A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
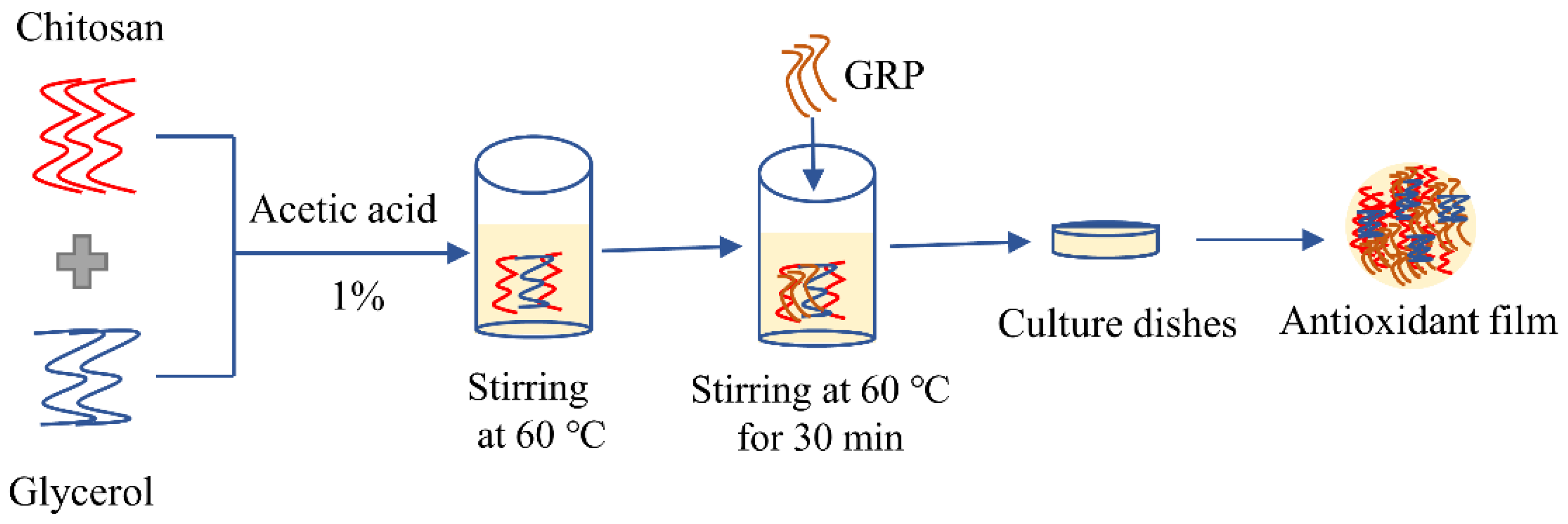
2.2. Preparations of Ginseng Residue Polysaccharides and Films
2.3. Determination of Physical Properties of Films
2.3.1. Thickness, Density, and Opacity
2.3.2. Moisture Content and Water Vapor Permeability
2.3.3. Mechanical Properties
2.4. Assays of Thermal Properties of Films
2.5. Assays of Structural Properties of Films
2.6. Assays of Microstructure of Films
2.7. Evaluations of Preservation Performance of Fresh-Cut Melon with Films
2.7.1. Fresh-Cut Melon Preparation
2.7.2. Determination of Quality Parameters and Antioxidant Parameters of Fresh-Cut Melon
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of GRP on Physical Properties of the Polysaccharide Films
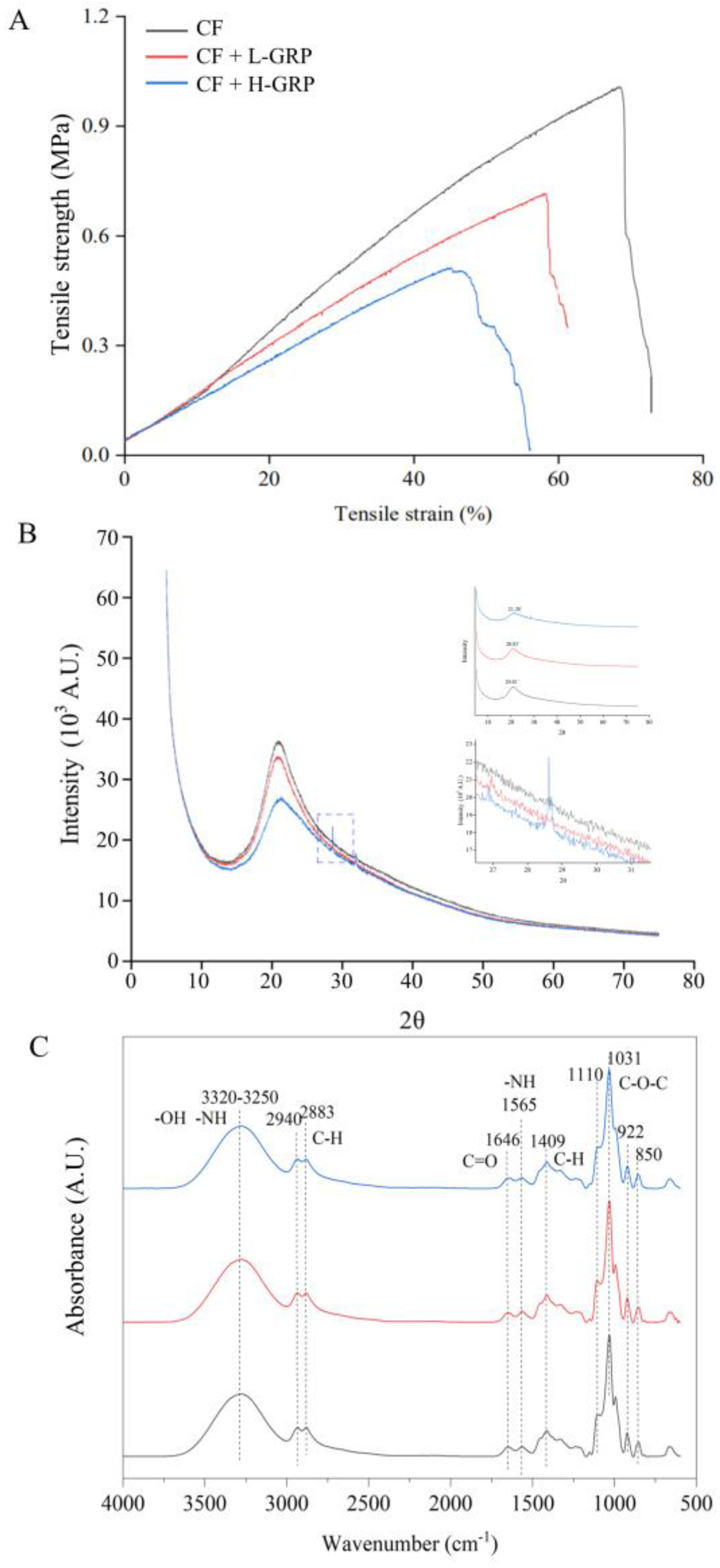
3.2. Effects of GRP on Mechanical Property and Chemical Structure of the Polysaccharide Films
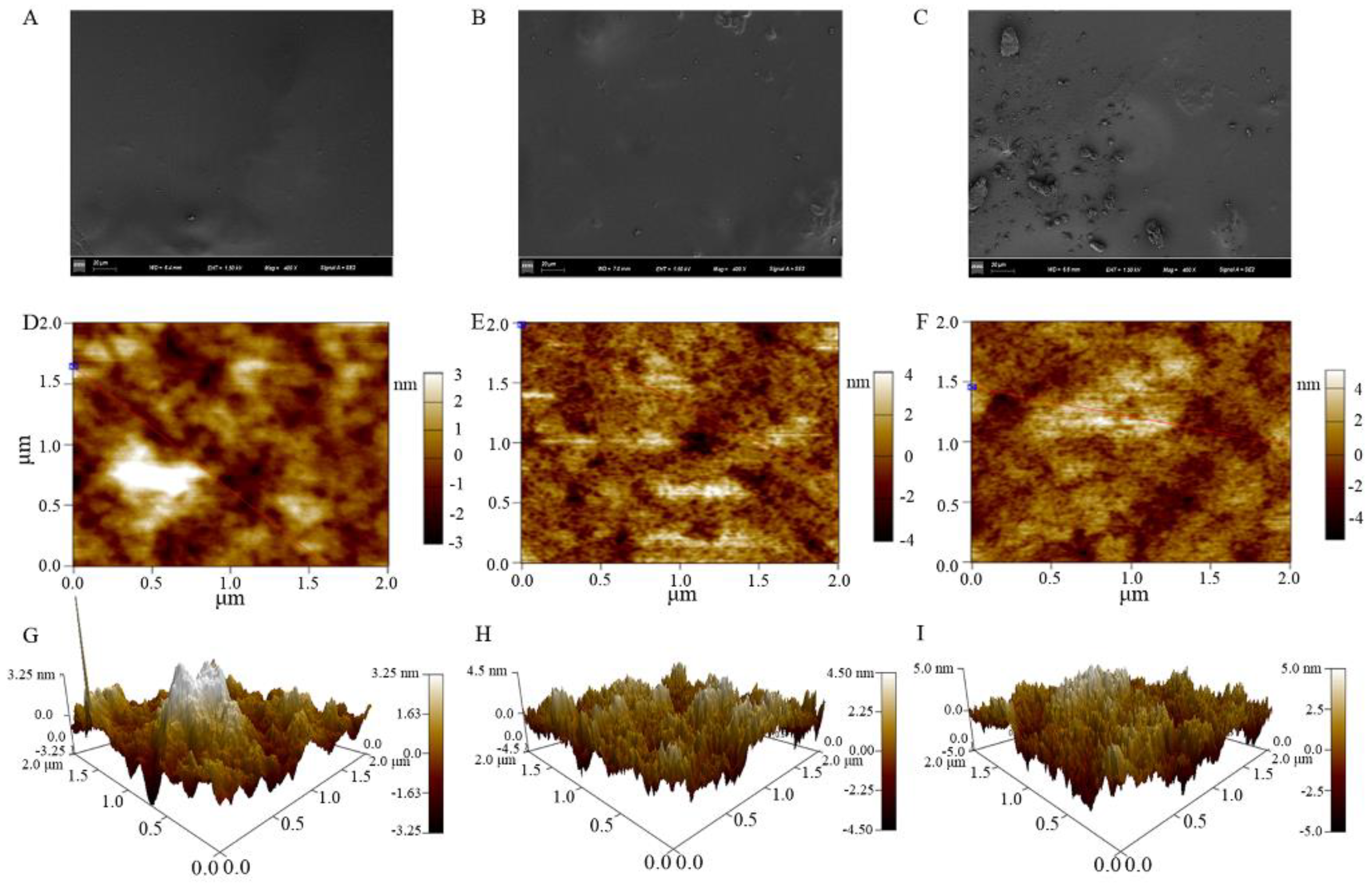
3.3. Effects of GRP on Microstructure of the Polysaccharide Films
3.4. Effects of the Composite Film on Preservation of Fresh-Cut Melon
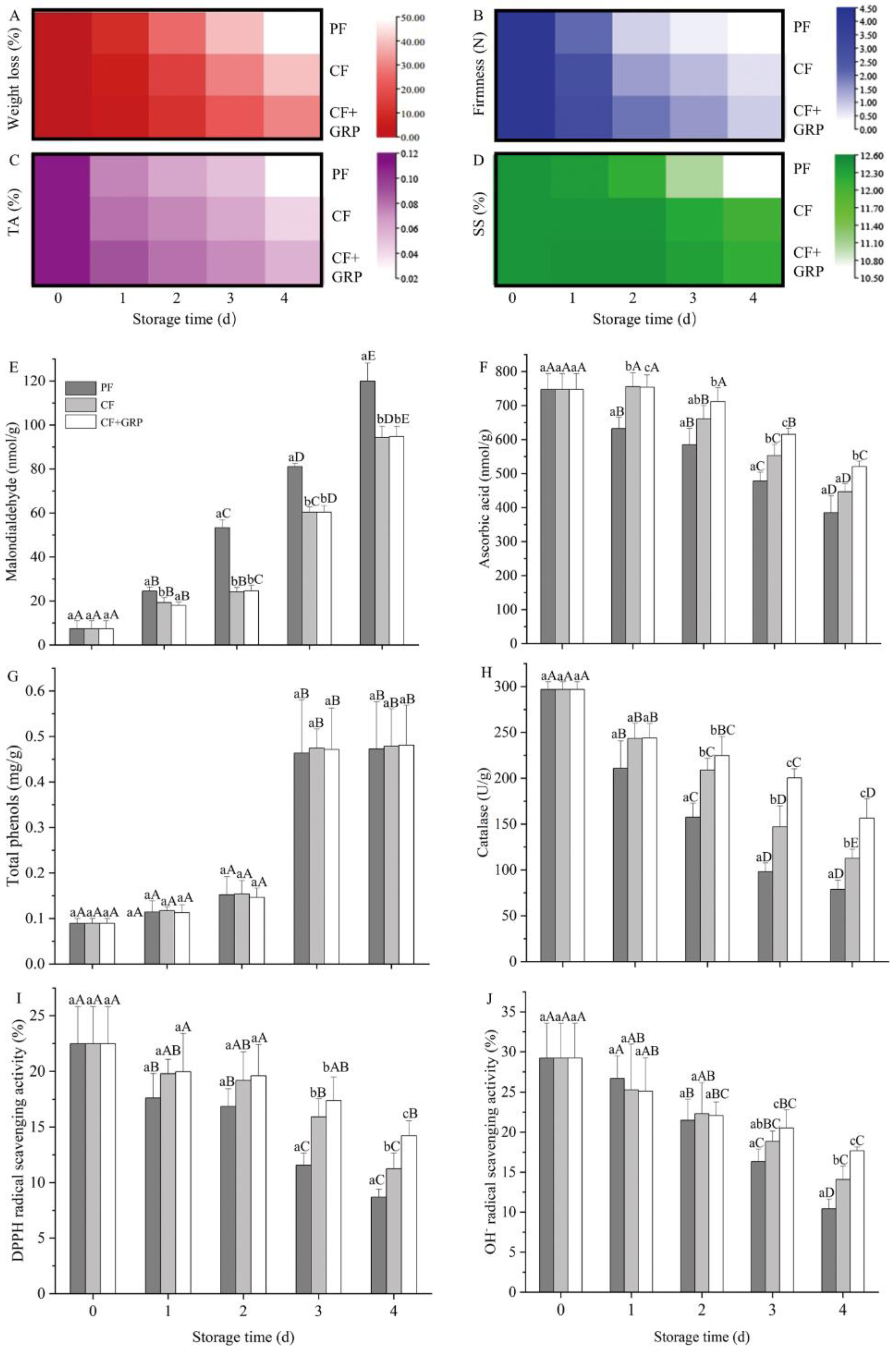
3.4.1. Effects on Appearance and Four Quality Parameters of the Fresh-Cut Melon
3.4.2. Effects on Six Antioxidant Indexes of Fresh-Cut Melon
3.4.3. Possible Mechanisms and PCA and Correlation Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque, B.; Lidon, F.C.; Barreiro, M.G. A case study on the flavor properties of melon (Cucumis melo L.) cultivars. Fruits 2006, 61, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.C.; Lea, J.M. Quality changes in cantaloupe during growth, maturation, and in stored fresh-cut cubes prepared from fruit harvested at various maturities. J. Am. Soc. Hortic. Sci. 2006, 132, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Shalit, M.; Katzi, N.; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M. Acetyl-CoA: Alcohol acetyltransferase activity and aroma formation in ripening melon fruits. J. Agric. Food Chem. 2001, 49, 794. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; De Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.D.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Vieira, M.G.; Silva, M.A.; Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Waibel, K.H.; Brian, H.; Moore, M.; Whisman, B.; Gomez, R. Safety of chitosan bandages in shellfish allergic patients. Mil. Med. 2011, 176, 1153–1156. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Xin, Y.; Chen, F.; Lai, S.; Yang, H. Influence of chitosan-based coatings on the physicochemical properties and pectin nanostructure of chinese cherry. Postharvest Biol. Technol. 2017, 133, 64–71. [Google Scholar]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of post-harvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active Edible Films Fortified with Natural Extracts: Case Study with Fresh-Cut Apple Pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Pérez Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101 (Suppl. C), 882–888. [Google Scholar] [CrossRef]
- Pérez Córdoba, L.J.; Sobral, P.J.A. Physical and antioxidant properties of films based on gelatin, gelatin-chitosan or gelatin-sodium caseinate blends loaded with nanoemulsified active compounds. J. Food Eng. 2017, 213, 47–53. [Google Scholar] [CrossRef]
- Parra, D.F.; Tadini, C.C.; Ponce, P.; Lugão, A.B. Mechanical properties and water vapor transmission in some blends of cassava starch edible films. Carbohydr. Polym. 2004, 58, 475–481. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Lu, R.; Xu, J.; Hu, K.; Liu, Y. Preparation of Chitosan/Corn Starch/Cinnamaldehyde Films for Strawberry Preservation. Foods 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Hu, M.; Gao, Z.; Li, M.; Jiang, Y. Apple polyphenols delay senescence and maintain edible quality in litchi fruit during storage. Postharvest Biol. Technol. 2019, 157, 110976. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Xu, Z.; Wang, Y. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of aloe barbadensis miller irrigated with sea water. Process Biochem. 2007, 42, 961–970. [Google Scholar]
- Priyadarshi, R.; Sauraj Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Duenas, M.T.; Guerrero, P.; de la Caba, K. Citric acid-incorporated fish gelatin/chitosan composite films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Shim, H.; Sah, H. Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry. Pharmaceutics 2020, 12, 626. [Google Scholar] [CrossRef]
- Don, T.M.; Liu, L.M.; Chen, M.; Huang, Y.C. Crosslinked complex films based on chitosan and ulvan with antioxidant and whitening activities. Algal Res. 2021, 58, 102423. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2019, 154, 1205–1214. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Rhim, J.W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, H.; Chen, J.; Li, S.; Huang, J.; Zhou, C. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. Carbohydr. Polym. 2016, 152, 832–840. [Google Scholar]
- Shashkov, A.S.; Liu, B.; Kenyon, J.J.; Popova, A.V.; Shneider, M.M.; Senchenkova, S.N.; Arbatsky, N.P.; Miroshnikov, K.A.; Lei, W.; Knirel, Y.A. Structures of the K35 and K15 capsular polysaccharides of Acinetobacter baumannii LUH5535 and LUH5554 containing amino and diamino uronic acids. Carbohydr. Res. 2017, 448, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhang, S.; Wang, Q.; Lu, X.; Lin, L.; Tian, Y.; Xiao, J.; Zheng, B. Characterization and hypoglycemic activity of a β-pyran polysaccharides from bamboo shoot (Leleba oldhami Nakal) shells. Carbohydr. Polym. 2016, 144, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; Berrios, J.D.J.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Tech. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Aday, M.S.; Temizkan, R.; Büyükcan, M.B.; Caner, C. An innovative technique for extending shelf life of strawberry: Ultrasound. LWT Food Sci. Tech. 2013, 52, 93–101. [Google Scholar] [CrossRef]
- Martiñon, M.E.; Moreira, R.G.; Castell-Perez, M.E.; Gomes, C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Sun, J.; You, X.; Li, L.; Peng, H.; Su, W.; Li, C.; He, Q.; Liao, F. Effects of a phospholipase D inhibitor on postharvest enzymatic browning and oxidative stress of litchi fruit. Postharvest Biol. Technol. 2011, 62, 288–294. [Google Scholar]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth gum impregnated with Satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Liu, J.; Kennedy, J.F.; Zhang, X.; Yin, H.; Wei, C.; Zhuo, C.; Wu, X. Preparation of alginate oligosaccharide and its effects on decay control and quality maintenance of harvested kiwifruit. Carbohydr. Polym. 2020, 242, 116462. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, M.; Geng, X.; Wang, H.; Ng, T.B. Characterization of Polysaccharides with Antioxidant and Hepatoprotective Activities from the Edible Mushroom Oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, S.; Khodaei, D.; Lacroix, M. Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries. Food Hydrocoll. 2021, 117, 106750. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Patre, D.D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Yu, S.H.; Tsai, M.L.; Lin, B.X.; Lin, C.W.; Mi, F.L. Tea catechins-crosslinked methylcellulose active films for inhibition of light irradiation and lipid peroxidation induced β-carotene degradation. Food Hydrocoll. 2015, 44, 491–505. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Hu, Y.; Zhu, Z.; Zhuang, C.; Zhao, Y.; Zhong, Y. The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. Int. J. Biol. Macromol. 2020, 151, 278–285. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, W.; Zheng, F.; Chen, F. Comparison of volatile compositions of 15 different varieties of Chinese jujube (Ziziphus jujuba Mill.). J. Food Sci. Technol. 2019, 56, 1631–1640. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Li, Y.; Cao, X.; Yao, F.; Shi, L.; Liu, Y. A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings 2022, 12, 468. https://doi.org/10.3390/coatings12040468
Sun J, Li Y, Cao X, Yao F, Shi L, Liu Y. A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings. 2022; 12(4):468. https://doi.org/10.3390/coatings12040468
Chicago/Turabian StyleSun, Jing, Yuanhang Li, Xinxin Cao, Fan Yao, Lingling Shi, and Yujun Liu. 2022. "A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon" Coatings 12, no. 4: 468. https://doi.org/10.3390/coatings12040468
APA StyleSun, J., Li, Y., Cao, X., Yao, F., Shi, L., & Liu, Y. (2022). A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings, 12(4), 468. https://doi.org/10.3390/coatings12040468





