Polymerisation Kinetics on FT-IR and Colorimetric Changes under UV Irradiation for a Commercial Polycyanoacrylate Adhesive, Addressed to Glass Restoration
Abstract
:1. Introduction
- (a)
- Satisfactory adhesion, either as a solution, emulsion, or as liquid pre-polymers. During the transition from liquid to gel or solid state, the contraction of the adhesive takes place and should not cause any physical damage to the object surface [1].
- (b)
- Reversibility upon request, usually by using solvents that dissolve linear polymers and swell the crosslinked ones. In the case of a lacquer coated on a smooth surface (glass or metal) total removal is possible; however, when the polymer is applied onto a porous material (paper or fabric) it seems impossible to remove the full amount of it.
- (c)
- Physical stability over time and towards external factors, since alterations, such as yellowing, brittleness, insolubility, contraction or expansion, fluidity, cracking, or dust absorption, are undesirable.
- (d)
- Certain acceptable aesthetics, provided that a conservation treatment should not alter the general appearance and interpretation of the object. (Interpretation means what the viewer, even the non-expert, perceives from the artifact, even if it is not visible to the eye, but can be perceived after scientific examination.) Generally, a conservation treatment must strengthen, reveal, and project the intention of the artist or manufacturer of the object and facilitate its interpretation for the world to see [2,3].
2. Materials and Methods
2.1. Polymerization Kinetics
2.2. Preparation of the Polycyanoacrylate Films
2.3. Ultraviolet Photooxidation
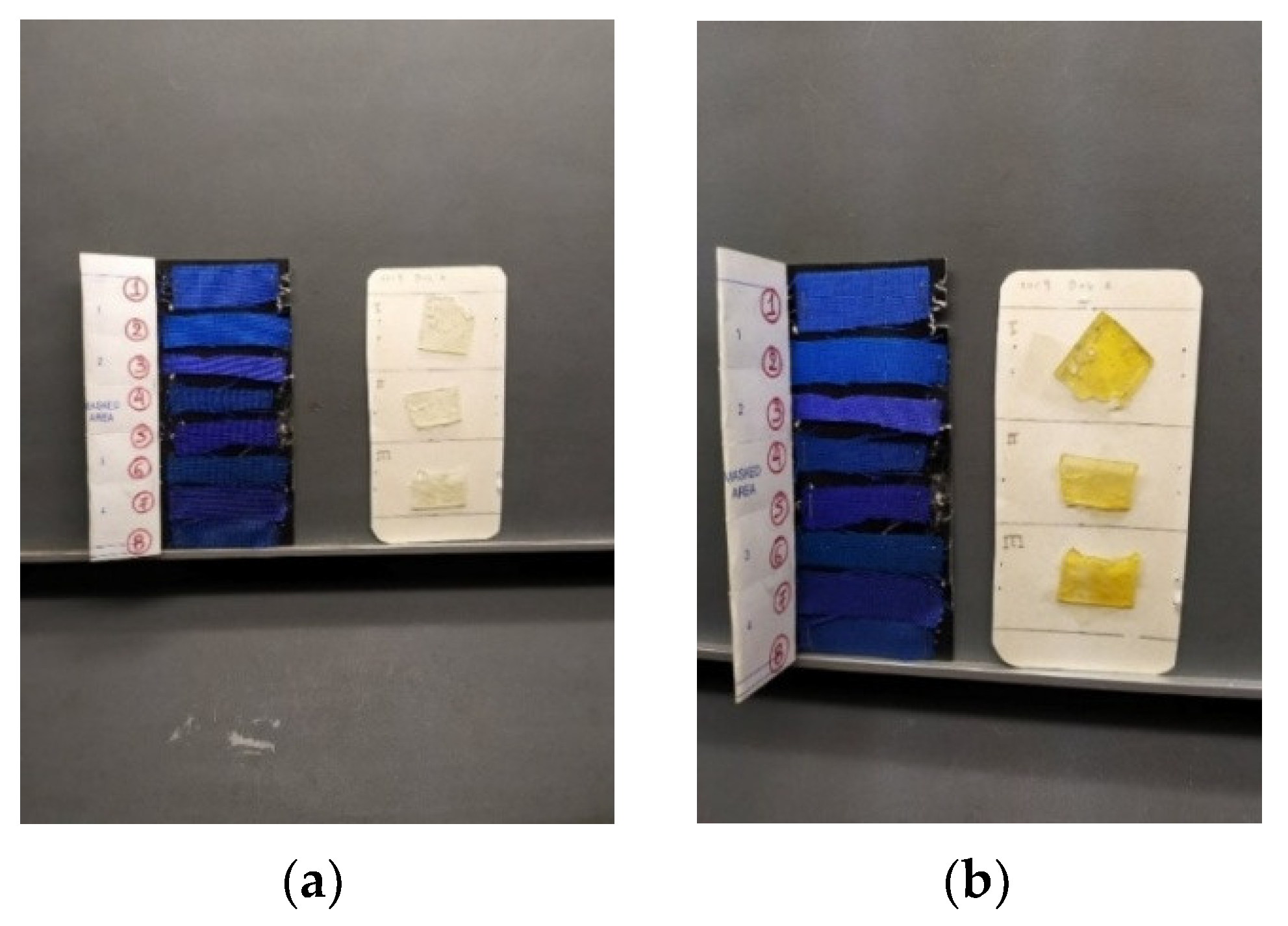
2.4. Visual Observation of Aged Samples
2.5. Colorimetry
3. Results
4. Discussion
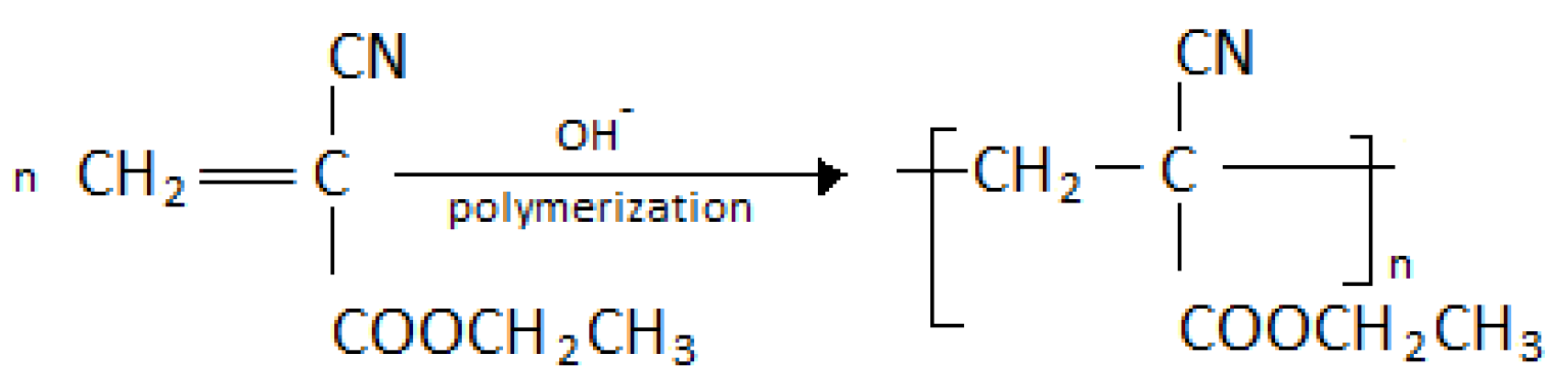
4.1. Polymerization Kinetics
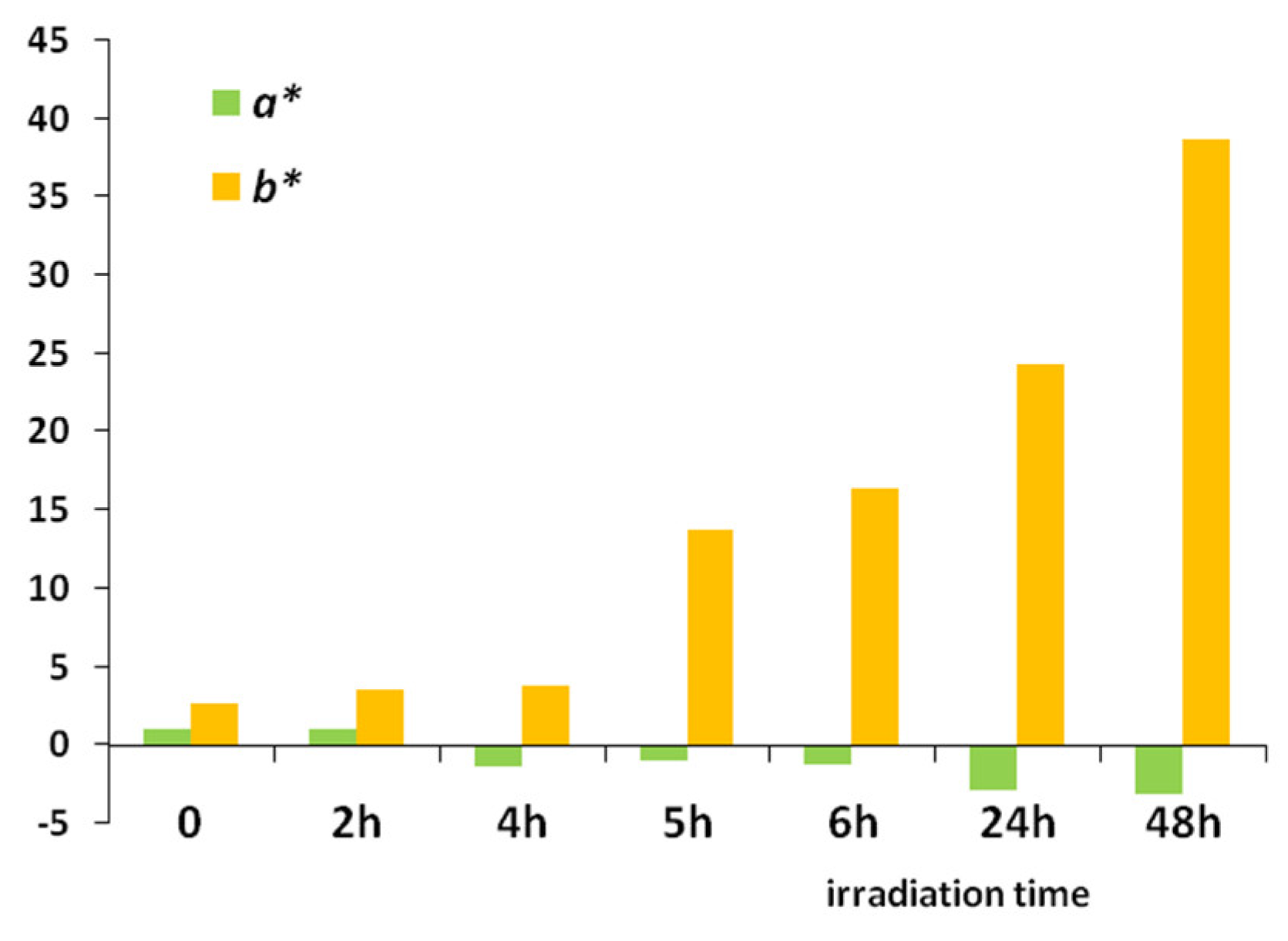
4.2. UV Weathering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, L.R.; Thickett, D. Testing Materials for Use in the Storage and Display of Antiquities—A Revised Methodology. Stud. Conserv. 1995, 40, 145–152. [Google Scholar]
- Baek, S.-S.; Jang, S.-J.; Hwang, S.-H. Preparation and Adhesion Performance of Transparent Acrylic Pressure Sensitive Adhesives: Effects of Substituent Structure of Acrylate Monomer. Int. J. Adhes. Adhes. 2016, 64, 72–77. [Google Scholar] [CrossRef]
- Machalická, K.; Eliášová, M. Adhesive Joints in Glass Structures: Effects of Various Materials in the Connection, Thickness of the Adhesive Layer, and Ageing. Int. J. Adhes. Adhes. 2017, 72, 10–22. [Google Scholar] [CrossRef]
- Blank, S.D. Rubber in Museums. AICCM Bull. 1988, 14, 53–93. [Google Scholar] [CrossRef]
- Castro, E.; Teresa, M.; Carbó, D. An Appraisal of the Properties of Adhesives Suitable for the Restoration of Spanish Medieval Ceramics. In The Conservation of Glass and Ceramics: Research, Practice and Training; Tennent, N.H., Ed.; Science Publishers: London, UK, 1999; pp. 114–132. [Google Scholar]
- Down, J.L. A Literature Review of Cyanoacrylate Adhesives. Stud. Conserv. 2001, 46, 35–38. [Google Scholar] [CrossRef]
- O’Donnell, A.; Sturge, T. The Conservation of Leather Artefacts: Case Studies from the Leather Conservation Centre. J. Am. Inst. Conserv. 2001, 40, 163–166. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Cheap non-toxic non-corrosive method of glass cleaning evaluated by contact angle, AFM, and SEM-EDX measurements. Environ. Sci. Pollut. Res. 2017, 24, 13373–13383. [Google Scholar] [CrossRef]
- Koob, S.P. Cleaning glass: A many-faceted issue. Objects Specialty Group Postprints. 2004, 11, 60–70. [Google Scholar]
- Homola, T.; Matoušek, J.; Kormunda, M.; Wu, L.Y.L.; Černák, M. Plasma treatment of glass surfaces using diffuse coplanar surface barrier discharge in ambient air. Plasma Chem. Plasma Processing 2013, 33, 881–894. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Zhao, S.; Dong, W. Hydrophobic and optical properties of silica antireflective coating prepared via sol-gel method. Mater. Res. Express 2001, 8, 046403. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Nano-porous sol-gel derived hydrophobic glass coating for increased light transmittance through greenhouse. Mater. Res. Bull. 2019, 116, 126–130. [Google Scholar] [CrossRef]
- Löbmann, P. Sol-gel processing of MgF2 antireflective coatings. Nanomaterials 2018, 8, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, P.; Borrelli, L.V. A Preliminary Note on the Use of Adhesives and Fillers in the Restoration of Ancient Materials with Special Reference to Glass. Stud. Conserv. 1975, 20, 201–205. [Google Scholar]
- Wartewig, S.; Neubert, R.H.H. Pharmaceutical Applications of Mid-IR and Raman Spectroscopy. Adv. Drug Deliv. Rev. 2005, 57, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kennedy, J.P. Polymerizability, Copolymerizability, and Properties of Cyanoacrylate. Polym. Adv. Technol. 2007, 18, 808–813. [Google Scholar] [CrossRef]
- Canale, A.J.; Goode, W.E.; Kinsinger, J.B.; Panchak, J.R.; Kelso, R.L.; Graham, R.K. Methyl α-cyanoacrylate. I. Free-radical Homopolymerization. J. Appl. Polym. Sci. 1960, 4, 231–236. [Google Scholar] [CrossRef]
- Han, M.G.; Kim, S. Controlled Degradation of Poly(ethyl cyanoacrylate-co-methyl methacrylate) (PECA-co-PMMA) Copolymers. Polymer 2009, 50, 1270–1280. [Google Scholar] [CrossRef]
- Baskaran, D.; Müller, A.H.E. Anionic Vinyl Polymerization. In Controlled and Living Polymerizations: From Mechanisms to Applications; Muller, A.H.E., Matyjaszewski, K., Eds.; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009; pp. 1–56. [Google Scholar]
- Whitaker, G.; Kincaid, B.J.; Raftery, D.P.; Van Hoof, N.; Regan, F.; Smyth, M.R.; Leonard, R.G. Potential of CE for the determination of inorganic and acidic anions in cyanoacrylate adhesives. Electrophoresis 2006, 27, 4532–4537. [Google Scholar] [CrossRef]
- Duffy, C.; Zetterlund, P.B.; Aldabbagh, F. Radical polymerization of alkyl 2-cyanoacrylates. Molecules 2018, 23, 465. [Google Scholar] [CrossRef] [Green Version]
- Estan-Cerezo, G.; Alonso, D.A.; Martín-Martínez, J.M. Structural and adhesion properties of poly (ethyl 2-cyanoacrylate) post-cured at different temperatures and times. J. Adhes. Sci. Technol. 2019, 33, 329–345. [Google Scholar] [CrossRef]
- Spathis, P.; Karagiannidou, E.; Magoula, A.-E. Influence of Titanium Dioxide Pigments on the Photodegradation of Paraloid Acrylic Resin. Stud. Conserv. 2003, 48, 57–64. [Google Scholar] [CrossRef]
- TDS Loctite® Super Attack Special Glass 3G. Henkel, ver 2018. Available online: https://dm.henkel-dam.com/is/content/henkel/tds (accessed on 10 June 2020).
- ISO Standards Regulation. Available online: https://www.iso.org/standard/3785.html (accessed on 12 February 2021).
- Jarikov, V.V.; Neckers, D.C. Anionic Photopolymerization of Methyl 2-Cyanoacrylate and Simultaneous Color Formation. Macromolecules 2000, 33, 7761–7764. [Google Scholar] [CrossRef]
- Tomlinson, S.K.; Ghita, O.R.; Hooper, R.M.; Evans, K.E. The Use of Near-Infrared Spectroscopy for the Cure Monitoring of an Ethyl Cyanoacrylate Adhesive. Vib. Spectrosc. 2006, 40, 133–141. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Vouvoudi, E.C.; Papadopoulos, G.D. Epoxy polymer Hxtal NYL-1™ used in restoration and conservation: Irradiation with short and long wavelengths and study of photo-oxidation by FT–IR spectroscopy. J. Cult. Herit. 2016, 18, 279–289. [Google Scholar] [CrossRef]
- Dey, T.; Carter, J.C.; Swift, K. SEM-EDX and FTIR analysis of archaeological ceramic potteries from southern Italy. Microscopy 2020, 69, 371–380. [Google Scholar] [CrossRef]
- Seetha, D.; Velraj, G. Determination of firing techniques of ancient artifacts by using FT-IR analysis. Chem. Sci. Rev. Lett. 2014, 2, 456–463. [Google Scholar]
- Thickett, D.; Pretzel, B. FTIR surface analysis for conservation. Herit. Sci. 2020, 8, 5. [Google Scholar] [CrossRef]
- López-Ballester, E.; Doménech-Carbó, M.T.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Study by FT-IR spectroscopy of ageing of adhesives used in restoration of archaeological glass objects. J. Mol. Struct. 1999, 482, 525–531. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, C.M.; Abidov, A.; Ravikumar, R.; Palanichamy, M.; Choi, E.Y.; Jang, H.T. Synthesis and Characterization of 1-octyl 2-cyano Acrylate for Wound Healing Applications. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Klemarczyk, P. Adhesion Studies of Mixtures of Ethyl Cyanoacrylate with a Difunctional Cyanoacrylate Monomer and with Other Electron-deficient Olefins. J. Adhes. 1999, 69, 293–306. [Google Scholar] [CrossRef]
- Han, M.G.; Kim, S.; Liu, S.X. Synthesis and degradation behavior of poly(ethyl cyanoacrylate). Polym. Degrad. Stab. 2008, 93, 1243–1251. [Google Scholar] [CrossRef]
- Flynn, J.H. Lifetime Prediction for Polymers from Thermal Analytical Experiments—Problems and How to Deal with Some of Them. Thermochim. Acta 1988, 134, 115–120. [Google Scholar] [CrossRef]
- Boxhammer, J. Shorter Test Times for Thermal- and Radiation-Induced Ageing of Polymer Materials. Polym. Test. 2001, 20, 719–724. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. UV ageing studies: Evaluation of lightfastness declarations of commercial acrylic paints. Anal. Bioanal. Chem. 2012, 402, 1567–1584. [Google Scholar] [CrossRef]
- Feller, R.L. Accelerating Aging—Photochemical and Thermal Aspects; The Getty Conservation Institute: Los Angeles, CA, USA, 1994. [Google Scholar]
- Feller, R.L. Further Studies on the International Blue-Wool Standards for the Exposure to Light. In Proceedings of the 5th Triennial Meeting, Zagreb, Croatia, 18 February 1978. [Google Scholar]
- Hattori, H.; Yoshizumi, K.; Crews, P.C. Wavelength Sensitivity of AATCC Blue Wool Lightfastness Standards under Light Radiation. Dye. Pigment. 2012, 92, 936–941. [Google Scholar] [CrossRef]
- Blum, M.-M.; John, H. Historical Perspective and Modern Applications of Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR). Drug Test. Anal. 2011, 4, 298–302. [Google Scholar] [CrossRef]
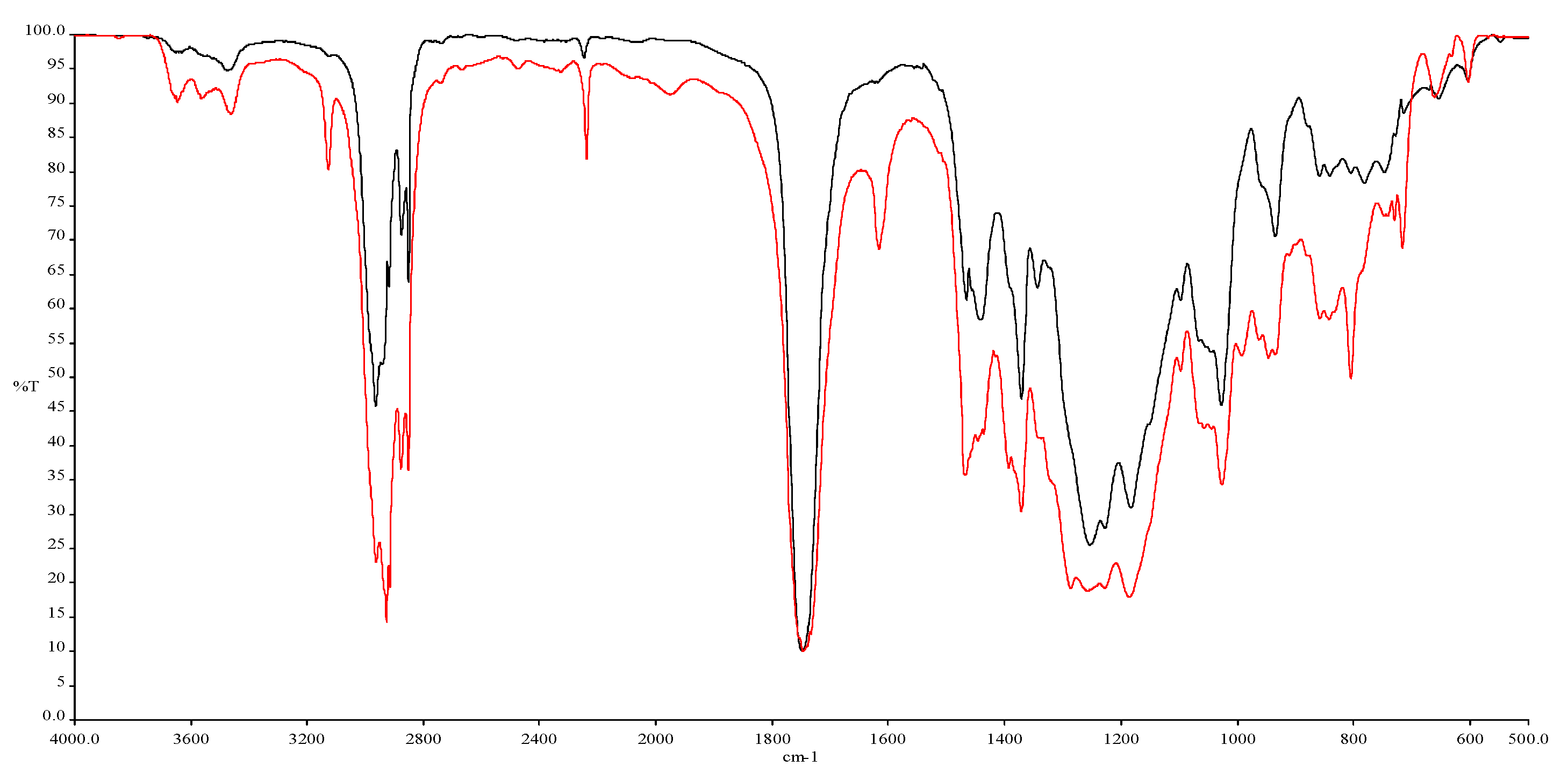


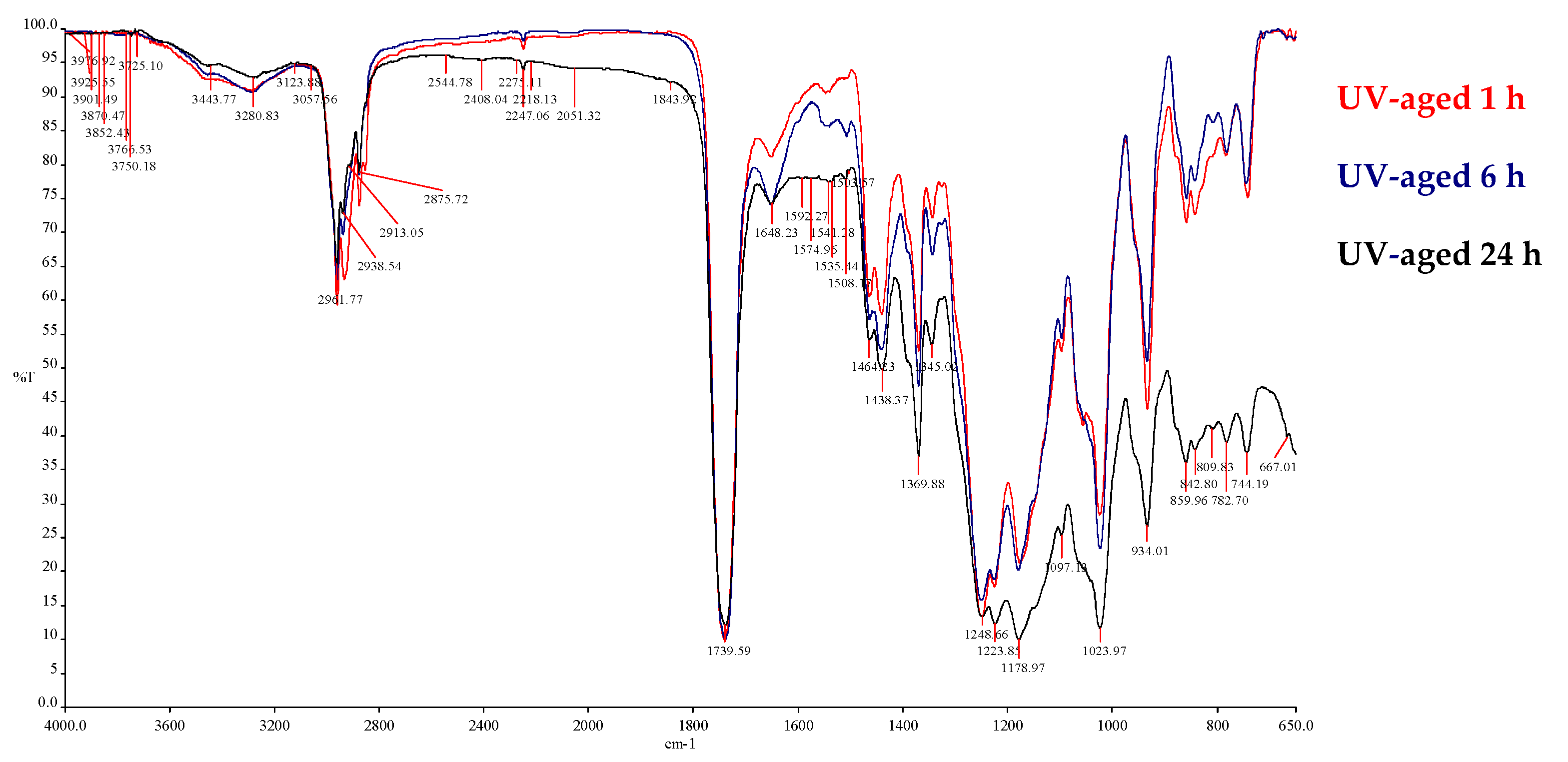
| v (cm−1) Absorbance | Peak Shape | Fun. Group |
|---|---|---|
| 3649–3462 | Weak, medium | O–H stretch |
| 3124 | Medium, sharp | =CH– and =CH2 stretch |
| 2957–2859 | Strong, Sharp | Asymmetric and symmetric stretching vibrations of –CH2– and –CH3 |
| 2237 | Medium, sharp | –C≡N stretch |
| 1749 | Sharp, strong | C=O stretch |
| 1617 | Medium, sharp | C=C stretch |
| 1457 | Medium, wide | C–H |
| 1370, 1342 | Medium | –CH2– and -CH3 scissoring and bending region |
| 1255, 1227, 1183 | Medium, sharp | –COO– |
| 1097 | Strong, sharp | C–O |
| 1027 | Strong, sharp | C–N |
| 934 | Medium | C–O–H bend |
| 881 | Medium | C=C bend |
| 730–667 | Medium | Deformation frequencies of C–H bonds |
| t | L* | α* | b* | C* | h° | R | ΔE* | k/s |
|---|---|---|---|---|---|---|---|---|
| 0 | 44.8 | 1.1 | 2.7 | 33.7 | 81.0 | 34.7 | 1.1 | 16.4 |
| 2 h | 44.1 | 1.1 | 3.6 | 34.0 | 82.3 | 31.5 | 3.3 | 14.8 |
| 4 h | 42.9 | −1.3 | 3.8 | 28.6 | 83.6 | 26.7 | 11.5 | 12.4 |
| 5 h | 46.9 | −1.0 | 13.8 | 24.6 | 85.0 | 26.6 | 19.3 | 12.3 |
| 6 h | 31.4 | −1.2 | 16.4 | 26.8 | 87.5 | 24.5 | 25.8 | 11.3 |
| 24 h | 31.2 | −2.8 | 24.3 | 32.3 | 92.6 | 9.5 | 39.0 | 3.8 |
| 48 h | 30.4 | −3.1 | 38.7 | 26.8 | 95.7 | 8.7 | 44.9 | 3.4 |
| Exposure Time | Blue Indicators | Grey Scale |
|---|---|---|
| 15 min | No change | 5 |
| 30 min | >> | 4 |
| 45 min | >> | 4 |
| 60 min | >> | 3 |
| 90 min | >> | 3 |
| 2 h | >> | 2 |
| 3 h | >> | 2 |
| 6 h | >> | 1 |
| 24 h | >> | 1 |
| 48 h | >> | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vouvoudi, E.C.; Morfis, P.D.; Verros, G.D.; Achilias, D.S. Polymerisation Kinetics on FT-IR and Colorimetric Changes under UV Irradiation for a Commercial Polycyanoacrylate Adhesive, Addressed to Glass Restoration. Coatings 2022, 12, 490. https://doi.org/10.3390/coatings12040490
Vouvoudi EC, Morfis PD, Verros GD, Achilias DS. Polymerisation Kinetics on FT-IR and Colorimetric Changes under UV Irradiation for a Commercial Polycyanoacrylate Adhesive, Addressed to Glass Restoration. Coatings. 2022; 12(4):490. https://doi.org/10.3390/coatings12040490
Chicago/Turabian StyleVouvoudi, Evangelia C., Panagiotis D. Morfis, George D. Verros, and Dimitris S. Achilias. 2022. "Polymerisation Kinetics on FT-IR and Colorimetric Changes under UV Irradiation for a Commercial Polycyanoacrylate Adhesive, Addressed to Glass Restoration" Coatings 12, no. 4: 490. https://doi.org/10.3390/coatings12040490
APA StyleVouvoudi, E. C., Morfis, P. D., Verros, G. D., & Achilias, D. S. (2022). Polymerisation Kinetics on FT-IR and Colorimetric Changes under UV Irradiation for a Commercial Polycyanoacrylate Adhesive, Addressed to Glass Restoration. Coatings, 12(4), 490. https://doi.org/10.3390/coatings12040490








