Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5
Abstract
:1. Introduction
2. Element Selection Basis
2.1. The Principles of High-Entropy Alloy and Biomaterial Design
2.2. The Influence of Elements on Mg Alloys
3. Performance Simulation
3.1. Theoretical Basis
- Assuming G (Total Gibbs Free Energy) of the system reaches the minimum value G min, the chemical potentials of each component i are equal. Equation (1) is the molar Gibbs Free Energy of each term.
- B.
- Assuming the solute diffusion in the solid phase can be ignored, and the solute in the liquid phase diffuses rapidly and completely, Equation (2) calculates the alloy composition in the solid phase. Equation (3) calculates the fraction of solid formed.
- C.
- Assuming that non-equilibrium conditions are met, Equation (4) calculates the relative properties of each phase based on the alloy composition of each phase. Equation (5) calculates the overall properties of the material according to the law of mixing.
3.2. Simulation Conditions
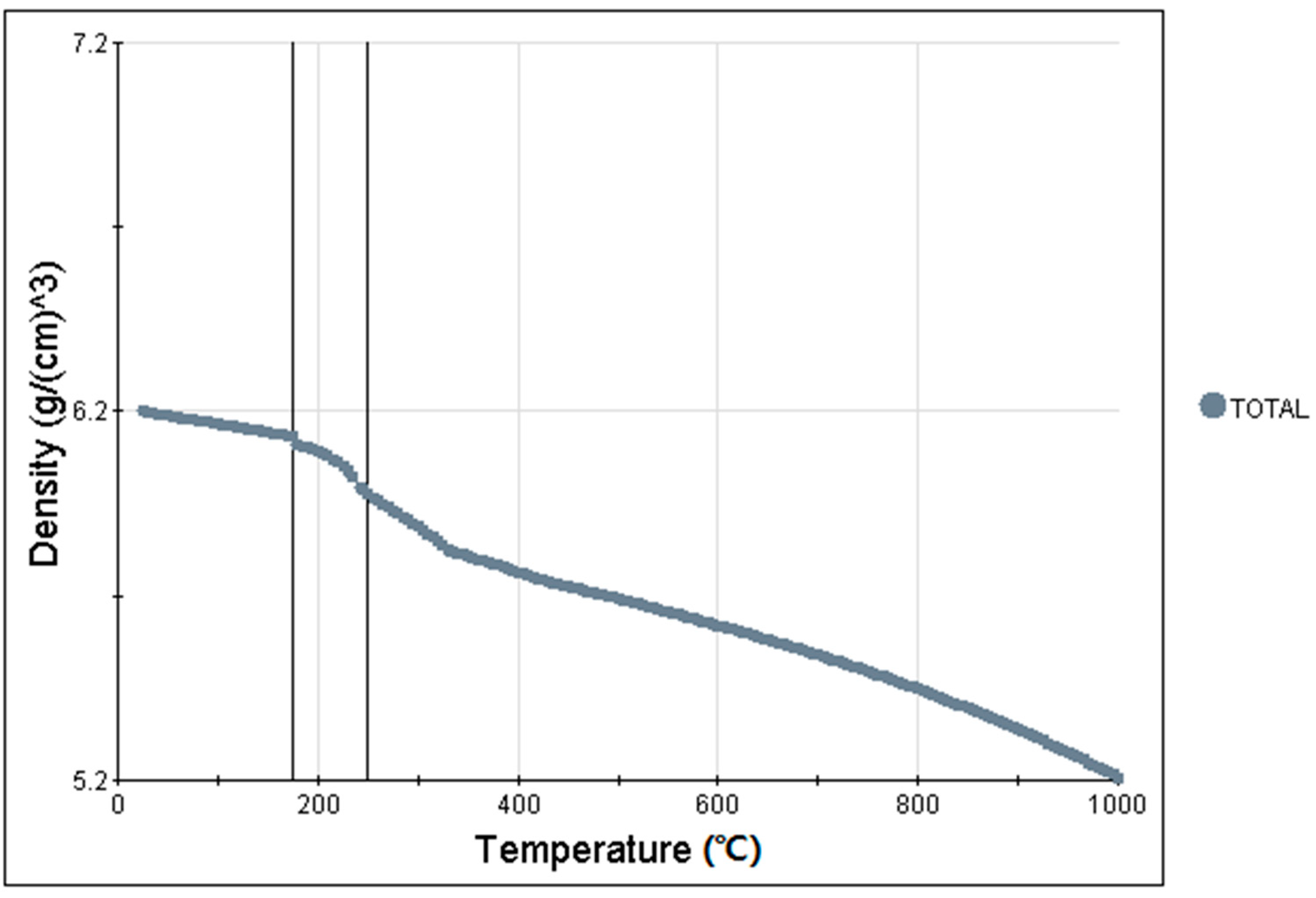
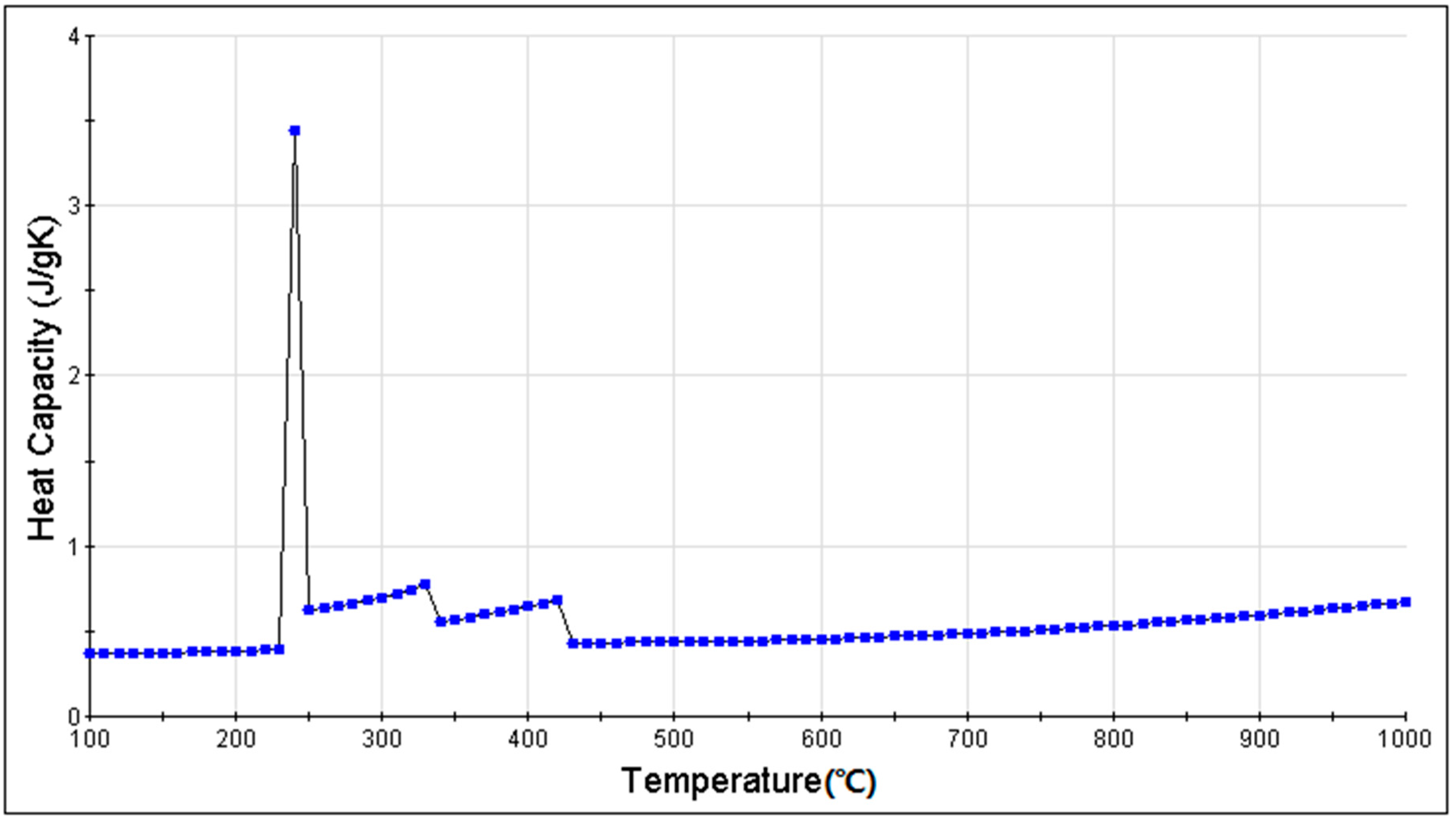
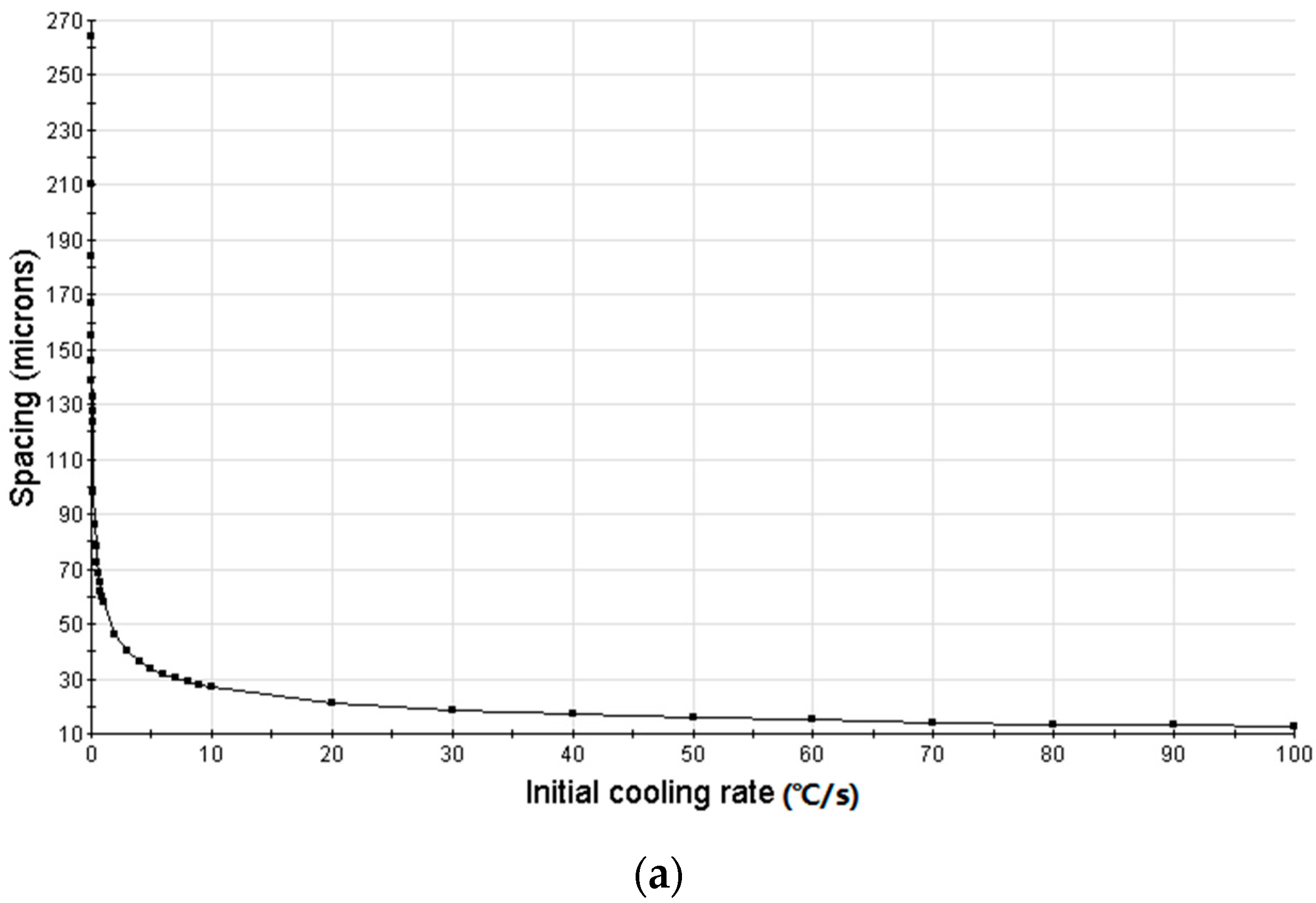
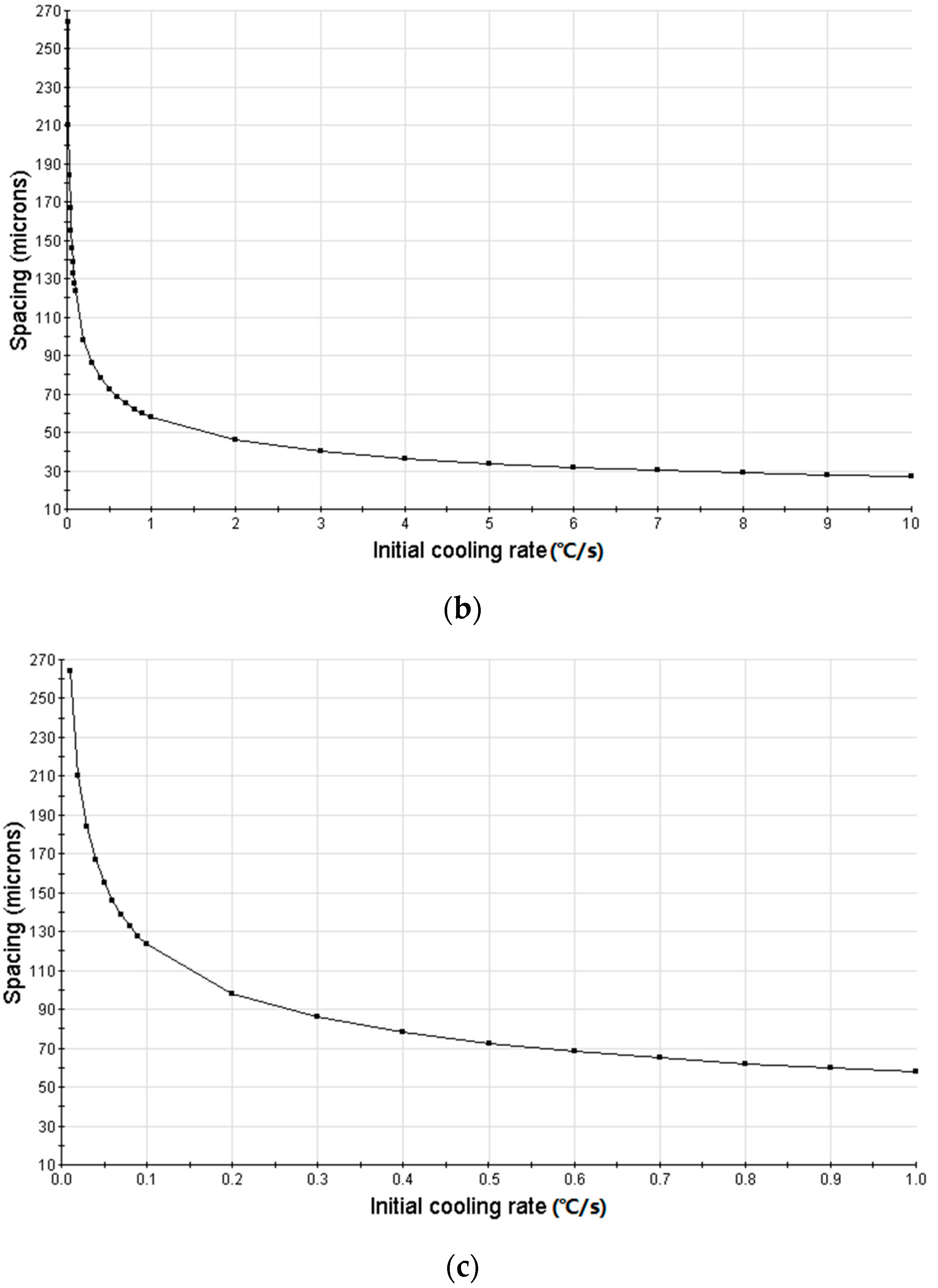
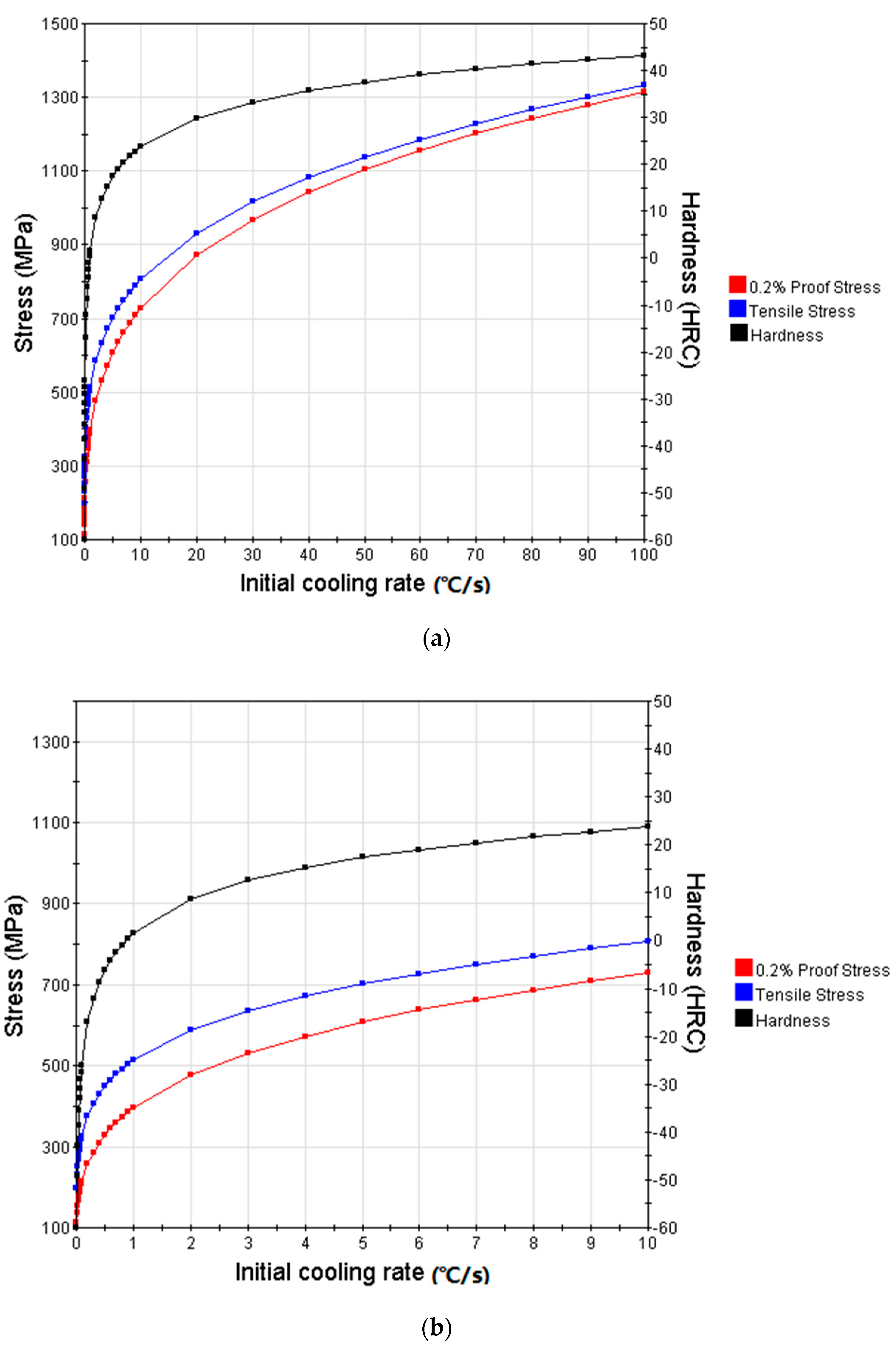
3.3. Simulation Results and Analysis
4. Preparation Method
4.1. As-Cast Alloy Preparation
4.2. Experimental Preparation
5. Results
5.1. XRD and SEM
5.2. Mechanical Properties
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRO Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Fini, M.; Nicoli Aldini, N.; Torricelli, P.; Giavaresi, G.; Borsari, V.; Lenger, H.; Bernauer, J.; Giardino, R.; Chiesa, R.; Cigada, A. A new austenitic stainless steel with engligible nickel content: An in vitro and in vivo comparative investigation. Biomaterials 2003, 24, 4929–4939. [Google Scholar] [CrossRef]
- Khandan, A.; Abdellahi, M.; Barenji, R.V.; Ozada, N.; Karamian, E. Introducing natural hydroxyapatite-diopside (NHA-Di) nano-bioceramic coating. Ceram. Int. 2015, 41, 12355–12363. [Google Scholar] [CrossRef]
- MA, X.M.; YANG, Y.J.; SUN, W. Current Research Progress of β-type Ti-Nb-Ta-Zr Alloys for Biomedical Applications. Mater. Rep. 2008, 8, 344–347. [Google Scholar]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231. [Google Scholar] [CrossRef]
- Wang, Y.F.; He, L.; Guo, W. Research and Application of Medical Titanium Alloy. Titan. Ind. Prog. 2015, 32, 1–6. [Google Scholar]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Master. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ba, Z.X.; Wang, Q.; Wu, Y.J.; Wang, Z.Z.; Wang, Q. Uniform corrosion behavior of GZ51K alloy with long period stacking ordered structure for biomedical application. Corros. Sci. 2014, 88, 1–5. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater Sci. Eng. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Lin, X.; Yang, X.M.; Tan, L.L.; Li, M.; Wang, X.; Zhang, Y.; Yang, K.; Hua, Z.Q.; Qiu, J.H. In vitro degradation and biocompatibility of a strontium-containing micro-arc oxidation coating on the biodegradable ZK60 magnesium alloy. Appl. Surf. Sci. 2014, 288, 718–726. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Chen, J.H.; Zou, Z.Y. Effects of tensile and compressive deformation on corrosion behaviour of a Mg–Zn alloy. Corros. Sci. 2015, 90, 445–450. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Seong, J.W.; Kim, W.J. Development of biodegradable Mg–Ca alloy sheets with enhanced strength and corrosion properties through the refinement and uniform dispersion of the Mg2Ca phase by high-ratio differential speed rolling. Acta Biomater. 2015, 11, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, Y.; Bi, Y.Z.; Li, Y.; Zheng, Y.F. Improved cytocompatibility of Mg-1Ca alloy modified by Zn ion implantation and deposition. Mater. Lett. 2017, 205, 87–89. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. Mg ZnCa glasses without clinically observablehydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Zhong, S.; Xi, T.; Wang, J.; Wang, W. Corrosion of, and cellular responses to Mg-Zn-Ca bulkmetallic glasses. Biomaterials 2010, 31, 1093–1103. [Google Scholar] [CrossRef]
- Witte, F.; Reifenrath, J.; Muller, P.; Crostack, H.-A.; Nellesen, J.; Bach, F.; Bormann, D.; Rudert, M. Cartilage Repair on Magnesium Scaffolds Used as a Subchondral Bone Replacement. Mater. Und Werkst. 2006, 37, 504–508. [Google Scholar] [CrossRef]
- Waizy, H.; Dickmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2013, 28, 667. [Google Scholar] [CrossRef]
- Dickmann, J.; Bauer, S.; Weizbauer, A.; Willbold, E.; Windhagen, H.; Helmecke, P.; Lucas, A.; Reifenrath, J.; Nolte, I.; Ezechieli, M. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: A pilot in vivo study in rabbits. Mater. Sci. Eng. C 2016, 59, 1100–1109. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Kenan, C.; Chuanbin, G. Research progress on biodegradable medial magnesium-based materials. Int. J. Stomatol. 2021, 48, 322–328. [Google Scholar]
- Kumar, A.; Pandey, P.M. Development of Mg based biomaterial with improved mechanical degradation properties using powder metallurgy. J. Magnes. Alloy. 2020, 8, 883–898. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 Update of Bone Physiology and Wolff’s Law for Clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar]
- Raffa, M.L.; VNguyen, H.; Hernigou, P.; Flouzat-Lachaniette, C.H.; Haiat, G. Stress Shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ration. Orthop Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Microstructure, Mechanical Properties and the Mechanism of Film Layer Formation and Degradation of Mg Alloys Containing Ca and Nd. Ph.D. Thesis, School of Materials Science and Engineering, University of Science and Technology, Beijing, China, 2019. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Knight, P.; Chang, I.T.H.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloy. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys—Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Toda-Caraballo, I.; Rivera-Diaz-del-Castillo, P.E. Modelling solid solution hardening in high entropy alloys. Acta Mater. 2015, 85, 14–23. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Gupta, M. High Entropy Alloys—Innovations, Advances, and Applications, 1st ed.; CRC Press—Tayior & Francis Group: Boca Raton, FL, USA, 2020. [Google Scholar]
- Yin, B.; Curtin, W.A. First-principles-based Prediction of Yield Strength in the RhIrPdPtNiCu High-Entropy Alloy. NPJ Comput. Mater. 2019, 5, 14. [Google Scholar]
- Kasem, M.R.; Hoshi, K.; Jha, R.; Katsuno, M.; Yamashita, A.; Goto, Y.; Matsuda, T.D.; Aoki, Y.; Mizuguchi, Y. Superconducting Properties of High-entropy-alloy Tellurides M-Te (M: Ag, In, Cd, Sn, Sb, Pb, Bi) with a NaCl-type Structure. Appl. Phys. Express 2020, 13, 033001. [Google Scholar] [CrossRef]
- Shivam, V.; Sanjana, V.; Mukhopadhyay, N.K. Phase Evolution and Thermal Stability of Mechanically Alloyed AlCrFeCoNiZn High-Entropy Alloy. Trans. Indian Inst. Met. 2020, 73, 821–830. [Google Scholar] [CrossRef]
- Poletti, M.G.; Branz, S.; Fiore, G.; Szost, B.A.; Crichton, W.A.; Battezzati, L. Equilibrium High Entropy Phases in X-NbTaTiZr (X = Al, V, Cr and Sn) Multiprincipal Component Alloys. J. Alloy. Compd. 2016, 655, 139–146. [Google Scholar] [CrossRef]
- Singh, N.; Shadangi, Y.; Mukhopadhyay, N.K. Phase Evolution and Thermal Stability of Low-Density MgAlSiCrFe High-Entropy Alloy Processed Through Mechanical Alloying. Trans. Indian Inst. Met. 2020, 73, 2377–2386. [Google Scholar] [CrossRef]
- Sandar, T.K.; Manoj, G. Microstructural Evolution in MgAlLiZnCaY and MgAlLiZnCaCu Multicomponent High Entropy Alloys. Mater. Sci. Forum 2018, 928, 183–187. [Google Scholar]
- Zong, X.; Zhang, J.; Liu, W.; Zhang, Y.; You, Z.; Xu, C. Corrosion Behaviors of Long-Period Stacking Ordered Structure in Mg Alloys Used in Biomaterials: A Review. Adv. Eng. Mater. 2018, 20, 1800017. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals-Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, Z.; Zhu, S.; Guan, S. Effetcs of Sr Addition on Microstructure, Mechanical and Corrosion Properties of Biodegradable Mg-Zn-Ca Alloy. J. Alloy. Compd. 2020, 838, 155611. [Google Scholar] [CrossRef]
- Chen, K.; Xie, X.; Tang, H.; Sun, H.; Qin, L.; Zheng, Y.; Gu, X.; Fan, Y. In Vitro and in Vivo Degradation Behavior of Mg-2Sr-Ca and Mg-2Sr-Zn alloys. Bioact. Mater. 2020, 5, 275–285. [Google Scholar] [CrossRef]
- Sheng, L.; Du, B.; Hu, Z.; Qiao, Y.; Xiao, Z.; Wang, B.; Xu, D.; Zheng, Y.; Xi, T. Effects of Annealing Treatment on Microstructure and Tensile Behavior of the Mg-Zn-Y-Nd Alloy. J Magnes Alloys. 2020, 8, 601–613. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, C.; Fan, H.; Fan, Y.; Jiang, Q.; Song, P.; Fan, H.; Chen, Y.; Zhang, X. Bio-functional Design, Application and Trends in Metallic Biomaterials. Int. J. Mol. Sci. 2018, 19, 24. [Google Scholar] [CrossRef] [Green Version]
- Ostrakhovitch, E.A.; Cherian, M.G. Tin. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier: London, UK, 2015; Chapter 42; pp. 839–859. [Google Scholar]
- Pouria, A.; Bandegani, H.; Pourbaghi-Masouleh, M.; Hesaraki, S.; Alizadeh, M. Physicochemical Properties and Cellular Responses of Strontium-Doped Gypsum Biomaterials. Bioinorg. Chem. Appl. 2012, 2012, 976495. [Google Scholar] [CrossRef]
- Skerfving, S.; Bergdahl, I.A. Lead. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier: London, UK, 2015; Chapter 31; pp. 599–643. [Google Scholar]
- Ren, W.L.; Li, Q.A.; Shi, Y.J. Effect of Bi on microstructure and mechanical properties of AZ81 magnesium alloy. Rare Met. Cem. Carbides 2010, 38, 34–37. [Google Scholar]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M.; Friberg, L. Handbook on the Toxicology of Metals, 3rd ed.; Academic Press: Burlington, FA, USA, 2007; Chapter 22; pp. 433–443. [Google Scholar]
- Chen, G.; Fan, P.G.; Peng, X.D.; Cao, P.J.; Cai, W. Effect of Sr addition on the microstructure and the properties of AZ91 magnesium alloy. Light Alloy. Processing Technol. 2008, 36, 15–18. [Google Scholar]
- Qin, W.; Kolooshani, A.; Kolahdooz, A.; Saber-Samandari, S.; Khazaei, S.; Khandan, A.; Ren, F.; Toghraie, D. Coating the magnesium implants with reinforced nanocomposite nanoparticles for use in orthopedic applications. Colloids Surf. A Physicochem. Eng. Aspects. 2021, 621, 126581. [Google Scholar] [CrossRef]
- Karamian, E.; Motamedi, M.R.K.; Khandan, A.; Soltani, P.; Maghsoudi, S. An in virto evaluation of novel NHA/zircon plasma coating on 316L stainless steel dental implant. Prog. Nat. Sci. Mater. Int. 2014, 24, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Guangyin, Y.; Jia, Z.; Wenjiang, D. Research Progress of Mg-Based Alloys as Degradable Biomedical Materials. Mater. China 2011, 30, 44–50. [Google Scholar]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion fatigue behaviors of two biomedical Mg alloys—AZ91D and WE43—In simulated body fluid. Acta Biomater. 2010, 5, 4605–4613. [Google Scholar] [CrossRef]





| Mg at.% | Zn at.% | Sn at.% | Sr at.% | Bi at.% | O at.% | |
|---|---|---|---|---|---|---|
| Spectrogram 1 | 9.86 | 1.65 | 2.31 | 2.35 | 20.39 | 63.43 |
| Spectrogram 2 | 50.60 | 0.25 | 27.34 | 0.34 | 0.08 | 21.39 |
| Spectrogram 3 | 41.94 | 0.29 | 39.48 | 0.18 | 0.36 | 17.75 |
| Spectrogram 4 | 9.22 | 21.95 | 34.86 | 0.65 | 0.27 | 33.06 |
| Spectrogram 5 | 10.17 | 0.74 | 2.37 | 2.34 | 22.37 | 62.01 |
| Spectrogram 6 | 10.59 | 1.12 | 3.51 | 3.72 | 20.21 | 60.85 |
| Spectrogram 7 | 12.29 | 1.44 | 5.87 | 3.50 | 17.60 | 59.30 |
| Spectrogram 8 | 50.86 | 0.15 | 27.03 | 0.28 | 0.12 | 21.55 |
| Spectrogram 9 | 9.24 | 1.34 | 2.48 | 2.33 | 18.52 | 66.08 |
| Spectrogram 10 | 13.29 | 1.37 | 3.29 | 3.00 | 13.92 | 65.14 |
| Spectrogram 11 | 39.09 | 0.65 | 25.51 | 0.30 | 0.07 | 34.38 |
| Properties | Natural Bone | Magnesium | Ti Alloy | Co-Cr Alloy | Stainless Steel | Mg30Zn30Sn30Sr5Bi5 |
|---|---|---|---|---|---|---|
| Density | 1.8–2.1 | 1.74–2.0 | 4.4–4.5 | 8.3–9.2 | 7.9–8.1 | 4.47 |
| Elastic modulus | 3–20 | 41–45 | 110–117 | 230 | 189–205 | 17.98 |
| Compressive yield strength | 130–180 | 65–100 | 758–1117 | 450–1000 | 170–310 | 192.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Ju, D.; Liu, Q. Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5. Coatings 2022, 12, 531. https://doi.org/10.3390/coatings12040531
Ma B, Ju D, Liu Q. Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5. Coatings. 2022; 12(4):531. https://doi.org/10.3390/coatings12040531
Chicago/Turabian StyleMa, Beiyi, Dongying Ju, and Qian Liu. 2022. "Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5" Coatings 12, no. 4: 531. https://doi.org/10.3390/coatings12040531
APA StyleMa, B., Ju, D., & Liu, Q. (2022). Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5. Coatings, 12(4), 531. https://doi.org/10.3390/coatings12040531






