q.L.; software, C.Z.; validation, Q.L.; formal analysis, Y.K.; investigation, Y.K.; resources, C.Z. and Z.L.; data curation, Y.K.; writing—original draft preparation, C.Z.; writing—review and editing, C.L.; visualization, C.L.; supervision, Z.L.; project administration, C.Z.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the Key Laboratory of Power Station Energy Transfer Conversion and System and the Fundamental Research Funds for the Central Universities (Grant No. 2017XS059) for the funding sources.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asphahani, A.I.; Nicholas, A.F.; Silence, W.L.; Meyer, T.H. High Performance Alloys for Solving Severe Corrosion Problems in Flue Gas Desulfurization Systems. Mater. Corros. 1989, 40, 409–417. [Google Scholar] [CrossRef]
- Dahl, L. Corrosion in Flue Gas Desulfurization Plants and Other Low Temperature Equipment. Mater. Corros. 1992, 43. [Google Scholar] [CrossRef]
- Rajendran, N.; Rajeswari, S. Evaluation of High Ni-Cr-Mo Alloys for the Construction of Sulfur Dioxide Scrubber Plants. J. Mater. Eng. Perform. 1996, 5, 46–50. [Google Scholar] [CrossRef]
- Bordziłowski, J.; Darowicki, K. Anti-Corrosion Protection of Chimneys and Flue Gas Ducts. Anti-Corros. Methods Mater. 1998, 45, 388–396. [Google Scholar] [CrossRef]
- Darowicki, K.; Krakowiak, S. Durability Evaluation of Ni-Cr-Mo Super Alloys in a Simulated Scrubbed Flue Gas Environment. Anti-Corros. Methods Mater. 1999, 46, 19–22. [Google Scholar] [CrossRef]
- CICIND. Model Code for Steel Chimneys, Revision 1—1990, Amendment A—2002; CICIND (International Committee on Industrial Construction): Zurich, Switzerland, 2002. [Google Scholar]
- Honga, S.J.; Honga, S.H.; Doh, J.M. Materials for Flue Gas Desulfurization Systems Operating in Korea and Their Failures. Mater. High Temp. 2007, 24, 289–293. [Google Scholar] [CrossRef]
- Shoemaker, L.; Crum, J.; Maitra, D. Recent Experience with Stainless Steels in FGD Air Pollution Control Service. In NACE—International Corrosion Conference Series; NACE International: Houston, TX, USA, 2011. [Google Scholar]
- Zeng, Y.; Li, K.; Hughes, R.; Luo, J.L. Corrosion Mechanisms and Materials Selection for the Construction of Flue Gas Component in Advanced Heat and Power Systems. Ind. Eng. Chem. Res. 2017, 56, 14141–14154. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, Z.G. Corrosion Evaluation of One Wet Desulfurization Equipment—Flue Gas Desulfurization Unit. Fuel Process. Technol. 2018, 181, 279–293. [Google Scholar] [CrossRef]
- Pan, P.; Chen, H.; Liang, Z.; Zhao, Q. Desulfurized Flue Gas Corrosion Coupled with Deposits in a Heating Boiler. Corros. Sci. 2018, 131, 126–136. [Google Scholar] [CrossRef]
- Mishra, A. Performance of Corrosion-Resistant Alloys in Concentrated Acids. Acta Metall. Sin. Engl. Lett. 2017, 30, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Liu, Z.; Chen, S.; Liu, C. Corrosion Behavior of a Ni-Cr-Mo Alloy Coating Fabricated by Laser Cladding in a Simulated Sulfuric Acid Dew Point Corrosion Environment. Coatings 2020, 10, 849. [Google Scholar] [CrossRef]
- Priyantha, N.; Jayaweera, P.; Macdonald, D.D.; Sun, A. An Electrochemical Impedance Study of Alloy 22 in NaCl Brine at Elevated Temperature. I. Corrosion Behavior. J. Electroanal. Chem. 2004, 572, 409–419. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Sun, A.; Priyantha, N.; Jayaweera, P. An Electrochemical Impedance Study of Alloy-22 in NaCl Brine at Elevated Temperature: II. Reaction Mechanism Analysis. J. Electroanal. Chem. 2004, 572, 421–431. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Shoesmith, D.W.; McIntyre, N.S.; Noël, J.J. Effects of Temperature and Potential on the Passive Corrosion Properties of Alloys C22 and C276. J. Electrochem. Soc. 2003, 150, B120. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Noël, J.J.; McIntyre, S.; Shoesmith, D.W. Cr, Mo and W Alloying Additions in Ni and Their Effect on Passivity. Electrochim. Acta 2004, 49, 3015–3027. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Noël, J.J.; McIntyre, N.S.; Shoesmith, D.W. The Open-Circuit Ennoblement of Alloy C-22 and Other Ni-Cr-Mo Alloys. JOM 2005, 57, 31–35. [Google Scholar] [CrossRef]
- Gray, J.J.; Hayes, J.R.; Gdowski, G.E.; Viani, B.E.; Orme, C.A. Influence of Solution PH, Anion Concentration, and Temperature on the Corrosion Properties of Alloy 22. J. Electrochem. Soc. 2006, 153, B61. [Google Scholar] [CrossRef]
- Jakupi, P.; Zagidulin, D.; Noël, J.J.; Shoesmith, D.W. The Impedance Properties of the Oxide Film on the Ni-Cr-Mo Alloy-22 in Neutral Concentrated Sodium Chloride Solution. Electrochim. Acta 2011, 56, 6251–6259. [Google Scholar] [CrossRef]
- Zhang, X.; Zagidulin, D.; Shoesmith, D.W. Characterization of Film Properties on the NiCrMo Alloy C-2000. Electrochim. Acta 2013, 89, 814–822. [Google Scholar] [CrossRef]
- Zhang, X.; Shoesmith, D.W. Influence of Temperature on Passive Film Properties on Ni-Cr-Mo Alloy C-2000. Corros. Sci. 2013, 76, 424–431. [Google Scholar] [CrossRef]
- Luo, H.; Gao, S.; Dong, C.; Li, X. Characterization of Electrochemical and Passive Behaviour of Alloy 59 in Acid Solution. Electrochim. Acta 2014, 135, 412–419. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, G.S.; Li, D.G. A Pre-Passive State Observed for the Passive Film Formed on Alloy 625 in a Hydrochloric Acid Solution. Appl. Surf. Sci. 2018, 431, 197–201. [Google Scholar] [CrossRef]
- Sun, H.; Wu, X.; Han, E.H. Effects of Temperature on the Protective Property, Structure and Composition of the Oxide Film on Alloy 625. Corros. Sci. 2009, 51, 2565–2572. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.R.; Zhang, L.; Feng, Z.; Lu, M.X. Comparison Study on the Semiconductive and Dissolution Behaviour of 316L and Alloy 625 in Hydrochloric Acid Solution. Acta Metall. Sin. Engl. Lett. 2020, 33, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.R.; Gray, J.J.; Szmodis, A.W.; Orme, C.A. Influence of Chromium and Molybdenum on the Corrosion of Nickel-Based Alloys. Corrosion 2006, 62, 491–500. [Google Scholar] [CrossRef]
- Henderson, J.D.; Li, X.; Filice, F.P.; Zagidulin, D.; Biesinger, M.C.; Kobe, B.; Shoesmith, D.W.; Ogle, K.; Noël, J.J. Investigating the Role of Mo and Cr during the Activation and Passivation of Ni-Based Alloys in Acidic Chloride Solution. J. Electrochem. Soc. 2021, 168, 021509. [Google Scholar] [CrossRef]
- Li, X.; Ogle, K. The Passivation of Ni-Cr-Mo Alloys: Time Resolved Enrichment and Dissolution of Cr and Mo during Passive-Active Cycles. J. Electrochem. Soc. 2019, 166, C3179–C3185. [Google Scholar] [CrossRef]
- Li, X.; Henderson, J.D.; Filice, F.P.; Zagidulin, D.; Biesinger, M.C.; Sun, F.; Qian, B.; Shoesmith, D.W.; Noël, J.J.; Ogle, K. The Contribution of Cr and Mo to the Passivation of Ni22Cr and Ni22Cr10Mo Alloys in Sulfuric Acid. Corros. Sci. 2020, 176, 109015. [Google Scholar] [CrossRef]
- Haemers, T.A.M.; Rickerby, D.G.; Lanza, F.; Geiger, F.; Mittemeijer, E.J. Laser Cladding of Stainless Steel with Hastelloy. Adv. Eng. Mater. 2001, 3, 242–245. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Wang, Y.; Tang, J. A Comparative Study on the High Temperature Corrosion of TP347H Stainless Steel, C22 Alloy and Laser-Cladding C22 Coating in Molten Chloride Salts. Corros. Sci. 2014, 83, 396–408. [Google Scholar] [CrossRef]
- Hidouci, A.; Pelletier, J.M.; Ducoin, F.; Dezert, D.; El Guerjouma, R. Microstructural and Mechanical Characteristics of Laser Coatings. Surf. Coat. Technol. 2000, 83, 396–408. [Google Scholar] [CrossRef]
- Barnes, S.; Timms, N.; Bryden, B.; Pashby, I. High Power Diode Laser Cladding. J. Mater. Processing Technol. 2003, 138, 411–416. [Google Scholar] [CrossRef]
- Cui, C.; Guo, Z.; Liu, Y.; Xie, Q.; Wang, Z.; Hu, J.; Yao, Y. Characteristics of Cobalt-Based Alloy Coating on Tool Steel Prepared by Powder Feeding Laser Cladding. Opt. Laser Technol. 2007, 39, 1544–1550. [Google Scholar] [CrossRef]
- Tuominen, J.; Vuoristo, P.; Mäntylä, T.; Latokartano, J.; Vihinen, J.; Andersson, P.H. Microstructure and corrosion behavior of high power diode laser deposited Inconel 625 coatings. J. Laser Appl. 2003, 15, 55. [Google Scholar] [CrossRef]
- Li, X.-Z.; Liu, Z.-D.; Li, H.-C.; Wang, Y.-T.; Li, B. Investigations on the behavior of laser cladding Ni–Cr–Mo alloy coating on TP347H stainless steel tube in HCl rich environment. Surf. Coat. Technol. 2013, 232, 627–639. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Gao, Y.; Zheng, C. High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S. Coatings 2020, 10, 617. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Zhang, Y.-F.; Bai, S.-L.; Liu, Z.-D. Microstructures, mechanical properties and corrosion resistance of Hastelloy C22 coating produced by laser cladding. J. Alloys Compd. 2013, 553, 253–258. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Bai, S.-L.; Liu, Z.-D. Corrosion behavior of Hastelloy C22 coating produced by laser cladding in static and cavitation acid solution. Trans. Nonferrous Met. Soc. China 2014, 24, 1610–1618. [Google Scholar] [CrossRef]
- Moskovits, P. Low-Temperature Boiler Corrosion and Deposits—A Literature Review. Ind. Eng. Chem. 1959, 51, 1305–1312. [Google Scholar] [CrossRef]
- Hodge, F.G.; Silence, W.L.; Wright, D. Predicting the Corrosivity of an Operating FGD System. Power Eng. 1994, 98, 30–34. [Google Scholar]
- Yoshio, T.; Katsuo, S. Corrosion-Resistant Ni-Cr-Mo Alloys in Hot Concentrated Sulfuric Acid with Active Carbon. Fuel Energy Abstr. 1996, 198, 145–152. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Sun, F.L.; Lv, S.J.; Li, X.G. A New Steel with Good Low-Temperature Sulfuric Acid Dew Point Corrosion Resistance. Mater. Corros. 2012, 63, 598–606. [Google Scholar] [CrossRef]
- ASTM International. Designation: G1-03 (Reapproved 2017), Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. Designation: G31-12a, Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Wang, X.; Gao, Y.; Li, K.; Yan, J.; Li, Y.; Feng, J. Effect of Yttrium on the Corrosion Behaviour of 09CrCuSb Alloy in Concentrated Sulphuric Acid. Corros. Sci. 2013, 69, 369–375. [Google Scholar] [CrossRef]
- Chang, J.H.; Chou, J.M.; Hsieh, R.I.; Lee, J.L. Corrosion Behaviour of Vacuum Induction-Melted Ni-Based Alloy in Sulphuric Acid. Corros. Sci. 2010, 52, 2323–2330. [Google Scholar] [CrossRef]
- Al’tpeter, E.; Kirkheiner, R.; Rokel’, M.; Uait, F. Corrosion Resistance of Alloys in Sulfuric Acid at Various Concentrations and Temperatures. Chem. Pet. Eng. 1995, 31, 395–399. [Google Scholar] [CrossRef]
- Bellanger, G.; Rameau, J.J. Behaviour of Hastelloy C22 steel in sulphate solutions at pH 3 and low temperatures. J. Mater. Sci. 1996, 31, 2097–2108. [Google Scholar] [CrossRef]
- Mishra, A.; Ramamurthy, S.; Biesinger, M.; Shoesmith, D. The activation/depassivation of nickel–chromium–molybdenum alloys in bicarbonate solution: Part I. Electrochim. Acta 2013, 100, 118–124. [Google Scholar] [CrossRef]
- Dou, Y.; Han, S.; Wang, L.; Wang, X.; Cui, Z. Characterization of the passive properties of 254SMO stainless steel in simulated desulfurized flue gas condensates by electrochemical analysis, XPS and ToF-SIMS. Corros. Sci. 2019, 165, 108405. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Pei, R.; Liu, S.; Wang, S.-L.; Dong, L.-J.; Zhou, L.-J.; Xi, Y.-C.; Bai, S.-L. Microstructure and corrosion behavior of different clad zones in multi-track Ni-based laser-clad coating. Surf. Coat. Technol. 2020, 402, 126310. [Google Scholar] [CrossRef]
- Jabs, T.; Borthen, P.; Strehblow, H. X-ray Photoelectron Spectroscopic Examinations of Electrochemically Formed Passive Layers on Ni-Cr Alloys. J. Electrochem. Soc. 1997, 144, 1231–1243. [Google Scholar] [CrossRef]
- Bakare, M.; Voisey, K.; Roe, M.; McCartney, D. X-ray photoelectron spectroscopy study of the passive films formed on thermally sprayed and wrought Inconel 625. Appl. Surf. Sci. 2010, 257, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hu, H.; Liu, C.; Zheng, Y. The effect of fluoride ions on the corrosion behavior of pure titanium in 0.05M sulfuric acid. Electrochim. Acta 2014, 135, 526–535. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Zhong, M.; Ge, F.; Gao, H.; Man, C.; Liu, C.; Wang, X. Electrochemical Behavior and Surface Characteristics of Pure Titanium during Corrosion in Simulated Desulfurized Flue Gas Condensates. J. Electrochem. Soc. 2018, 165, C542–C561. [Google Scholar] [CrossRef]
- Mogoda, A.; Ahmad, Y.; Badawy, W. Corrosion Behaviour of Ti–6Al–4V Alloy in Concentrated Hydrochloric and Sulphuric Acids. J. Appl. Electrochem. 2004, 34, 873–878. [Google Scholar] [CrossRef]
- Escrivà-Cerdán, C.; Blasco-Tamarit, E.; Garcia, D.; Garcia-Anton, J.; Guenbour, A. Passivation behaviour of Alloy 31 (UNS N08031) in polluted phosphoric acid at different temperatures. Corros. Sci. 2012, 56, 114–122. [Google Scholar] [CrossRef]
- De Souza, K.A.; Robin, A. Influence of concentration and temperature on the corrosion behavior of titanium, titanium-20 and 40% tantalum alloys and tantalum in sulfuric acid solutions. Mater. Chem. Phys. 2007, 103, 351–360. [Google Scholar] [CrossRef]
- Cardoso, M.; Amaral, S.; Martini, E. Temperature effect in the corrosion resistance of Ni–Fe–Cr alloy in chloride medium. Corros. Sci. 2008, 50, 2429–2436. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.Q.; Gao, S.J.; Li, X.G.; Zou, S.W. The influence mechanism of Fe3+on corrosion behavior of Ti6Al4V in sulfuric acid solutions. Mater. Corros. 2013, 66, 251–256. [Google Scholar] [CrossRef]
- Gray, J.; Orme, C. Electrochemical impedance spectroscopy study of the passive films of alloy 22 in low pH nitrate and chloride environments. Electrochim. Acta 2007, 52, 2370–2375. [Google Scholar] [CrossRef]
- Escrivà-Cerdán, C.; Blasco-Tamarit, E.; Garcia, D.; Garcia-Anton, J.; Akid, R.; Walton, J. Effect of temperature on passive film formation of UNS N08031 Cr–Ni alloy in phosphoric acid contaminated with different aggressive anions. Electrochim. Acta 2013, 111, 552–561. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Ni, H.; Hao, W.; Man, C.; Chen, S.; Wang, X.; Liu, Z.; Li, X. Influence of temperature on the electrochemical and passivation behavior of 2507 super duplex stainless steel in simulated desulfurized flue gas condensates. Corros. Sci. 2017, 118, 31–48. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Zheng, Y.; Ke, W.; Qiao, Y. Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid. Corros. Sci. 2015, 103, 50–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).