Electrochromic and Capacitive Properties of WO3 Nanowires Prepared by One-Step Water Bath Method
Abstract
1. Introduction
2. Materials and Methods
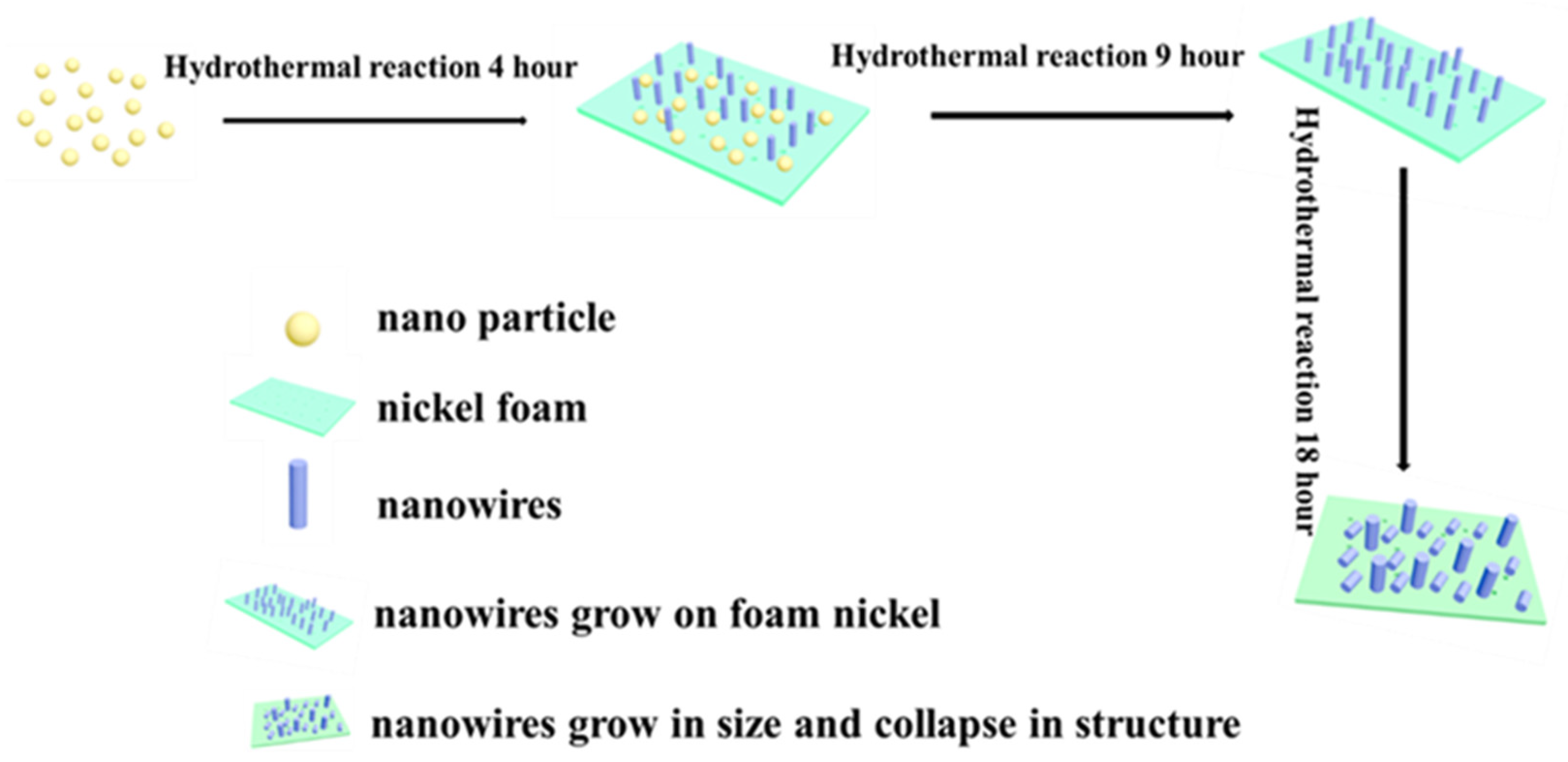
2.1. Synthesis and Deposition of WO3 Nanowires
2.2. Microstructure and Phase Characterization
2.3. Electrochemical Test
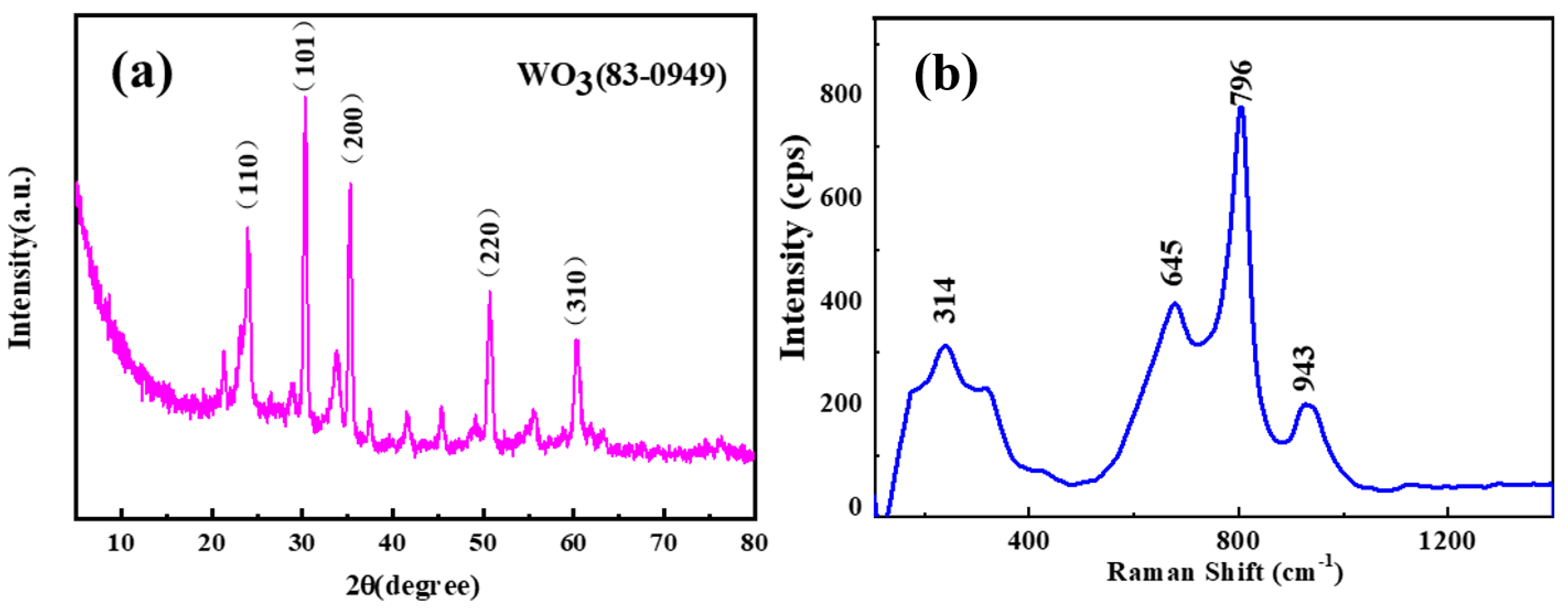
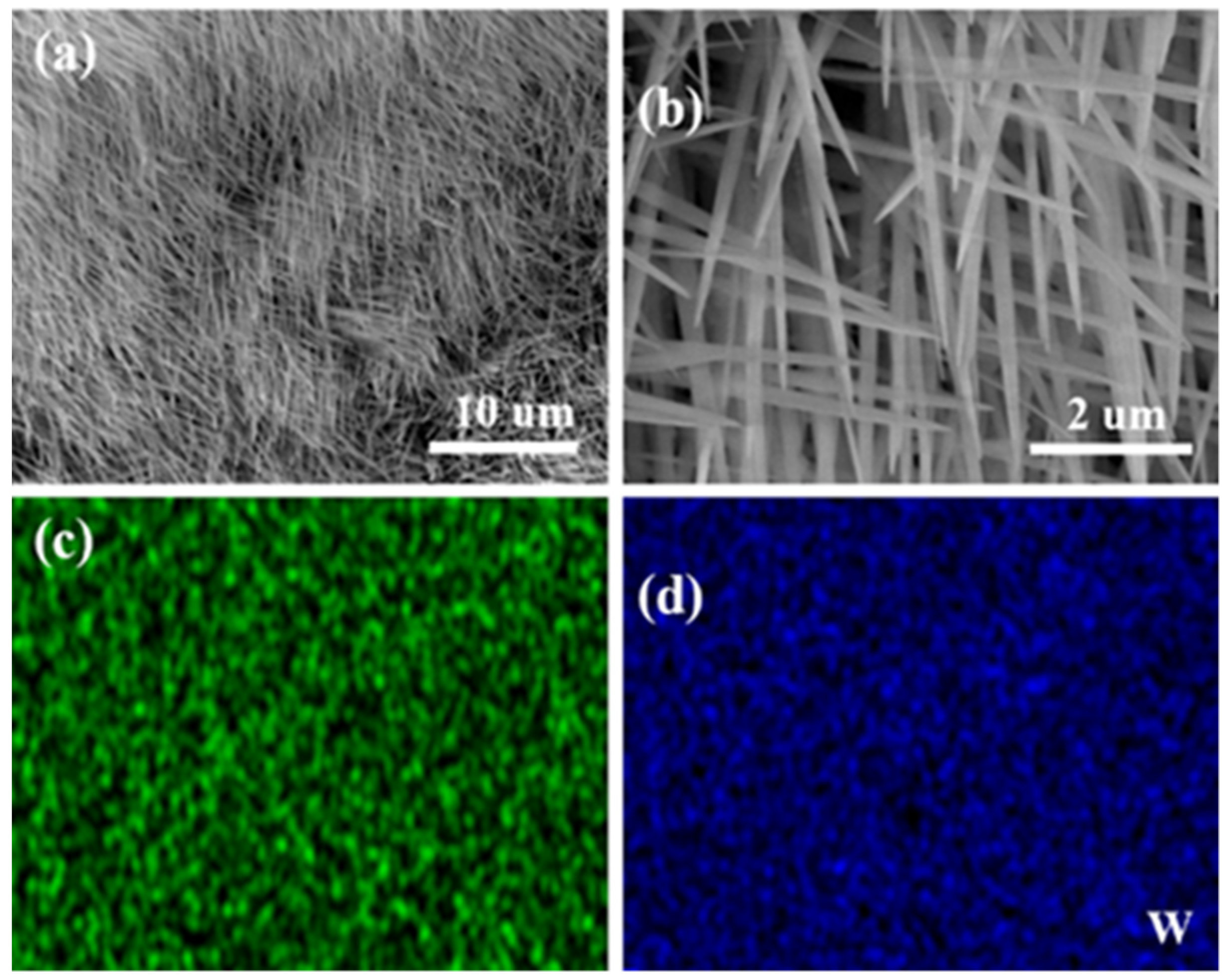
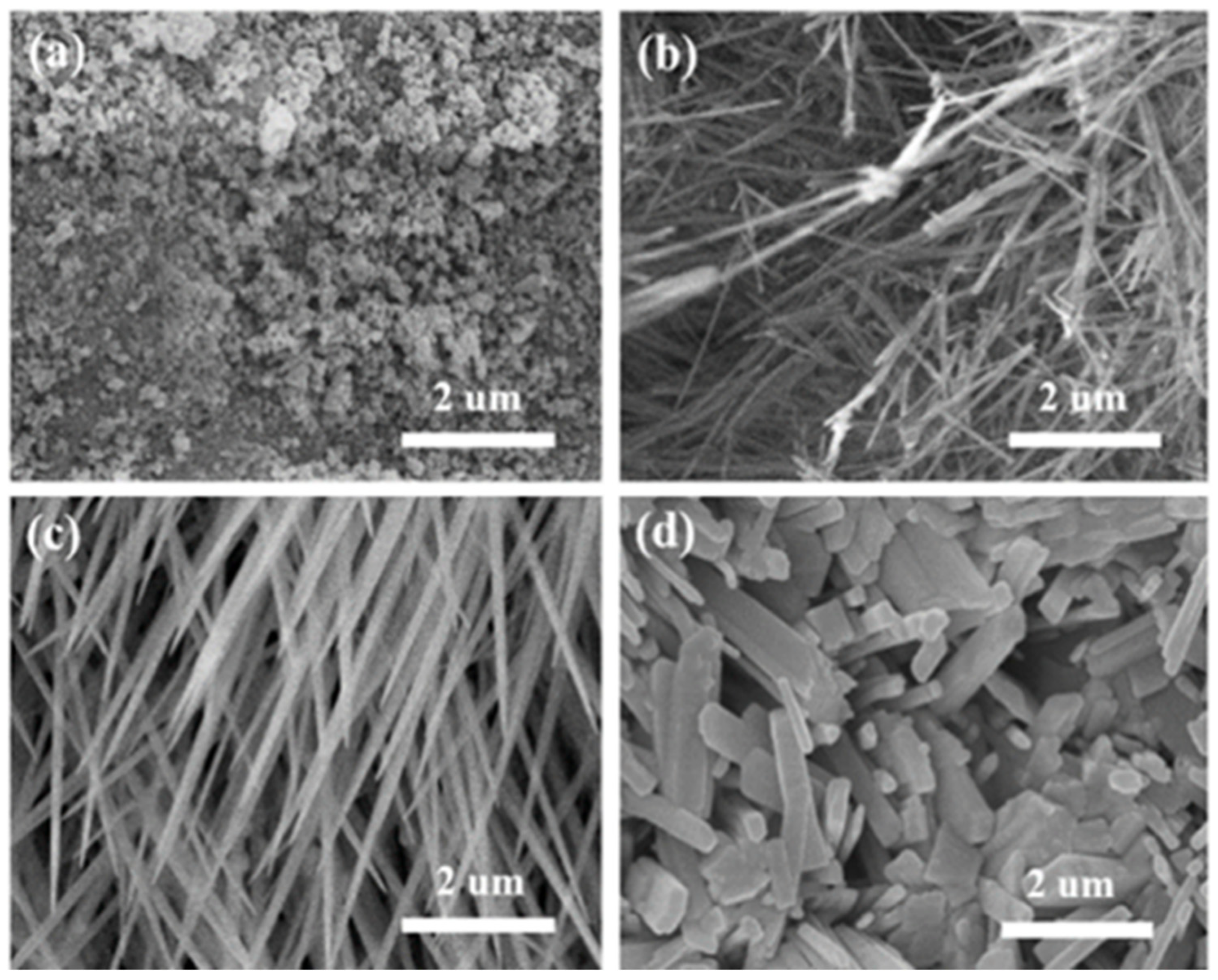
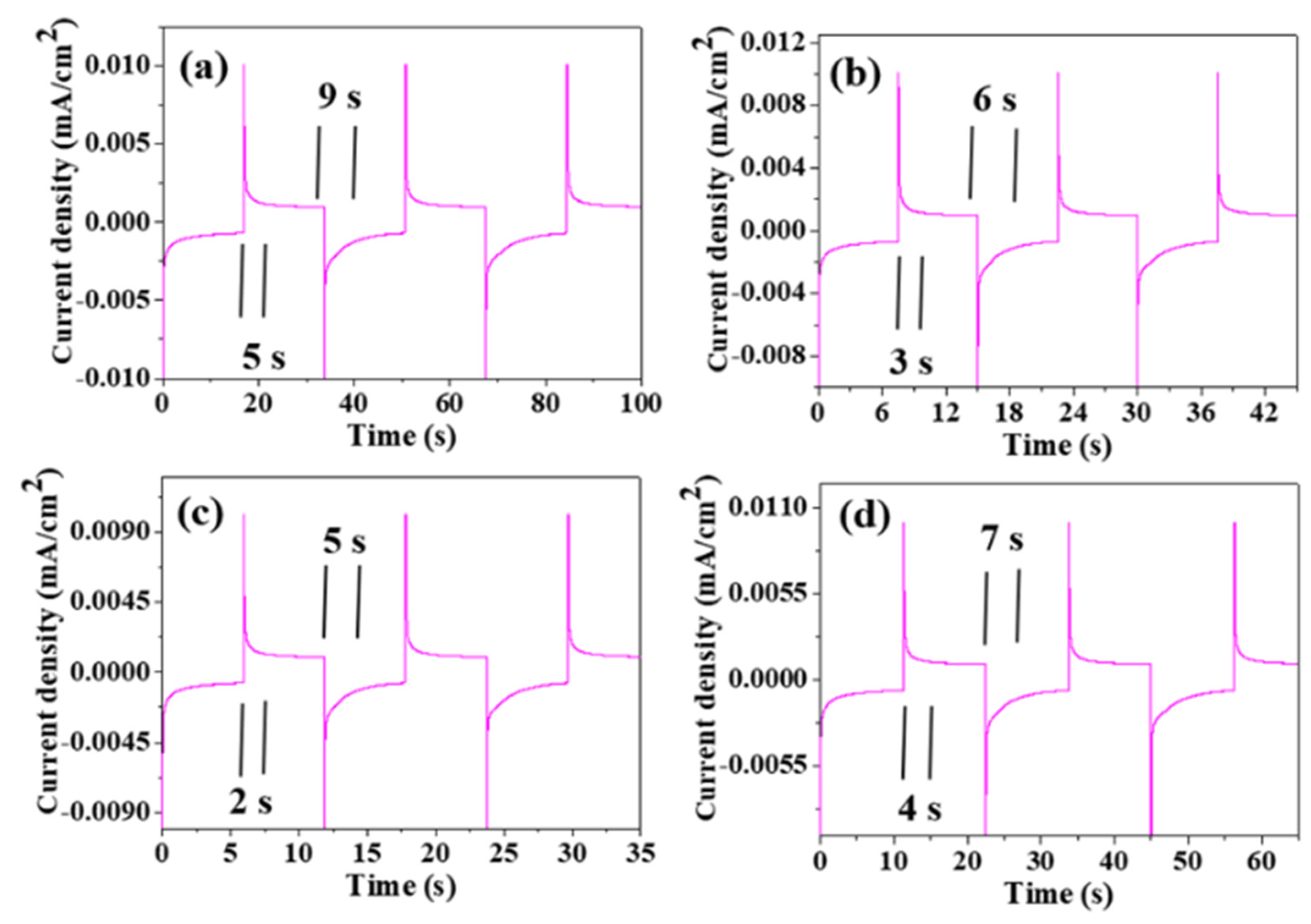
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhang, M.; Zhang, H.; Jiang, Z.; Liu, C.; Cai, W. A review on energy, environment and economic assessment in remanufacturing based on life cycle assessment method. J. Clean. Prod. 2020, 255, 120160. [Google Scholar] [CrossRef]
- Xu, H.; Yu, K.; Pan, M.; Yao, Z.; Zhao, T.; Jiang, Z. A mechanically durable, excellent recyclable 3D hierarchical Ni3S2@ Ni foam photothermal membrane. Green Energy Environ. 2020, 7, 492–499. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, D.; Zou, L.; Wang, Q.; Liu, C. A Review on the Properties and Applications of WO3 Nanostructure−Based Optical and Electronic Devices. Nanomaterials 2021, 11, 2136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, H.; Hopmann, E.; Elezzabi, A.Y. Nanostructured inorganic electrochromic materials for light applications. Nanophotonics 2021, 10, 825–850. [Google Scholar] [CrossRef]
- Stoycheva, T.; Annanouch, F.; Gràcia, I.; Llobet, E.; Blackman, C.; Correig, X.; Vallejos, S. Micromachined gas sensors based on tungsten oxide nanoneedles directly integrated via aerosol assisted CVD. Sens. Actuat. B-Chem. 2014, 198, 210–218. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge storage in WO3 polymorphs and their application as supercapacitor electrode material. Results Phys. 2019, 12, 2012–2020. [Google Scholar] [CrossRef]
- Patil, S.S.; Mane, R.M.; Khot, K.V.; Mali, S.S.; Hong, C.K.; Bhosale, P.N. Surfactant assisted approach to development of efficient WO3 photoanode for natural dye sensitized solar cell. Sol. Energy. 2021, 220, 371–383. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Shi, Y.; Cui, J.; Shu, X.; Wang, Y.; Qin, Y.; Liu, J.; Tan, H.H.; Wu, Y. Crystalline WO3 nanowires array sheathed with sputtered amorphous shells for enhanced electrochromic performance. Appl. Surf. Sci. 2019, 498, 143796. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Correction: Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2019, 7, 10119. [Google Scholar] [CrossRef]
- Klinke, C.; Hannon, J.B.; Gignac, L.; Reuter, K.; Avouris, P. Tungsten oxide nanowire growth by chemically induced strain. J. Phys. Chem. B 2005, 109, 17787–17790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spanu, D.; Recchia, S.; Schmuki, P.; Altomare, M. Thermal-Oxidative Growth of Substoichiometric WO3−x Nanowires at Mild Conditions. Phys. Status Solidi 2020, 14, 2000235. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Q.; Liu, Q.; Zhai, J.; Diao, X. Enhanced electrochromic performance of 2D grid-structured WO3 thin films. Thin Solid Films 2018, 653, 188–193. [Google Scholar] [CrossRef]
- Zheng, F.; Xi, C.; Xu, J.; Yu, Y.; Yang, W.; Hu, P.; Li, Y.; Zhen, Q.; Bashir, S.; Liu, J.L. Facile preparation of WO3 nano-fibers with super large aspect ratio for high performance supercapacitor. J. Alloys Compd. 2019, 772, 933–942. [Google Scholar] [CrossRef]
- Bose, R.J.; Illyasukutty, N.; Tan, K.; Rawat, R.; Matham, M.V.; Kohler, H.; Pillai, V.M. Preparation and characterization of Pt loaded WO3 films suitable for gas sensing applications. Appl. Surf. Sci. 2018, 440, 320–330. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Xiao, J.; Zhao, X.; Li, H.; An, Q.; Zhai, S.; Wang, K.; Wei, L.; Tong, Y. Three-dimensional hierarchical porous lignin-derived carbon/WO3 for high-performance solid-state planar micro-supercapacitor. Int. J. Biol. Macromol. 2021, 190, 11–18. [Google Scholar] [CrossRef]
- Yao, Z.; Di, J.; Yong, Z.; Zhao, Z.; Li, Q. Aligned coaxial tungsten oxide–carbon nanotube sheet: A flexible and gradient electrochromic film. Chem. Commun. 2012, 48, 8252–8254. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, C.; Hao, J.; Jiao, Y.; Xu, Y.; Wang, J. Electrochromic Performance and Capacitor Performance of α-MoO3 Nanorods Fabricated by a One-Step Procedure. Coatings 2021, 11, 783. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Wang, W.; Hou, J.; Gao, L.; Gu, X.; Su, G. Nickel oxide film with tertiary hierarchical porous structure and high electrochromic performance and stability. Mater. Chem. Phys. 2021, 269, 124738. [Google Scholar] [CrossRef]
- Ju, X.; Yang, F.; Zhu, X.; Jia, X. Zinc ion intercalation/deintercalation of metal organic framework-derived nanostructured NiO@C for low-transmittance and high-performance electrochromism. ACS Sustain. Chem. Eng. 2020, 8, 12222–12229. [Google Scholar] [CrossRef]
- Chang-Jian, C.W.; Cho, E.C.; Yen, S.C.; Ho, B.C.; Lee, K.C.; Huang, J.H.; Hsiao, Y.S. Facile preparation of WO3/PEDOT: PSS composite for inkjet printed electrochromic window and its performance for heat shielding. Dye. Pigment. 2018, 148, 465–473. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, M.; Chen, W.; Zhang, Y.; Shu, X.; Qin, Y.; Zhang, X.; Shen, H.; Wu, Y. Rational construction of porous amorphous WO3 nanostructures with high electrochromic energy storage performance: Effect of temperature. J. Non-Cryst. Solids 2020, 549, 120337. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Fisher, A.; Lee, J.Y. Al 3+ intercalation/de-intercalation-enabled dual-band electrochromic smart windows with a high optical modulation, quick response and long cycle life. Energy Environ. Sci. 2018, 11, 2884–2892. [Google Scholar] [CrossRef]
- Chavan, H.S.; Hou, B.; Ahmed, A.T.A.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Cha, S.; Inamdar, A.I.; Im, H. Nanoflake NiMoO4 based smart supercapacitor for intelligent power balance monitoring. Sol. Energy Mat. Sol. C 2018, 185, 166–173. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Q.; Yang, X.; Liu, M.; Sun, Q.; Wang, Z.L. Hybrid piezo/triboelectric-driven self-charging electrochromic supercapacitor power package. Adv. Energy Mater. 2018, 8, 1800069. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, H.; Shen, W.; Miao, X.; Zhang, M.; Guo, M. Bifunctional aligned hexagonal/amorphous tungsten oxide core/shell nanorod arrays with enhanced electrochromic and pseudocapacitive performance. J. Mater. Chem. A 2019, 7, 16867–16875. [Google Scholar] [CrossRef]
- Bathe, S.R.; Patil, P. WO3 thin films doped with Ru by facile chemical method with enhanced electrochromic properties for electrochromic window application. Mater. Sci. Eng. B 2020, 257, 114542. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Z.; Liu, H.; Ma, T. Biomass-derived highly porous nitrogen-doped graphene orderly supported NiMn2O4 nanocrystals as efficient electrode materials for asymmetric supercapacitors. Appl. Surf. Sci. 2020, 507, 145065. [Google Scholar] [CrossRef]
- Rodrigues, S.; Munichandraiah, N.; Shukla, A. A review of state-of-charge indication of batteries by means of ac impedance measurements. J. Power Sources 2000, 87, 12–20. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Ray, C.; Jun, S.C. Direct growth of WO3 nanostructures on multi-walled carbon nanotubes for high-performance flexible all-solid-state asymmetric supercapacitor. Electrochim. Acta 2019, 308, 231–242. [Google Scholar] [CrossRef]
- Yang, F.; Jia, J.; Mi, R.; Liu, X.; Fu, Z.; Wang, C.; Liu, X.; Tang, Y. Fabrication of WO3·2H2O/BC hybrids by the radiation method for enhanced performance supercapacitors. Front. Chem. 2018, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H. Porous WO3@CuO composites derived from polyoxometalates@ metal organic frameworks for supercapacitor. Mater Lett. 2017, 206, 91–94. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High performance solid-state asymmetric supercapacitor using green synthesized graphene-WO3 nanowires nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Huang, J.; Nong, D.; Lin, B. Novel ternary composite reduced-graphene oxide/WO3/poly (p-phenylenediamine) and its electrochemical performance for energy storage. J. Mater. Sci.-Mater. Electron. 2018, 29, 9312–9320. [Google Scholar] [CrossRef]
- Koo, B.R.; Jo, M.H.; Kim, K.H.; Ahn, H.J. Multifunctional electrochromic energy storage devices by chemical cross-linking: Impact of a WO3·H2O nanoparticle-embedded chitosan thin film on amorphous WO3 films. Npg Asia Mater. 2020, 12, 10. [Google Scholar] [CrossRef]



| Material | Wavelength (nm) | Transmittance (%) | Coloration Efficiency (cm2 C−1) | Response Time (s) | Cyclic Stability | Ref. |
|---|---|---|---|---|---|---|
| NiO@C | 35% | 113.5 | 4.5, 8.5 | 90.1% (20,000) | [20] | |
| WO3 nanoparticles | 650 | 54.1% | 83.87 | 1.1, 1.2 | 80.3% (10,000) | [21] |
| Porous WO3 amorphous | 633 | 56.8 | 3.2, 5.6 | 95.7% (2000) | [22] | |
| WO3 | 633 | 121 | 16, 13 | 94.5% (2000) | [23] | |
| NiMoO4 nanoflake | 630 | 57% | 31.44 | 65% (2500) | [24] | |
| Ag/NiO nanowires | 550 | 65.7% | 51.9 | 80.7% (10,000) | [25] | |
| WOx nanorod | 800 | 67.7% | 101 | 15, 21 | 91.8% (2000) | [26] |
| WO3 platelets | 500 | 67 | 2.28, 1.98 | 87% (1000) | [27] | |
| WO3 Nanowires | 650 | 66% | 115.2 | 1.2, 2 | 98.4% (10,000) | This paper |
| Material | Current (A/g) | Specific Capacity (F/g) | Cyclic Stability | Ref. |
|---|---|---|---|---|
| hierarchical porous carbon/WO3 | 0.5 | 429.6 | 94.3% (5000) | [30] |
| WO3·2H2O/bamboo charcoal | 0.5 | 391 | 82% (10,000) | [31] |
| WO3 | 0.5 | 432 | 86.6% (10,000) | [16] |
| WO3@CuO | 1 | 284 | 85.2% (1500) | [32] |
| graphene−WO3 nanowire | 1 | 465 | 97.7% (2000) | [33] |
| WO3 | 1 | 508.9 | 91.42% (2000) | [34] |
| WO3·H2O | 2 | 154.0 | 91.5% (1000) | [35] |
| Amorphous WO3 | 1 | 436 | 93% (5000) | [14] |
| WO3 nanowires | 1 | 565 | 98.4% (10,000) | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, G.; Wang, J.; Gong, X.; Chang, J.; Jin, X.; Zhang, X.; Wang, J.; Hao, J.; Liu, B. Electrochromic and Capacitive Properties of WO3 Nanowires Prepared by One-Step Water Bath Method. Coatings 2022, 12, 595. https://doi.org/10.3390/coatings12050595
Liu X, Wang G, Wang J, Gong X, Chang J, Jin X, Zhang X, Wang J, Hao J, Liu B. Electrochromic and Capacitive Properties of WO3 Nanowires Prepared by One-Step Water Bath Method. Coatings. 2022; 12(5):595. https://doi.org/10.3390/coatings12050595
Chicago/Turabian StyleLiu, Xusong, Gang Wang, Jun Wang, Xue Gong, Jiang Chang, Xiangyang Jin, Xiang Zhang, Jing Wang, Jian Hao, and Baosheng Liu. 2022. "Electrochromic and Capacitive Properties of WO3 Nanowires Prepared by One-Step Water Bath Method" Coatings 12, no. 5: 595. https://doi.org/10.3390/coatings12050595
APA StyleLiu, X., Wang, G., Wang, J., Gong, X., Chang, J., Jin, X., Zhang, X., Wang, J., Hao, J., & Liu, B. (2022). Electrochromic and Capacitive Properties of WO3 Nanowires Prepared by One-Step Water Bath Method. Coatings, 12(5), 595. https://doi.org/10.3390/coatings12050595








