Microstructure and Wear Properties of HVAF Sprayed Cu-Zr-Al-Ag-Co Amorphous Coatings at Different Spray Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder and Coating
2.2. Characterization of Powders and Coatings
2.3. Microhardness and Wear Tests
3. Results and Discussion
3.1. Phase Formation and Thermal Properties
3.2. Characteristics of HVAF Sprayed Coatings
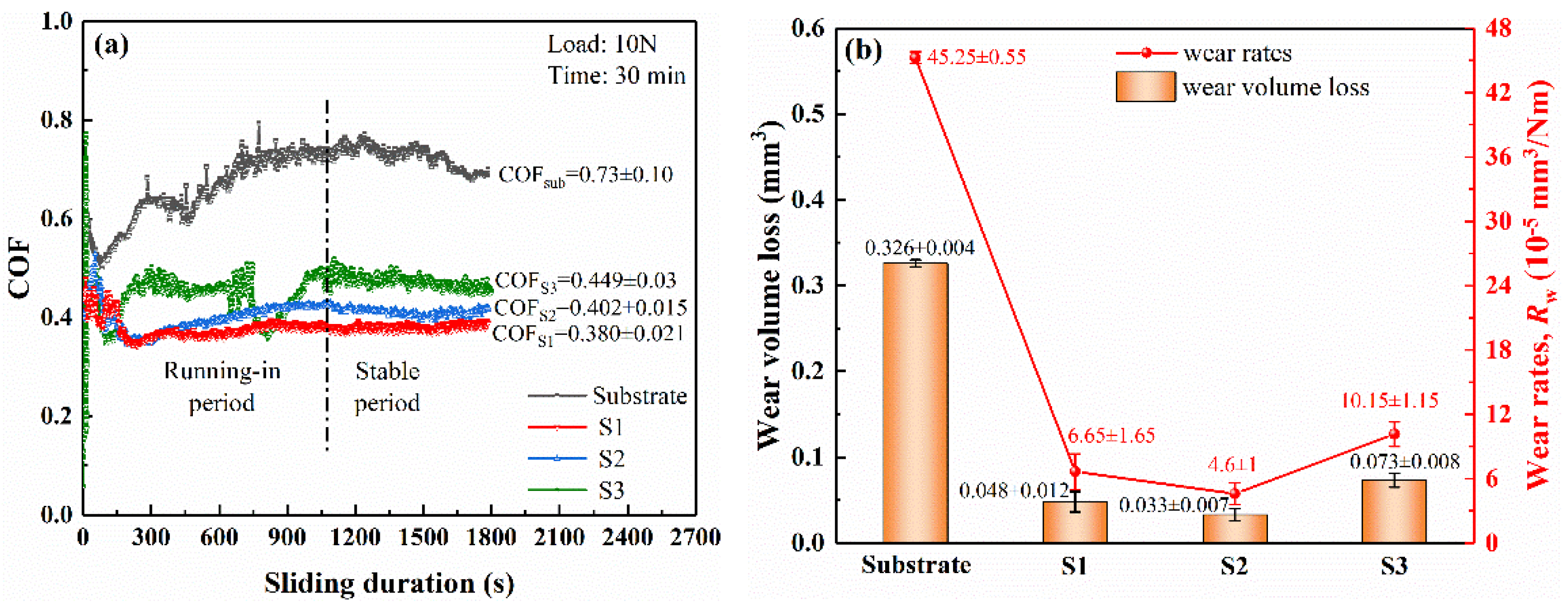
3.3. Wear Resistance Properties of HVAF Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahade, S.; Aranke, O.; Björklund, S.; Dizdar, S.; Awe, S.; Mušálek, R.; Lukáč, F.; Joshi, S. Influence of processing conditions on the microstructure and sliding wear of a promising Fe-based coating deposited by HVAF. Surf. Coat. Technol. 2021, 409, 126953. [Google Scholar] [CrossRef]
- Liao, Y.C.; Song, S.M.; Li, T.H.; Chiang, Y.L.; Tsai, P.H.; Nguyen, V.T.; Li, S.Y.; Jang, J.S.C.; Huang, J.C. Significant TRIP-effect improvement by manipulating ZrCu-B2 distribution in ZrCuAlCo-based bulk metallic glass composites via inoculating Ta particles. J. Alloys Compd. 2019, 774, 547–555. [Google Scholar] [CrossRef]
- Khan, M.M.; Nemati, A.; Rahman, Z.U.; Shah, U.H.; Asgar, H.; Haider, W. Recent advancements in bulk metallic glasses and their applications: A review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 233–268. [Google Scholar] [CrossRef]
- Si, C.; Duan, B.; Zhang, Q.; Cai, J.; Wu, W. Microstructure, corrosion-resistance, and wear-resistance properties of subsonic flame sprayed amorphous Fe-Mo-Cr-Co coating with extremely high amorphous rate. J. Mater. Res. Technol. 2020, 9, 3292–3303. [Google Scholar] [CrossRef]
- Sıkan, F.; Atabay, S.E.; Motallebzadeh, A.; Özerinç, S.; Kalay, I.; Kalay, Y.E. Effect of Sm on thermal and mechanical properties of Cu-Zr-Al bulk metallic glasses. Mater. Sci. Eng. A 2019, 743, 168–174. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, C.; Zhang, G.; Liao, H. Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: A review. Surf. Coat. Technol. 2019, 377, 124896. [Google Scholar] [CrossRef]
- Filipecka, K.; Pawlik, P.; Filipecki, J. The effect of annealing on magnetic properties, phase structure and evolution of free volumes in Pr-Fe-B-W metallic glasses. J. Alloys Compd. 2017, 694, 228–234. [Google Scholar] [CrossRef]
- Monfared, A.; Vali, H.; Faghihi, S. Biocorrosion and biocompatibility of Zr-Cu-Fe-Al bulk metallic glasses. Surf. Interface Anal. 2013, 45, 1714–1720. [Google Scholar] [CrossRef]
- Sohrabi, N.; Panikar, R.S.; Jhabvala, J.; Buch, A.R.; Mischler, S.; Logé, R.E. Laser coating of a Zr-based metallic glass on an aluminum substrate. Surf. Coat. Technol. 2020, 400, 126223. [Google Scholar] [CrossRef]
- Ye, X.; Bae, H.; Shin, Y.C.; Stanciu, L.A. In situ synthesis and characterization of Zr-based amorphous composite by laser direct deposition. Metall. Mater. Trans. A 2015, 46, 4316–4325. [Google Scholar] [CrossRef]
- Cai, B.; Yang, L. Enhanced corrosion and wear resistances of Zr-based alloy induced by amorphous/nanocrystalline coating. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 791–797. [Google Scholar] [CrossRef]
- Liu, Y.; Padmanabhan, J.; Cheung, B.; Liu, J.; Chen, Z.; Scanley, B.E.; Wesolowski, D.; Pressley, M.; Broadbridge, C.C.; Altman, S.; et al. Combinatorial development of antibacterial Zr-Cu-Al-Ag thin film metallic glasses. Sci. Rep. 2016, 6, 26950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.; Ma, J.; Cai, Y.; Liu, X.; Xie, S.; Wei, X.; Xu, G.; Shen, J. Characterization and elevated-temperature tribological performance of AC–HVAF-sprayed Fe-based amorphous coating. Surf. Coat. Technol. 2020, 387, 125535. [Google Scholar] [CrossRef]
- Xie, L.; Xiong, X.; Zeng, Y.; Wang, Y. The wear properties and mechanism of detonation sprayed iron-based amorphous coating. Surf. Coat. Technol. 2019, 366, 146–155. [Google Scholar] [CrossRef]
- Yugeswaran, S.; Kobayashi, A.; Suresh, K.; Rao, K.P.; Subramanian, B. Wear behavior of gas tunnel type plasma sprayed Zr-based metallic glass composite coatings. Appl. Surf. Sci. 2012, 258, 8460–8468. [Google Scholar] [CrossRef]
- Wang, Q.; Han, P.; Yin, S.; Niu, W.-J.; Zhai, L.; Li, X.; Mao, X.; Han, Y. Current research status on cold sprayed amorphous alloy coatings: A review. Coatings 2021, 11, 206. [Google Scholar] [CrossRef]
- Iqbal, A.; Siddique, S.; Maqsood, M.; Rehman, M.A.U.; Yasir, M. Comparative analysis on the structure and properties of Iron-based amorphous coating sprayed with the thermal spraying techniques. Coatings 2020, 10, 1006. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.L.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Chang, X.C.; Hou, W.L.; Wang, J.Q. Effect of processing parameters on the microstructures and corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings. Mater. Corros. 2013, 64, 801–810. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Q.; Jiao, J.; Wei, X.; Shen, J. Investigating the wear behavior of Fe-based amorphous coatings under nanoscratch tests. Metals 2017, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.R.; Li, J.W.; Jiao, J.; Chang, C.T.; Wang, G.; Shen, J.; Wang, X.M.; Li, R.W. Wear resistance of Fe-based amorphous coatings prepared by AC-HVAF and HVOF. Mater. Sci. Technol. 2017, 33, 65–71. [Google Scholar] [CrossRef]
- Guo, R.Q.; Zhang, C.; Chen, Q.; Yang, Y.; Li, N.; Liu, L. Study of structure and corrosion resistance of Fe-based amorphous coatings prepared by HVAF and HVOF. Corros. Sci. 2011, 53, 2351–2356. [Google Scholar] [CrossRef]
- Huang, F.; Kang, J.J.; Yue, W.; Fu, Z.Q.; Zhu, L.N.; She, D.S.; Liang, J.; Wang, C.B. Corrosion behavior of FeCrMoCBY amorphous coating fabricated by high-velocity air fuel Spraying. J. Therm. Spray Technol. 2019, 28, 842–850. [Google Scholar] [CrossRef]
- Gao, M.; Lu, W.; Yang, B.; Zhang, S.; Wang, J. High corrosion and wear resistance of Al-based amorphous metallic coating synthesized by HVAF spraying. J. Alloys Compd. 2018, 735, 1363–1373. [Google Scholar] [CrossRef]
- Si, C.; Wu, W. Excellent corrosion resistant amorphous coating prepared by gas atomization followed by AC-HVAF spray technology. Mater. Res. Express 2019, 6, 055202. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, S.; Sun, W.; Wang, J. Differences in dry sliding wear behavior between HVAF-sprayed amorphous steel and crystalline stainless steel coatings. J. Mater. Sci. Technol. 2019, 35, 865–874. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Hong, S.; Li, G.; Ying, G.; Guo, J.; Qin, Y. Fabrication and characterization of thermal-sprayed Fe-based amorphous/nanocrystalline composite coatings: An overview. J. Therm. Spray Technol. 2014, 23, 1157–1180. [Google Scholar] [CrossRef]
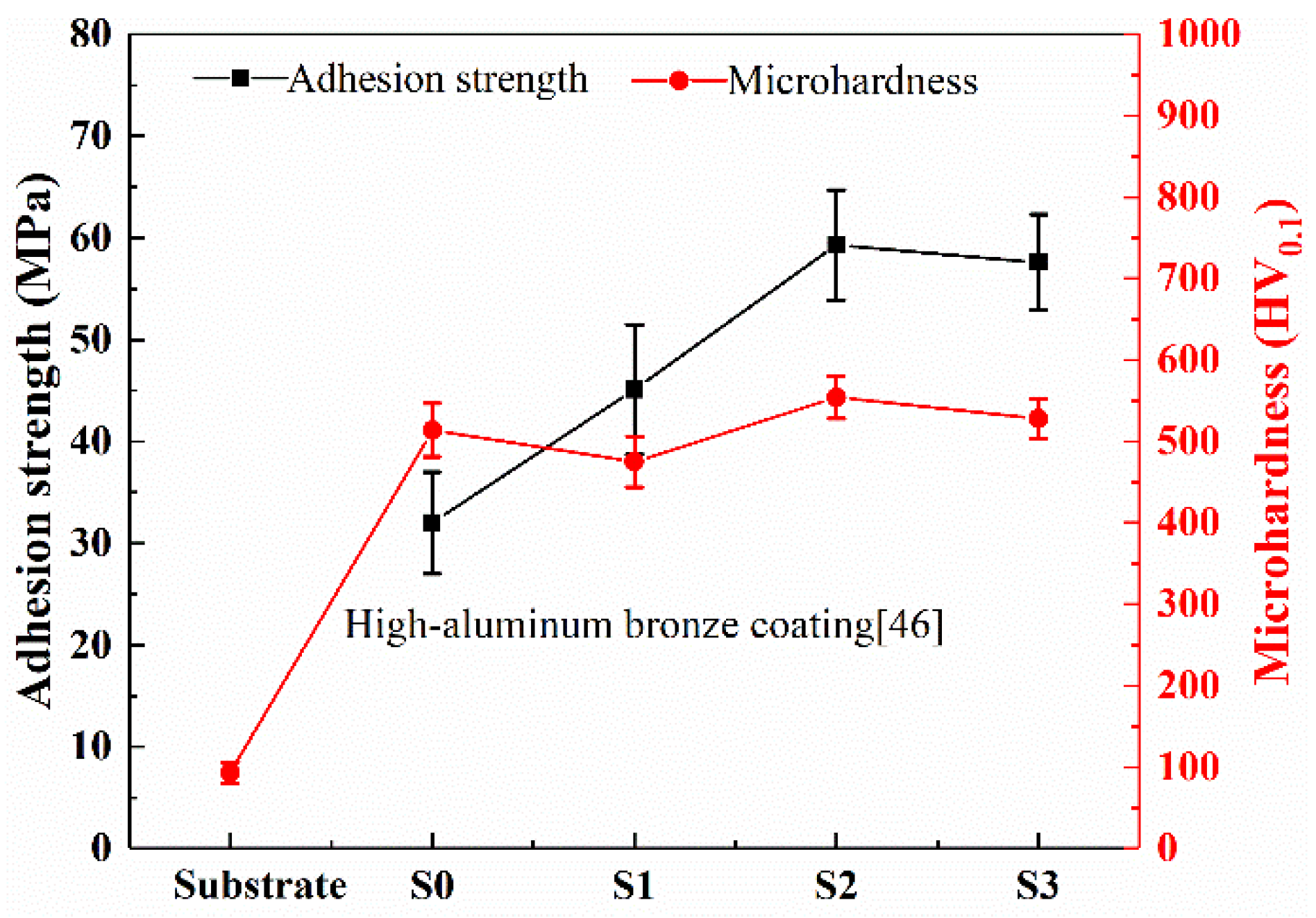
- Zhang, C.; Guo, R.Q.; Yang, Y.; Wu, Y.; Liu, L. Influence of the size of spraying powders on the microstructure and corrosion resistance of Fe-based amorphous coating. Electrochim. Acta 2011, 56, 6380–6388. [Google Scholar] [CrossRef]
- Movahedi, B.; Enayati, M.H.; Wong, C.C. Structural and thermal behavior of Fe-Cr-Mo-P-B-C-Si amorphous and nanocrystalline HVOF coatings. J. Therm. Spray Technol. 2010, 19, 1093–1099. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Zois, D.; Lekatou, A.; Vardavoulias, M. Preparation and characterization of highly amorphous HVOF stainless steel coatings. J. Alloys Compd. 2010, 504, S283–S287. [Google Scholar] [CrossRef]
- Wang, A.P.; Wang, Z.M.; Zhang, J.; Wang, J.Q. Deposition of HVAF-sprayed Ni-based amorphous metallic coatings. J. Alloys Compd. 2007, 440, 225–228. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, Z.; Leng, H.; Chou, K. Thermodynamic evaluation and experimental verification of the glass forming ability of Cu-Zr-based alloys. J. Non-Cryst. Solids 2021, 564, 120835. [Google Scholar] [CrossRef]
- Xu, Y.; Jeurgens, L.P.H.; Bo, H.; Lin, L.; Zhu, S.; Huang, Y.; Liu, Y.; Qiao, J.; Wang, Z. On the competition between synchronous oxidation and preferential oxidation in Cu-Zr-Al metallic glasses. Corros. Sci. 2020, 177, 108996. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Bouala, G.I.N.; Etiemble, A.; der Loughian, C.; Langlois, C.; Pierson, J.F.; Steyer, P. Silver influence on the antibacterial activity of multi-functional Zr-Cu based thin film metallic glasses. Surf. Coat. Technol. 2018, 343, 108–114. [Google Scholar] [CrossRef]
- Escher, B.; Kühn, U.; Eckert, J.; Rentenberger, C.; Pauly, S. Influence of Ag and Co additions on glass-forming ability, thermal and mechanical properties of Cu–Zr–Al bulk metallic glasses. Mater. Sci. Eng. A 2016, 673, 90–98. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, Y.; Wen, Y.; Wu, B.; Song, M. Effects of structural relaxation and crystallization on the corrosion resistance of an Fe-based amorphous coating. J. Non-Cryst. Solids. 2020, 550, 120378. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Zhao, X.Y.; Ma, L.Q. Co substituted Zr-Cu-Al-Ni metallic glasses with enhanced glass-forming ability and high plasticity. J. Non-Cryst. Solids 2017, 473, 120–124. [Google Scholar] [CrossRef]
- Gammer, C.; Escher, B.; Ebner, C.; Minor, A.M.; Karnthaler, H.P.; Eckert, J.; Pauly, S.; Rentenberger, C. Influence of the Ag concentration on the medium-range order in a CuZrAlAg bulk metallic glass. Sci. Rep. 2017, 7, 44903. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Lv, Y.; Sun, Q.; Hu, L.; Yang, X.; Yue, Y. Thermodynamic evidence for cluster ordering in Cu46Zr42Al7Y5 ribbons during glass transition. Sci. Bull. 2016, 61, 706–713. [Google Scholar] [CrossRef] [Green Version]
- List, A.; Gärtner, F.; Mori, T.; Schulze, M.; Assadi, H.; Kuroda, S.; Klassen, T. Cold spraying of amorphous Cu50Zr50 alloys. J. Therm. Spray Technol. 2015, 24, 108–118. [Google Scholar] [CrossRef]
- Kim, J.; Kang, K.; Yoon, S.; Kumar, S.; Na, H.; Lee, C. Oxidation and crystallization mechanisms in plasma-sprayed Cu-based bulk metallic glass coatings. Acta Mater. 2010, 58, 952–962. [Google Scholar] [CrossRef]
- Kim, J.; Kang, K.; Yoon, S.; Lee, C. Enhancement of metallic glass properties of Cu-based BMG coating by shroud plasma spraying. Surf. Coat. Technol. 2011, 205, 3020–3026. [Google Scholar] [CrossRef]
- Blink, J.; Farmer, J.; Choi, J.; Saw, C. Applications in the nuclear industry for thermal spray amorphous metal and ceramic coatings. Metall. Mater. Trans. A 2009, 40, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Moreau, C.; Cielo, P.; Lamontagne, M.; Dallaire, S.; Krapez, J.C.; Vardelle, M. Temperature evolution of plasma-sprayed niobium particles impacting on a substrate. Surf. Coat. Technol. 1991, 46, 173–187. [Google Scholar] [CrossRef]
- Feng, L.; Li, D.; An, G.; Wang, G.; Li, W.; Chang, J. Effect of powder particle size on microstructure and properties of low pressure cold sprayed high aluminium bronze coating. Mater. Res. Express 2020, 7, 026524. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Huang, L.; Huang, S.; Zheng, X.; Chen, G. Effect of feedstock particle sizes on wear resistance of plasma sprayed Fe-based amorphous coatings. Surf. Coat. Technol. 2014, 258, 495–502. [Google Scholar] [CrossRef]
- Michalak, M.; Sokołowski, P.; Szala, M.; Walczak, M.; Łatka, L.; Toma, F.L.; Björklund, S. Wear behavior analysis of Al2O3 coatings manufactured by APS and HVOF spraying processes using powder and suspension feedstocks. Coatings 2021, 11, 879. [Google Scholar] [CrossRef]
- Greer, A.L.; Rutherford, K.L.; Hutchings, I.M. Wear resistance of amorphous alloys and related materials. Int. Mater. Rev. 2002, 47, 87–112. [Google Scholar] [CrossRef]

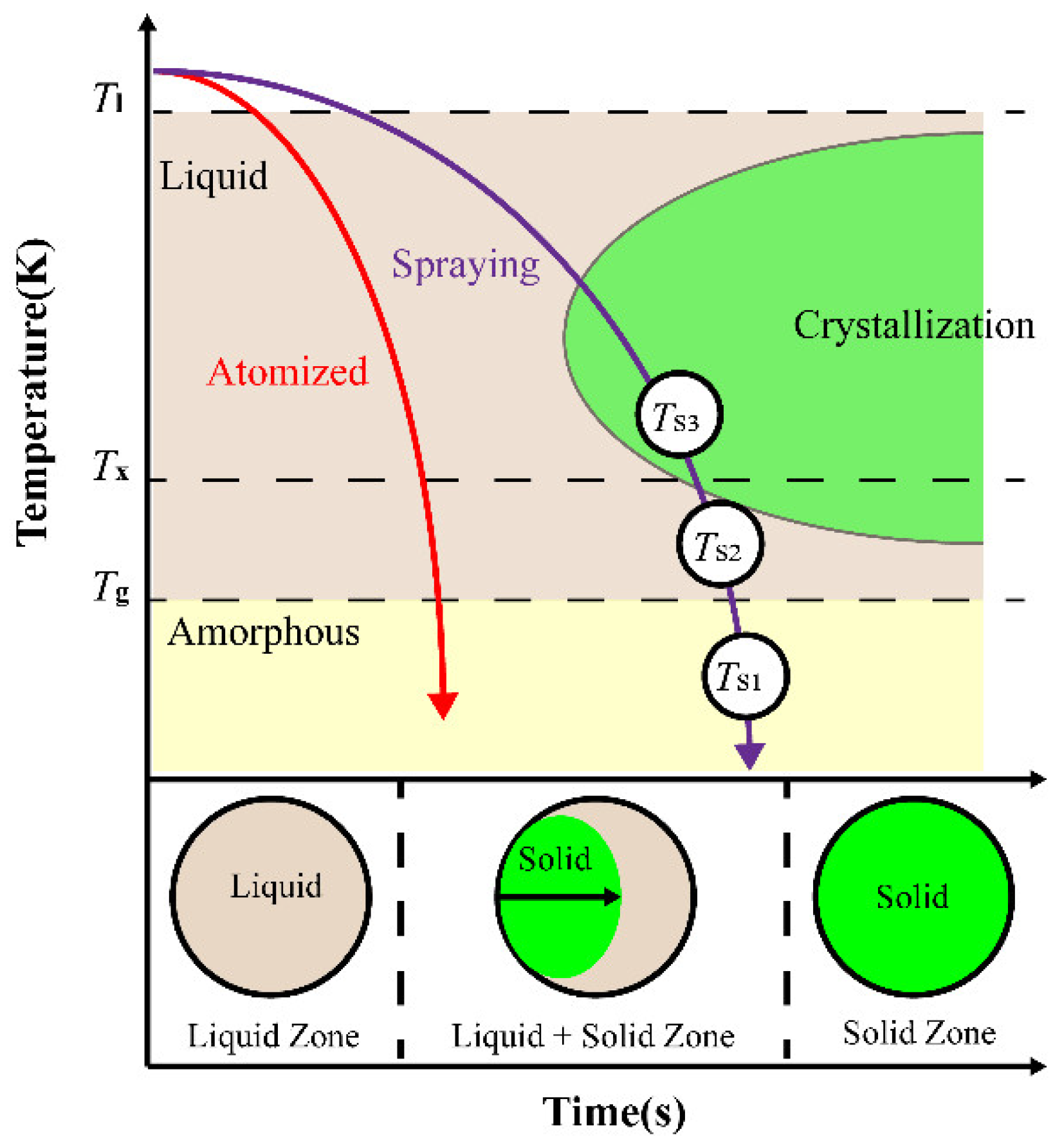
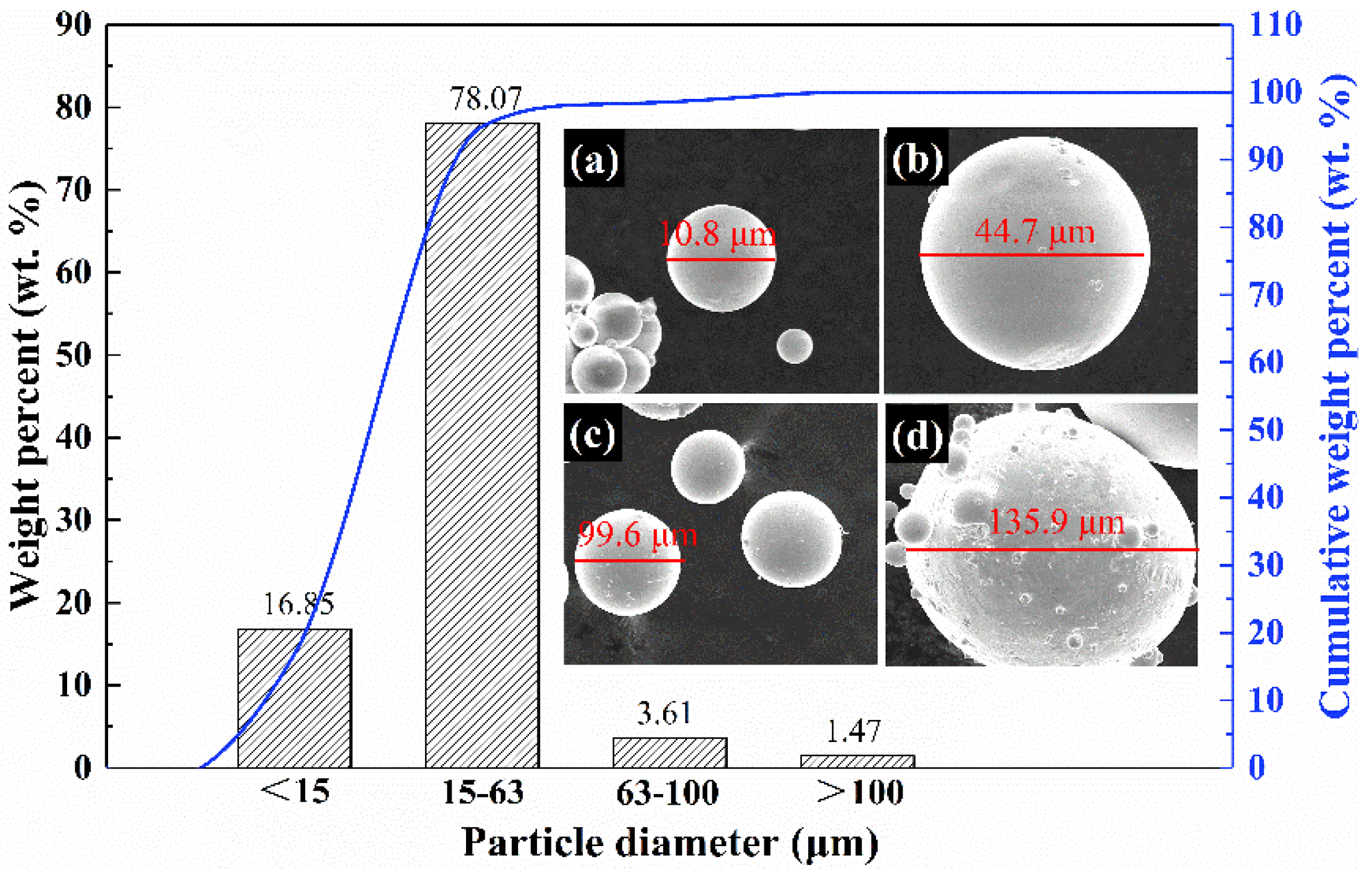
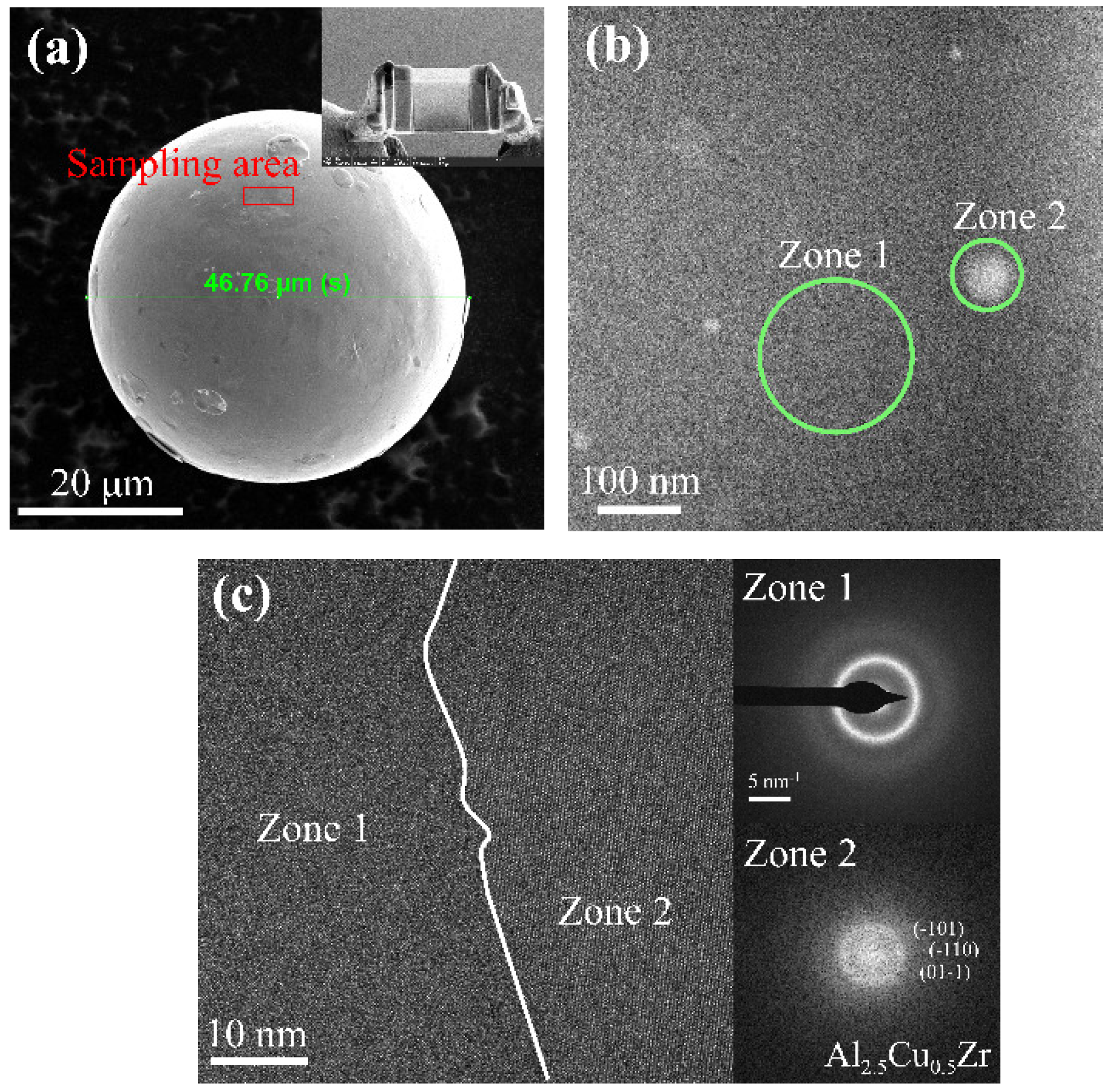

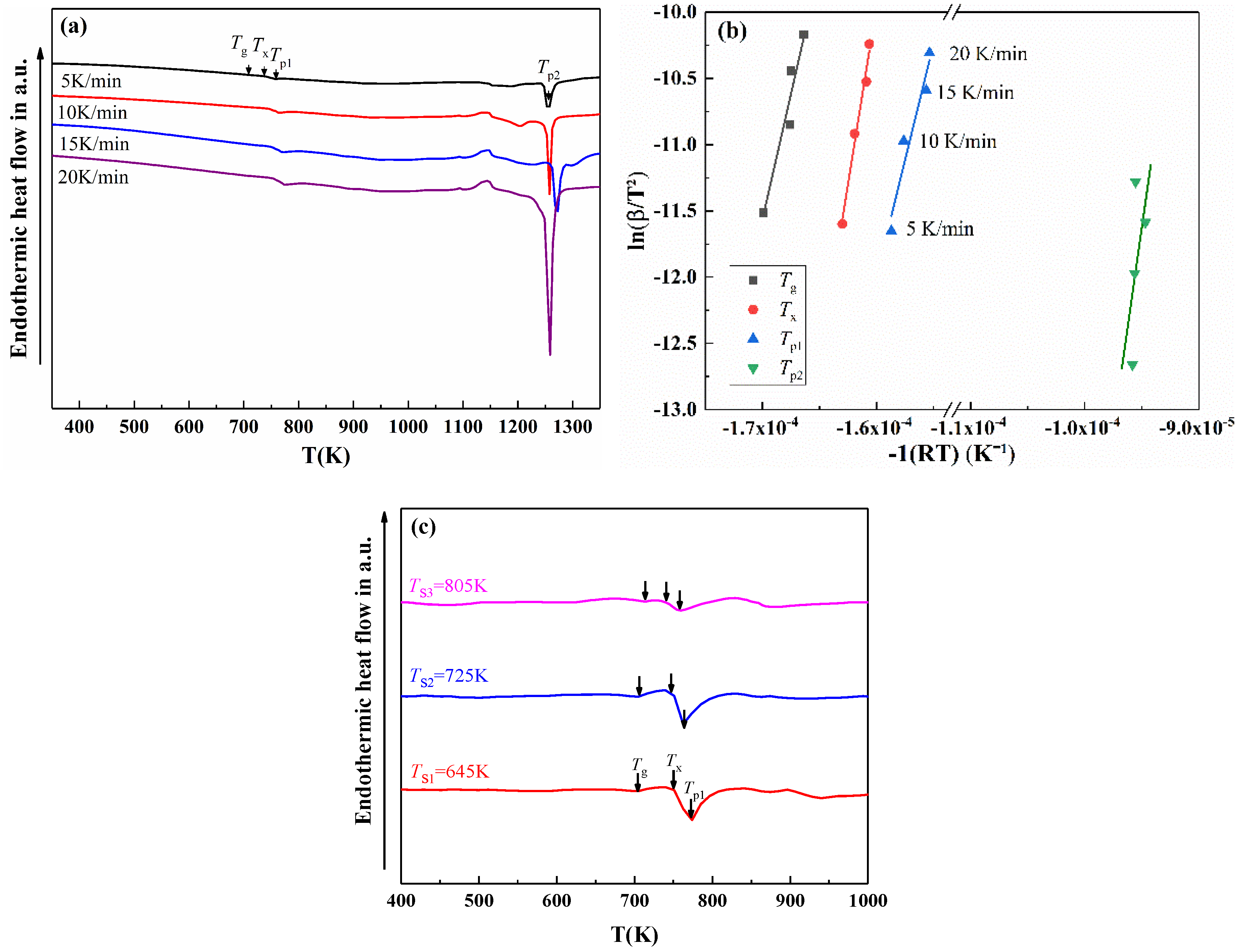




| Atomization Gas | Gas Pressure (MPa) | Gas Velocity (mm/s) | Superheat Temperature (°C) | Flow Diameter (mm) | Melt Velocity (kg/min) |
|---|---|---|---|---|---|
| N2 | 3.5 | 145 | 215 | 5–10 | 1.4 |
| No. | Spray Temperature (K) | Ratio of Air/Propane Pressure | N2 (L/min) | Spray Distance (mm) | Powder Feeding Rate (g/s) | Scan Rate (mm/s) | Coating Thickness (μm) |
|---|---|---|---|---|---|---|---|
| S1 | 645 | 90/80 | 35 | 160 | 0.6 | 400 | 560.6 ± 23 |
| S2 | 725 | 90/86 | 594.3 ± 16 | ||||
| S3 | 805 | 90/90 | 602.6 ± 15 |
| Heating Rates | Tg/K | Tx/K | Tp1/K | Tp2/K | ΔTx/K |
|---|---|---|---|---|---|
| 5 K | 707.9 | 737.9 | 757.9 | 1255.3 | 30.0 |
| 10 K | 717.8 | 742.8 | 763.1 | 1257.9 | 25.0 |
| 15 K | 718.1 | 747.6 | 772.8 | 1270.4 | 29.5 |
| 20 K | 723.0 | 748.8 | 774.2 | 1258.9 | 25.8 |
| Activation energy, E (kJ/mol) | 377.7 ± 7.4 | 537.9 ± 5.0 | 350.7 ± 6.6 | 619.5 ± 7.1 | \ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Dai, C.; Mao, W.; Ren, Z.; Wang, X.; Zhao, Y.; Han, G. Microstructure and Wear Properties of HVAF Sprayed Cu-Zr-Al-Ag-Co Amorphous Coatings at Different Spray Temperatures. Coatings 2022, 12, 458. https://doi.org/10.3390/coatings12040458
Wen S, Dai C, Mao W, Ren Z, Wang X, Zhao Y, Han G. Microstructure and Wear Properties of HVAF Sprayed Cu-Zr-Al-Ag-Co Amorphous Coatings at Different Spray Temperatures. Coatings. 2022; 12(4):458. https://doi.org/10.3390/coatings12040458
Chicago/Turabian StyleWen, Shu, Cuiying Dai, Weiguo Mao, Zhiqiang Ren, Xiaoming Wang, Yang Zhao, and Guofeng Han. 2022. "Microstructure and Wear Properties of HVAF Sprayed Cu-Zr-Al-Ag-Co Amorphous Coatings at Different Spray Temperatures" Coatings 12, no. 4: 458. https://doi.org/10.3390/coatings12040458






