Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ta and Ta/TaN Coating Deposition
2.2. Microstructural Characterization
2.3. Preparation of Simulated Body Fluid (SBF)
2.4. Impedance Characterization
2.5. Potentiodynamic Polarization
2.6. Electrochemical Deposition
3. Results and Discussion
3.1. Characterization by Electrochemical Impedance Spectroscopy
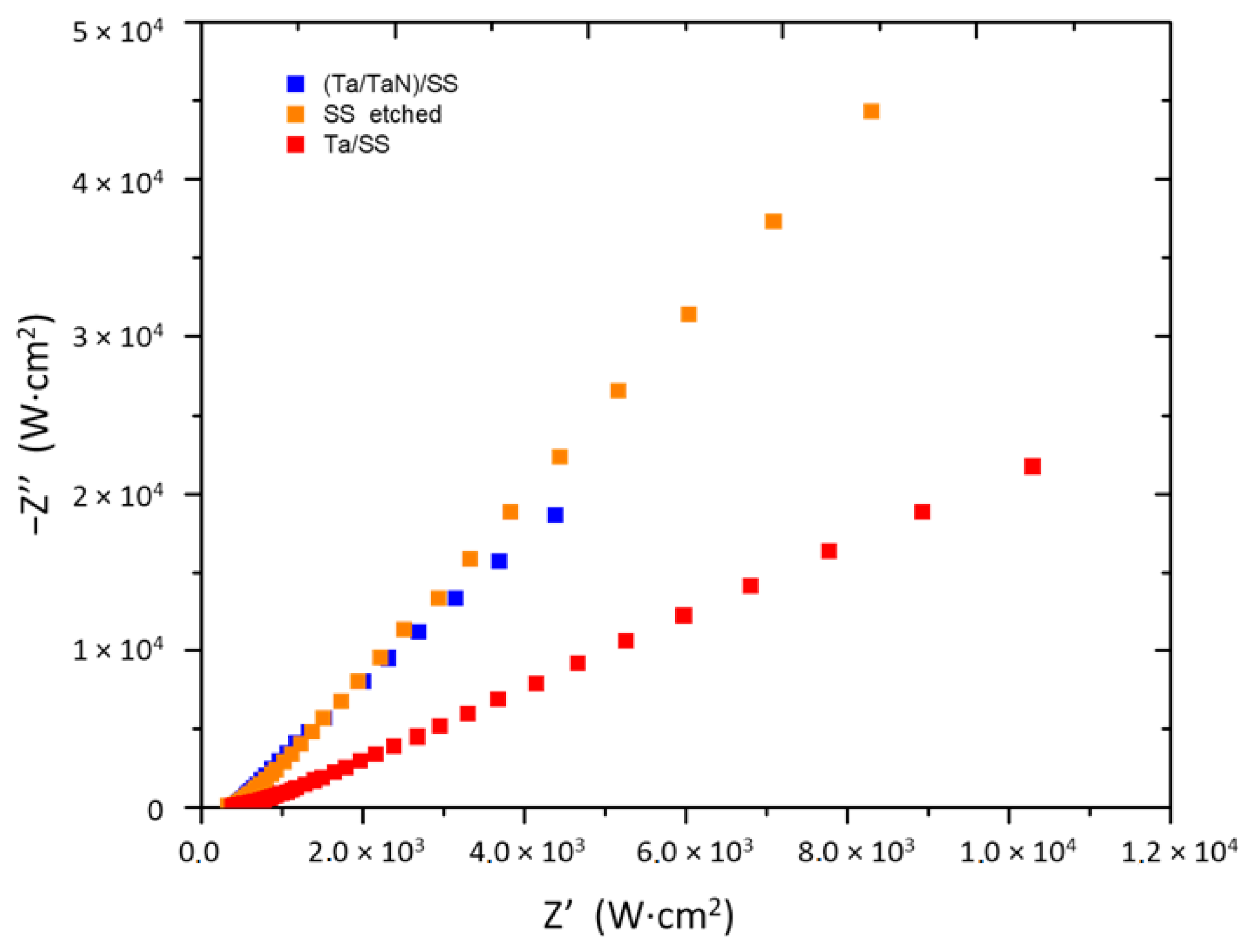

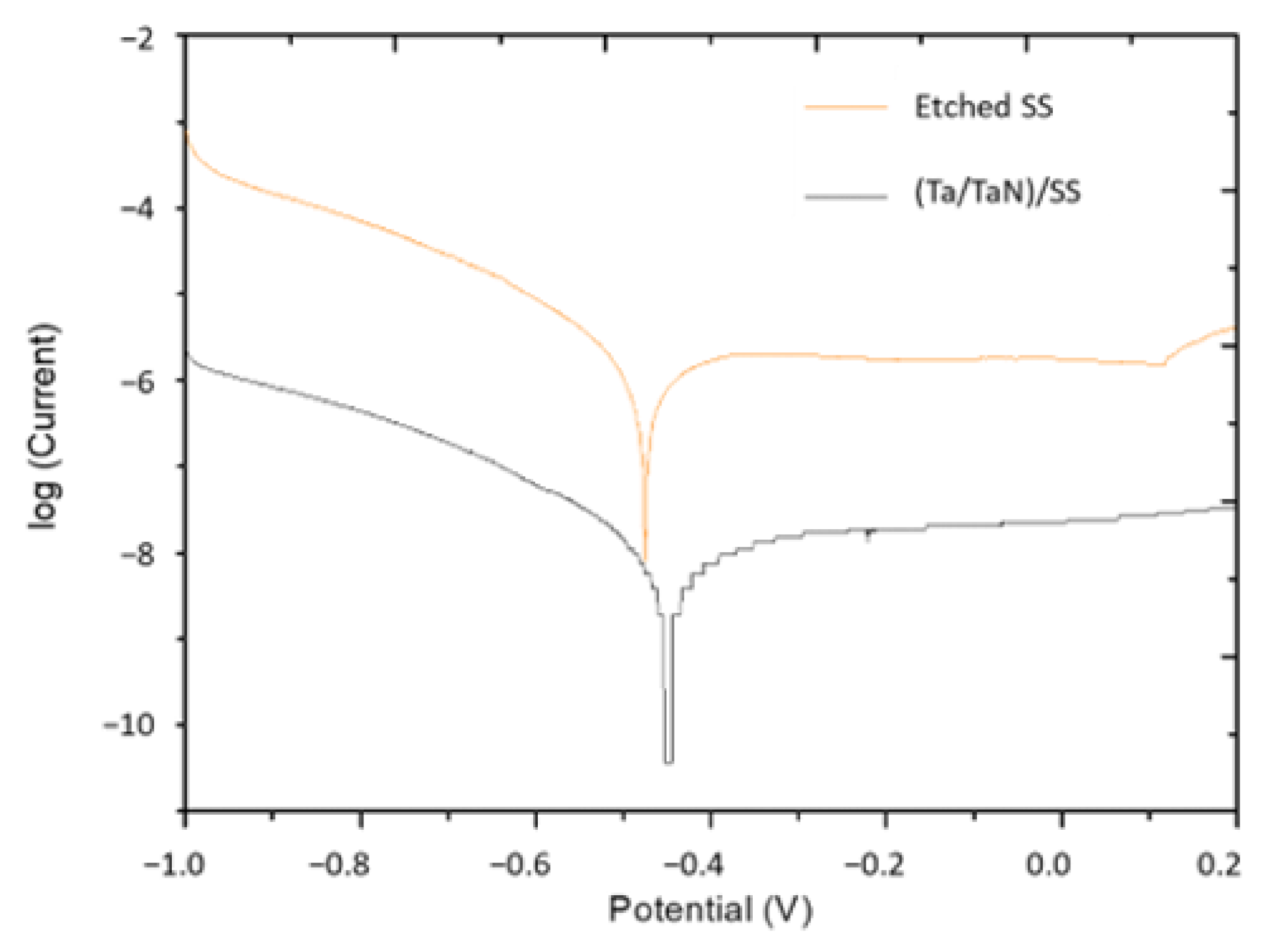

| Sample | Potential 1 (V) | Potential 2 (V) |
|---|---|---|
| Etched SS | −1.2 | −1.4 |
| (Ta/TaN)/SS | −1.4 | −1.7 |
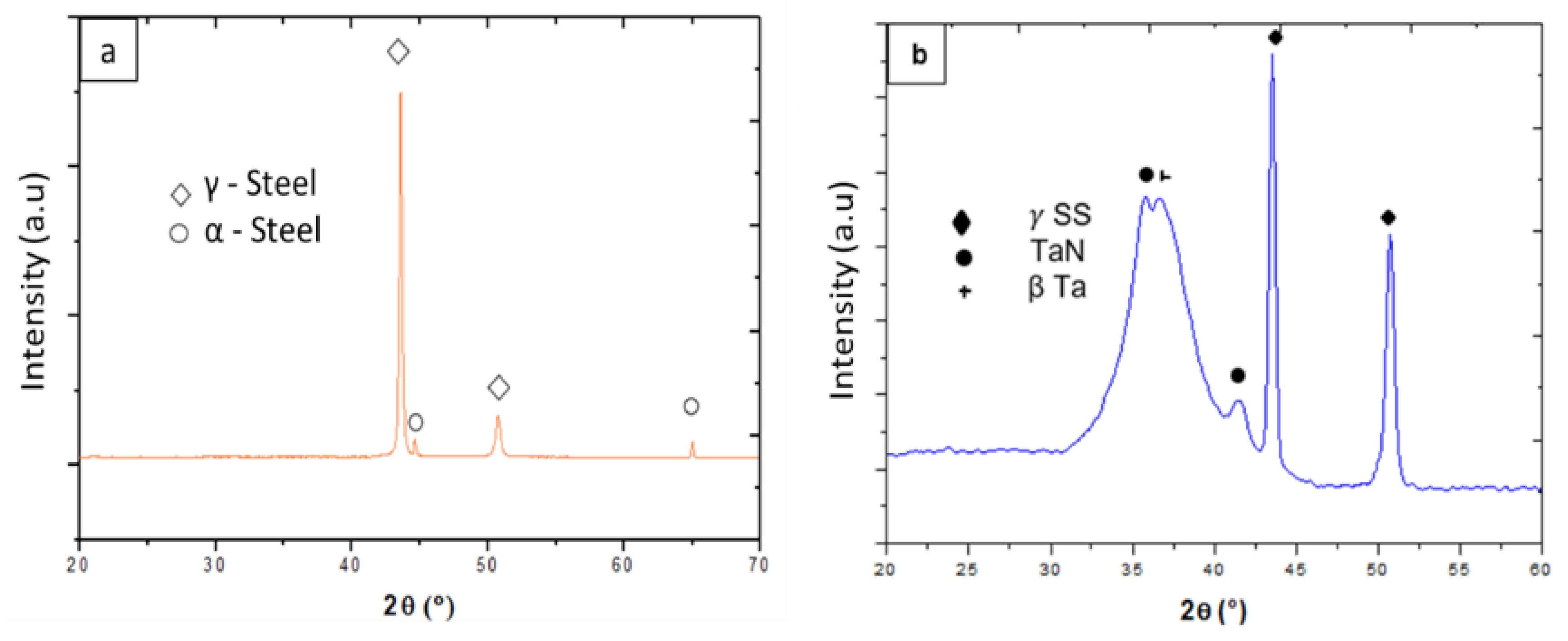
3.2. Characterization of Substrates
3.3. Characterization of Layers Formed on the Different Substrates by Electrodeposition
3.4. Mechanism of Formation of Hydroxyapatite

3.5. Contact Angle Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratner, B.; Hoffman, A.; Schoen, F.; Lemons, J. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: San Diego, CA, USA, 1996; pp. 527–573. [Google Scholar]
- Bergmann, C.; Stumpf, A. Dental Ceramics. Topics in Mining, Metallurgy and Materials Engineering; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; pp. 9–14. [Google Scholar]
- Dos Santos, V.; Brandalise, R.; Savaris, M. Engineering of Biomaterials. Topics in Mining. In Metallurgy and Materials Engineering; Springer: Berlin/Heidelberg, Germany, 2017; pp. 5–86. [Google Scholar]
- Rodil, S.E. Modificación Superficial De Biomateriales Metálicos. Rev. Latin Am. Metal. Mater. 2009, 29, 67–83. [Google Scholar]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Sun, H.; Yang, P.; Chen, J.; Wang, J.; Wan, G.; Huang, N.; Tian, X.; Wang, L.; Chu, P. Biomedical properties of tantalum nitride films synthesized by reactive magnetron sputtering. Thin Solid Films 2001, 398–399, 471–475. [Google Scholar] [CrossRef]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kim, H.-M.; Kokubo, T.; Ohtsuki, C.; Kato, H.; Nakamura, T. Mechanism of bonelike apatite formation on bioactive tantalum metal in a simulated body fluid. Biomaterials 2002, 23, 827–832. [Google Scholar] [CrossRef]
- Yang, Y.H.; Chen, D.J.; Wu, F.B. Microstructure, hardness, and wear resistance of sputtering TaN coating by controlling RF input power. Surf. Coat. Technol. 2016, 303, 32–40. [Google Scholar] [CrossRef]
- Flores, J.F.; Schorr, M.; Olaya, J.J. Corrosion protection of steels by PVD TaN thin films. Anti-Corros. Methods Mater. 2006, 53, 88–94. [Google Scholar] [CrossRef]
- Ma, G.; Lin, G.; Gong, S.; Liu, X.; Sun, G.; Wu, H. Mechanical and corrosive characteristics of Ta/TaN multilayer coatings. Vacuum 2013, 89, 244–248. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J.; Sheykholeslami, S.O.R.; Moosavifar, M. Biocompatibility assessment of graphene oxide-hydroxyapatite coating applied on TiO2 nanotubes by ultrasound-assisted pulse electrodeposition. Mater. Sci. Eng. C 2018, 87, 10–21. [Google Scholar] [CrossRef]
- Rigo, E.; Boschi, A.; Yoshimoto, M.; Allegrini, S.; Konig, B.; Carbonari, M. Evaluation in vitro and in vivo of biomimetic hydroxyapatite coated on titanium dental implants. Mater. Sci. Eng. C 2004, 24, 647–651. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, Y.; Park, S.; Jeong, S.; Kim, H.; Cho, C. Surface properties and cell response of fluoridated hydroxyapatite/TiO2 coated on Ti substrate. Curr. Appl. Phys. 2008, 9, 528–533. [Google Scholar] [CrossRef]
- Kim, H.-W.; Koh, Y.-H.; Li, L.-H.; Lee, S.; Kim, H.-E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol–gel method. Biomaterials 2003, 25, 2533–2538. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Wei, X.; Carl, L.; Jukka, L.; Hakan, E. Biomimetic Hydroxyapatite Deposition on Titanium Oxide Surfaces for Biomedical Application. In Advances in Biomimetics; Marko, C., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-191-6. Available online: http://www.intechopen.com/books/advances-inbiomimetics/biomimetic-hydroxyapatite-deposition-on-titanium-oxide-surfaces-for-biomedical-application (accessed on 20 February 2022).
- Forsgren, J.; Svahn, F.; Jarmar, T.; Engqvist, H. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomater. 2007, 3, 980–984. [Google Scholar] [CrossRef]
- Uribe, R.; Uvillús, A.; Bonilla, O.; Lascano, L.; González, G. Effect of Low Magnetic Field on Biomimetic Deposition of Hydroxyapatite on Titanium and BIOLINE Stainless Steel. Coatings 2021, 11, 1484. [Google Scholar] [CrossRef]
- Chakraborty, R.; Saha, P. A comparative study on surface morphology and electrochemical behaviour of hydroxyapatite-calcium hydrogen phosphate composite coating synthesized in-situ through electro chemical process under various deposition conditions. Surf. Interfaces 2018, 12, 160–167. [Google Scholar] [CrossRef]
- San, H.; Hu, J.; Zhang, Y.; Han, J.; Tang, S. Formation and in vitro mineralization of electrochemically deposited coatings prepared on micro-arc oxidized titanium alloy. J. Appl. Electrochem. 2019, 49, 485–501. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.; van Rijn, P.; Vakis, A.; Pei, Y. Crystal growth mechanism of calcium phosphate coatings on titanium by electrochemical deposition. Surf. Coatings Technol. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Katić, J.; Metikoš-Huković, M.; Škapin, S.; Petravić, M.; Varašanec, M. The potential-assisted deposition as valuable tool for producing functional apatite coatings on metallic materials. Electrochim. Acta 2014, 127, 173–179. [Google Scholar] [CrossRef]
- Abdel-Aal, E.; Dietrich, D.; Steinhaeuser, S.; Wielage, B. Electrocrystallization of nanocrystallite calcium phosphate coatings on titanium substrate at different current densities. Surf. Coat. Technol. 2008, 202, 5895–5900. [Google Scholar] [CrossRef]
- Schmidt, R.; Hoffmann, V.; Helth, A.; Gostin, P.F.; Calin, M.; Eckert, J.; Gebert, A. Electrochemical deposition of hydroxyapatite on beta-Ti-40Nb. Surf. Coat. Technol. 2016, 294, 186–193. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, K.; Das, S. Synthesis of calcium hydrogen phosphate and hydroxyapatite coating on SS316 substrate through pulsed electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, Q.; Lin, J.-H.; Lou, C.-W. Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds. Nanomaterials 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Que, L.X.; Van Anh, N.; Hoang, T.; Lam, T.D. Controlling the electrodeposition, morphology and structure of hydroxyapatite coating on 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 2037–2045. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2018, 94, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Matsuo, K.; Mizutani, N.; Hasegawa, J. Hydrothermal-Electrochemical Deposition of Calcium Phosphates on Various Metals. Dent. Mater. J. 1999, 18, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Peng, H.-K.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Zielinski, A.; Bartmanski, M. Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings 2020, 10, 782. [Google Scholar] [CrossRef]
- Peng, P.; Kumar, S.; Voelcker, N.; Szili, E.; Smart, R.; Griesser, H. Thin calcium phosphate coatings on titanium by electro-chemical deposition in modified simulated body fluid. J. Biomed. Mater. Res. 2016, 76, 347–355. [Google Scholar]
- Ban, S.; Maruno, S. Deposition of Calcium Phosphate on Titanium by Electrochemical Process in Simulated Body Fluid. Jpn. J. Appl. Phys. 1993, 32, L1577–L1580. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Zuo, Y.; Xiong, J. Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid. Chin. J. Chem. Eng. 2009, 17, 667–671. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Zhang, L.; Yin, X.; Guo, Y. In simulated body fluid performance of polymorphic apatite coatings synthesized by pulsed electrodeposition. Mater. Sci. Eng. C 2017, 79, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sandvik. SANDVIK BIOLINE 316LVM: Austenitic Stainless Steel for Implants. Alloy. Dig. 2000, 49, SS-784. [Google Scholar] [CrossRef]
- Jara, A.; Fraisse, B.; Flaud, V.; Fréty, N.; Gonzalez, G. Thin film deposition of Ta, TaN and Ta/TaN bi-layer on Ti and SS316-LVM substrates by RF sputtering. Surf. Coat. Technol. 2017, 309, 887–896. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.-M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.A. Evaluación Mediante Técnicas Electroquímicas del Comportamiento de Recubrimientos Alquídicos Utilizados en la Protección Anticorrosiva del Acero. Bachelor’s Thesis, Escuela Politecnica Nacional, Quito, Ecuador, 2012. [Google Scholar]
- Gladczuk, L.; Patel, A.; Paur, C.S.; Sosnowski, M. Tantalum films for protective coatings of steel. Thin Solid Films 2004, 467, 150–157. [Google Scholar] [CrossRef]
- Bernoulli, D.; Müller, U.; Schwarzenberger, M.; Hauert, R.; Spolenak, R. Magnetron sputter deposited tantalum and tantalum nitride thin films: An analysis of phase, hardness and composition. Thin Solid Films 2013, 548, 157–161. [Google Scholar] [CrossRef]
- Gladczuk, L.; Patel, A.; Demaree, J.D.; Sosnowski, M. Sputter deposition of bcc tantalum films with TaN underlayers for protection of steel. Thin Solid Films 2005, 476, 295–302. [Google Scholar] [CrossRef]
- Cheng, X.; Roscoe, S.G. Corrosion behavior of titanium in the presence of calcium phosphate and serum proteins. Biomaterials 2005, 26, 7350–7356. [Google Scholar] [CrossRef]
- Kuo, M.; Yen, S. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Tao, J.; Pang, Y.-C.; Wang, W.; Wang, T. Electrochemical deposition of hydroxyapatite coatings on titanium. Trans. Nonferrous Met. Soc. China 2006, 16, 633–637. [Google Scholar] [CrossRef]
- Nam, P.T.; Lam, T.D.; Huong, H.T.; Phuong, N.T.; Trang, N.T.T.; Hoang, T.; Huong, N.T.T.; Thang, L.B.; Drouet, C.; Grossin, D.; et al. Electrodeposition and Characterization of Hydroxyapatite on TiN/316LSS. J. Nanosci. Nanotechnol. 2015, 15, 9991–10001. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Abdulateef, N.E.; Abdulkareem, M.H. Evaluation of Surface Roughness of 316L Stainless Steel Substrate on Nanohydroxyapatite by Electrophoretic Deposition. Al-Nahrain J. Eng. Sci. 2018, 21, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Huynh, V.; Ngo, N.K.; Golden, T.D. Surface Activation and Pretreatments for Biocompatible Metals and Alloys Used in Biomedical Applications. Int. J. Biomater. 2019, 2019, 3806504. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012, 12, 251–263. [Google Scholar]
- Budevski, E.; Staikov, G.; Lorenz, W. Electrocrystallization: Nucleation and growth phenomena. Electrochim. Acta 2000, 45, 2559–2574. [Google Scholar] [CrossRef]
- Antonio, R.F.; Rangel, E.C.; Mas, B.A.; Duek, E.A.; Cruz, N.C. Growth of hydroxyapatite coatings on tantalum by plasma electrolytic oxidation in a single step. Surf. Coat. Technol. 2019, 357, 698–705. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.; Gilbert, P. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Liu, X.; Chu, P.K.; Ding, C. Nucleation and growth of calcium–phosphate on Ca-implanted titanium surface. Surf. Sci. 2006, 600, 651–656. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.; Muller, F.; Will, J.; Frederik, P.; De With, G.; Sommerdijk, N. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Kavitha, L. A comparative study on the direct and pulsed current electrodeposition of hydroxyapatite coatings on surgical grade stainless steel. Surf. Coat. Technol. 2012, 206, 2859–2869. [Google Scholar] [CrossRef]
- Manso, M.; Jiménez, C.; Morant, C.; Herrero, P.; Martínez-Duart, J.M. Apatite films produced by electrodeposition: Characterization by TEM and AFM. Surf. Interface Anal. 2001, 31, 1104–1109. [Google Scholar] [CrossRef]
- Strnad, G.; Chirila, N.; Petrovan, C.; Russu, O. Contact Angle Measurement on Medical Implant Titanium Based Biomaterials. Procedia Technol. 2016, 22, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Minagar, S.; Berndt, C.C.; Wen, C. Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization. J. Funct. Biomater. 2015, 6, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]

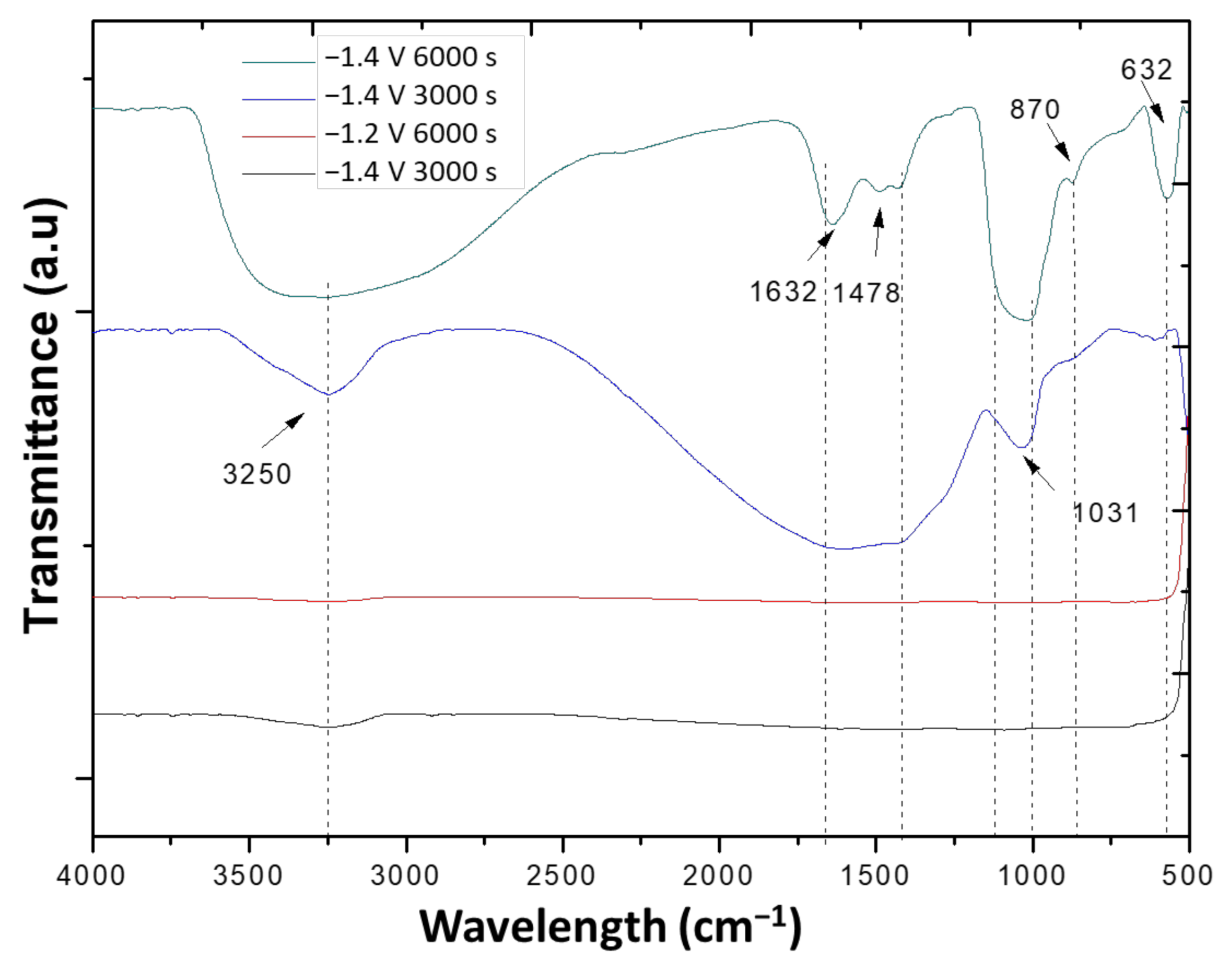
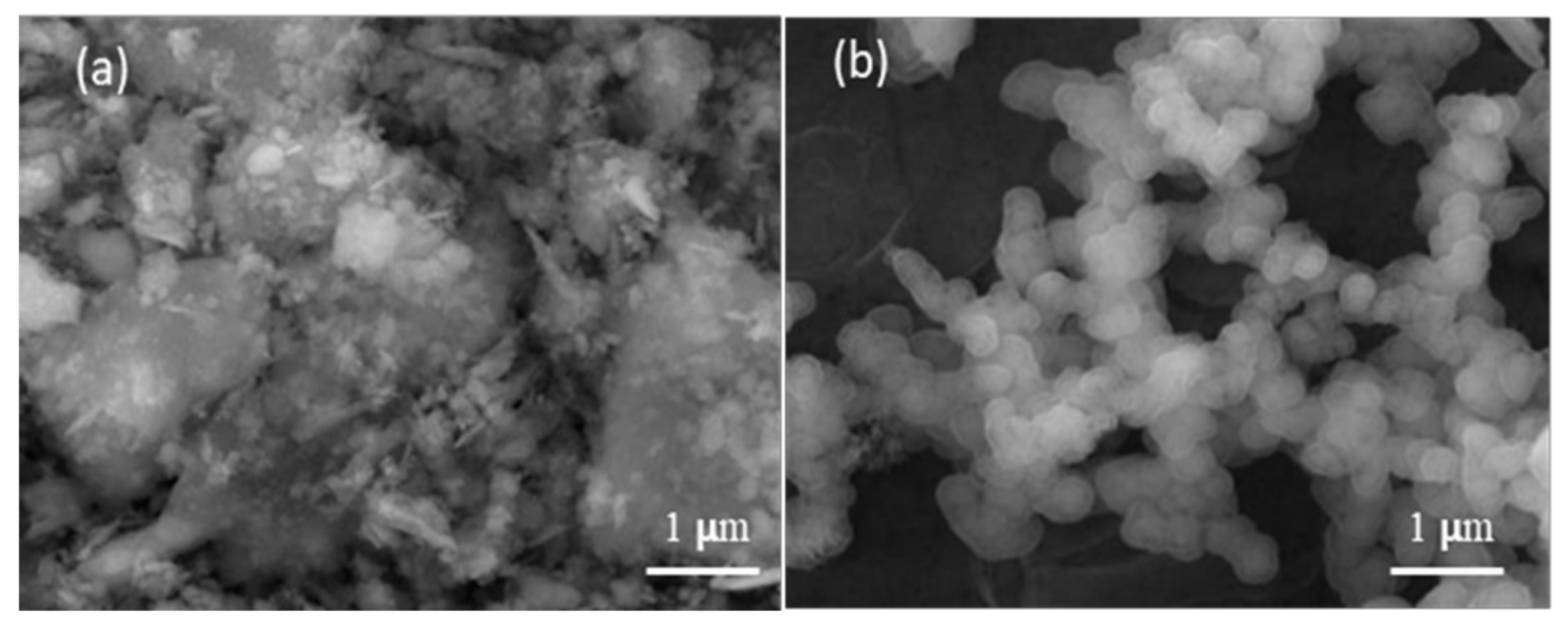
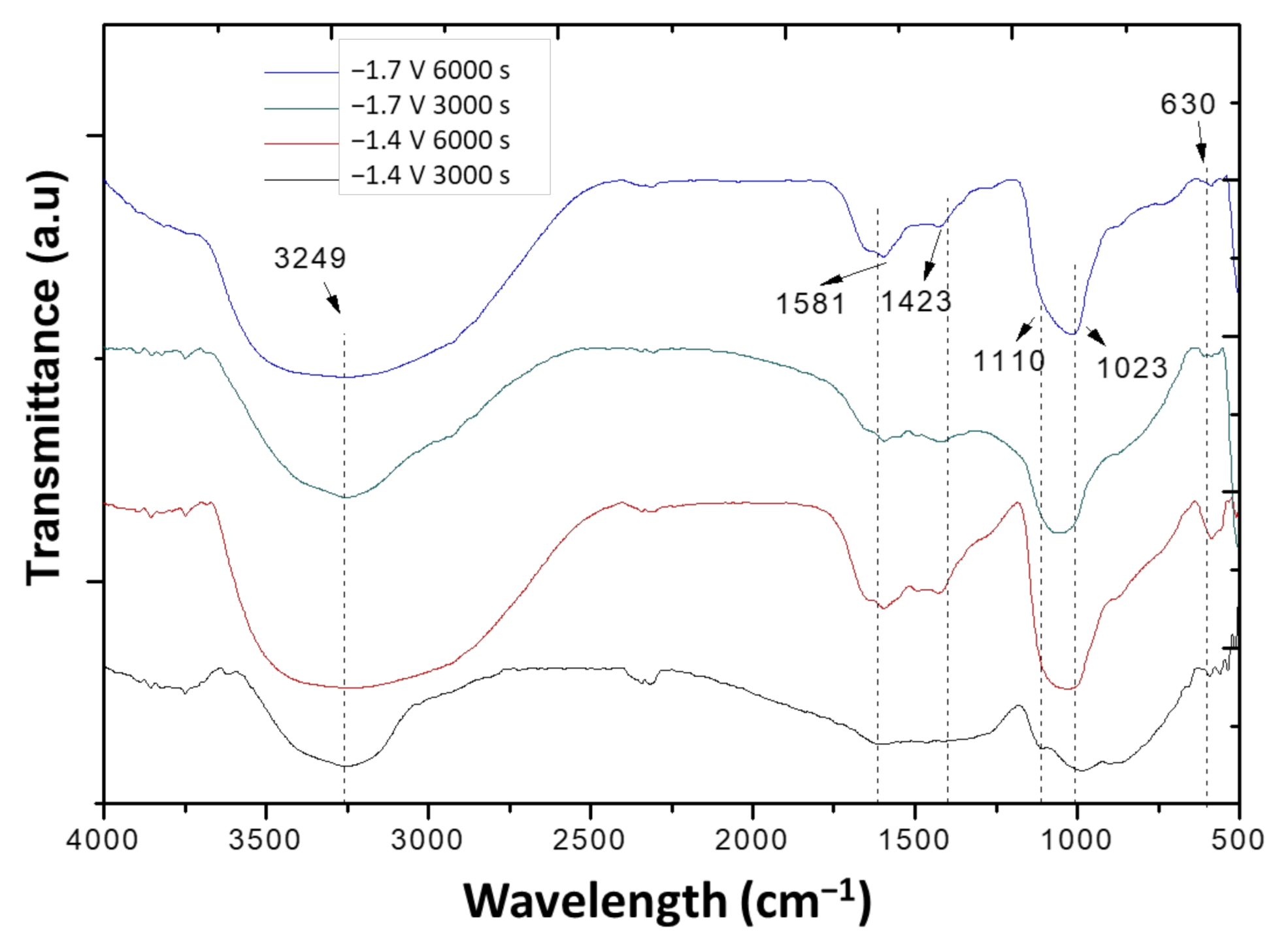


| Substrate | Potential (V) | Time (s) | Ca/P | Mass of Deposition of HAp (mg/mm2) |
|---|---|---|---|---|
| SS | −1.20 | 3000 | 1.20 | 0.056 |
| −1.20 | 6000 | 1.27 | 0.064 | |
| −1.40 | 3000 | 1.41 | 0.105 | |
| −1.40 | 6000 | 1.61 | 0.170 | |
| (Ta/TaN)/SS | −1.40 | 3000 | 1.25 | 0.021 |
| −1.40 | 6000 | 1.55 | 0.286 | |
| −1.70 | 3000 | 1.62 | 0.267 | |
| −1.70 | 6000 | 1.65 | 0.288 |
| Substrate | Before HAp Deposition | Applied Potentials (V) | After HAp Deposition | ||
|---|---|---|---|---|---|
| Angle (°) | 6000 s | Angle (°) | |||
| Ta/TaN/SS | 48.65 ± 0.93 |  | −1.7 | 31.00 ± 1.53 |  |
| SS | 46.73 ± 1.00 |  | −1.4 | 40.35 ± 1.29 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, R.; Uvillús, A.; Fernández, L.; Bonilla, O.; Jara, A.; González, G. Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium. Coatings 2022, 12, 440. https://doi.org/10.3390/coatings12040440
Uribe R, Uvillús A, Fernández L, Bonilla O, Jara A, González G. Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium. Coatings. 2022; 12(4):440. https://doi.org/10.3390/coatings12040440
Chicago/Turabian StyleUribe, Rafael, Andrea Uvillús, Lenys Fernández, Omar Bonilla, Angélica Jara, and Gema González. 2022. "Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium" Coatings 12, no. 4: 440. https://doi.org/10.3390/coatings12040440
APA StyleUribe, R., Uvillús, A., Fernández, L., Bonilla, O., Jara, A., & González, G. (2022). Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium. Coatings, 12(4), 440. https://doi.org/10.3390/coatings12040440






