Layer by Layer Optimization of Langmuir–Blodgett Films for Surface Acoustic Wave (SAW) Based Sensors for Volatile Organic Compounds (VOC) Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the SLP, HDAA, and CDAA LB Films
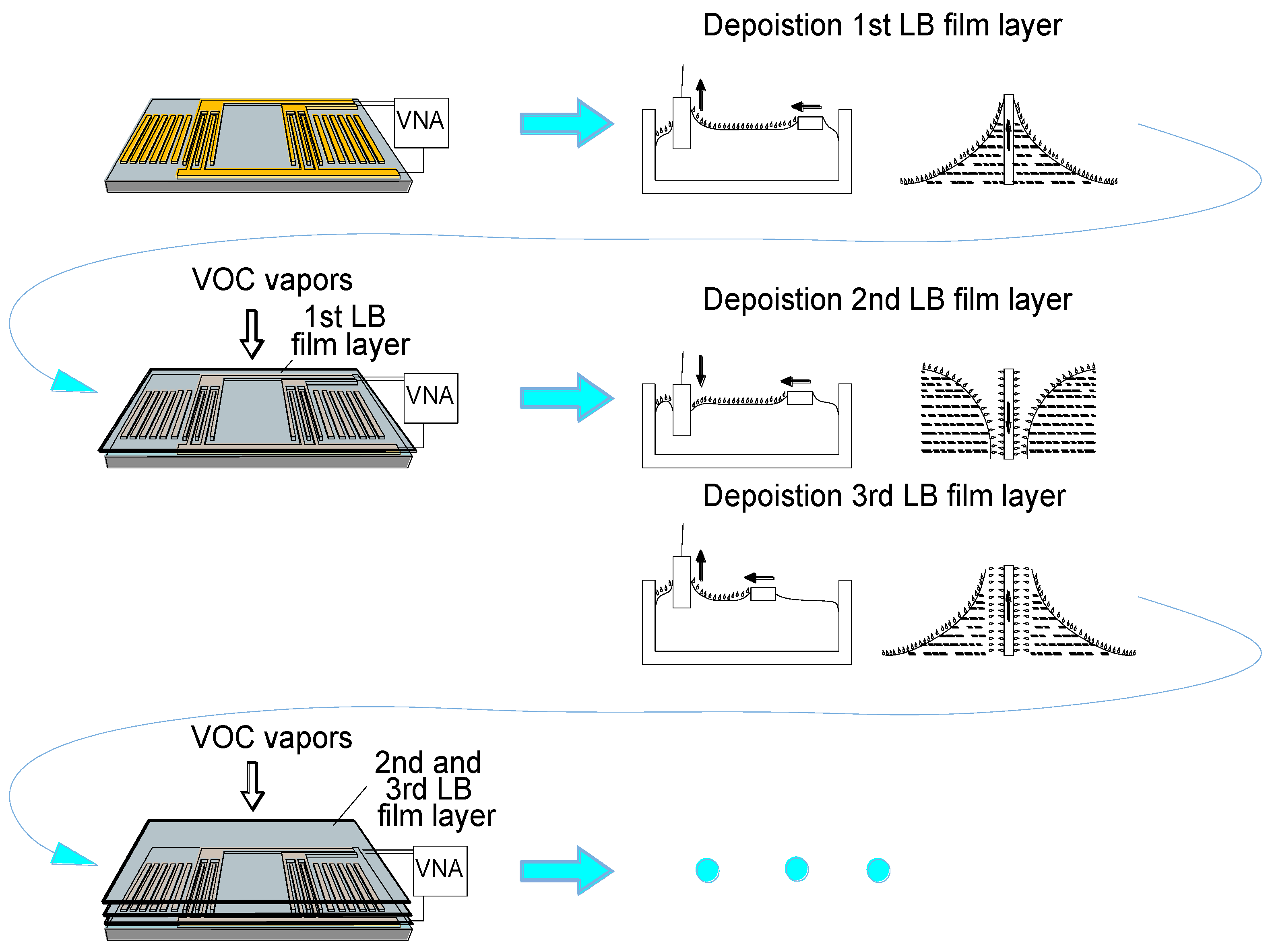
2.2. Deposition of the LB Films
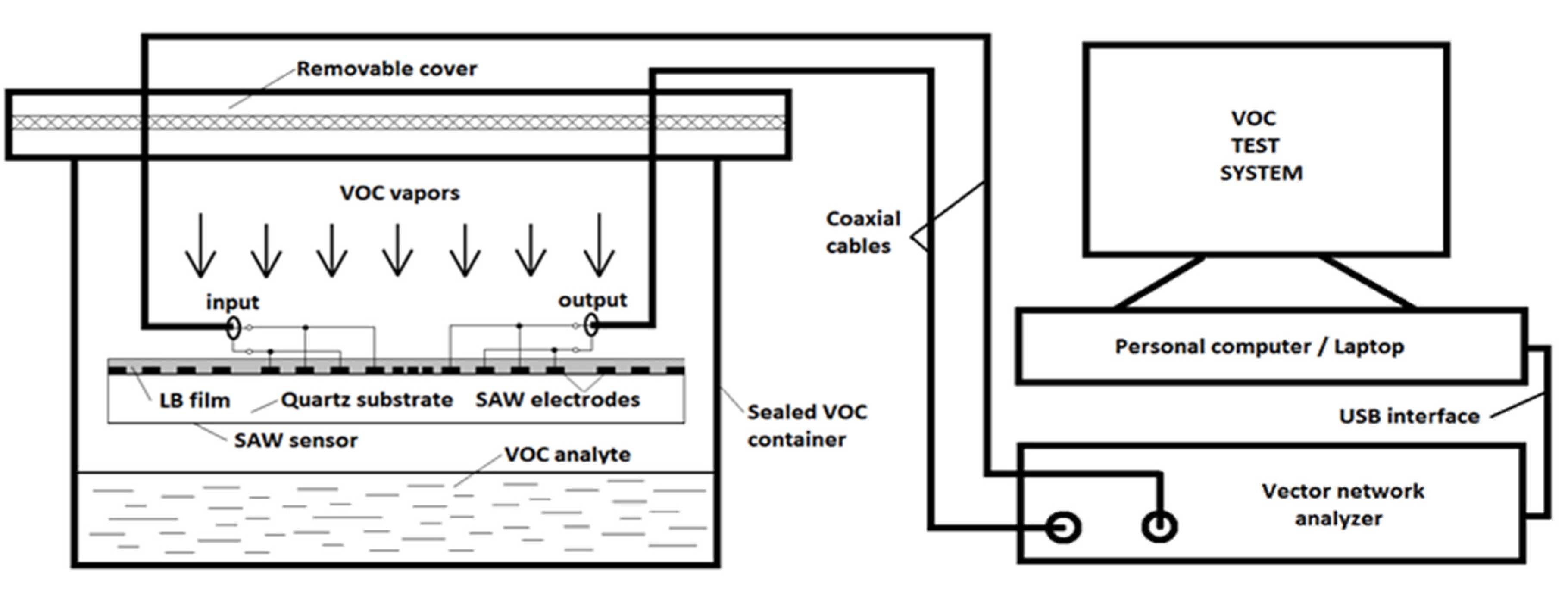
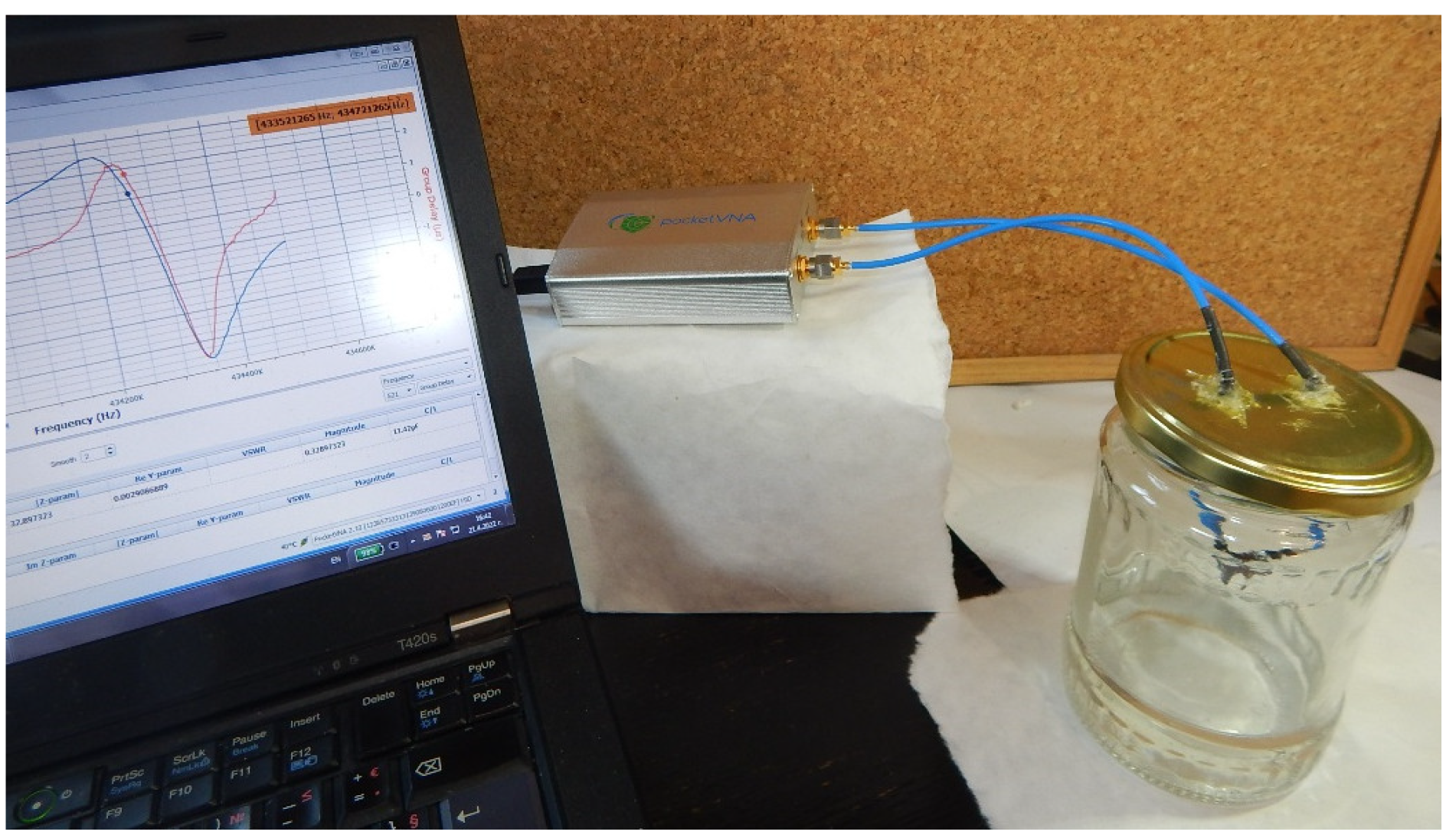
2.3. VOC Vapor Test Setup
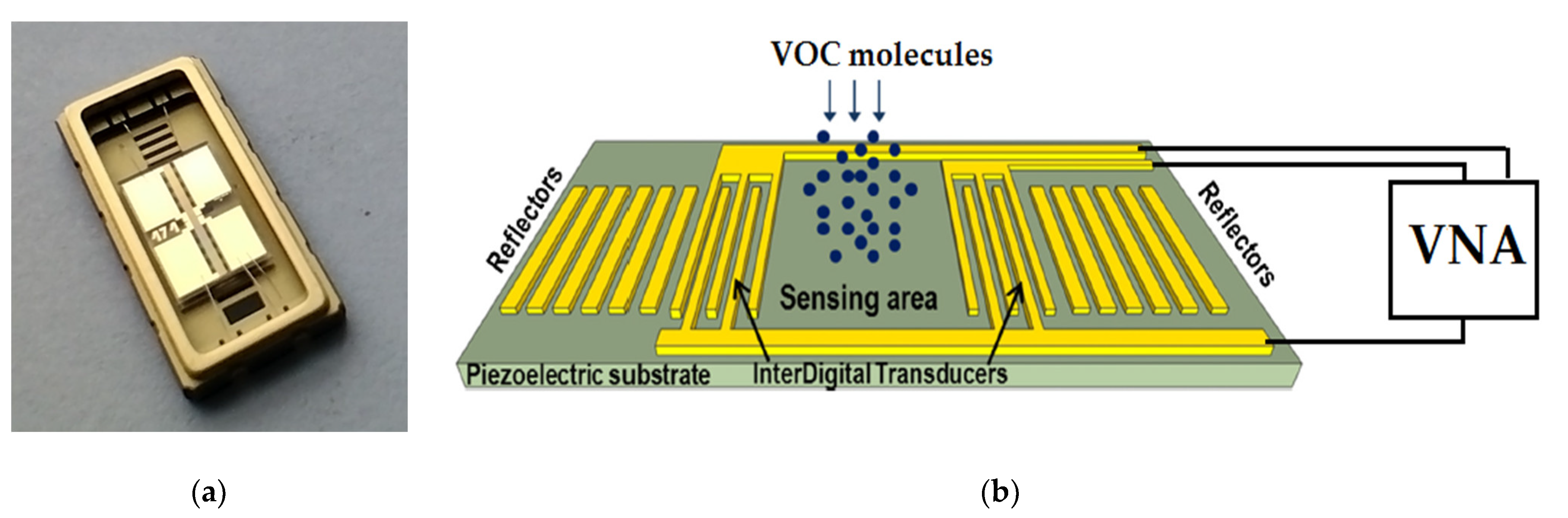
2.4. The Quartz Surface Microbalance (QSM) Device
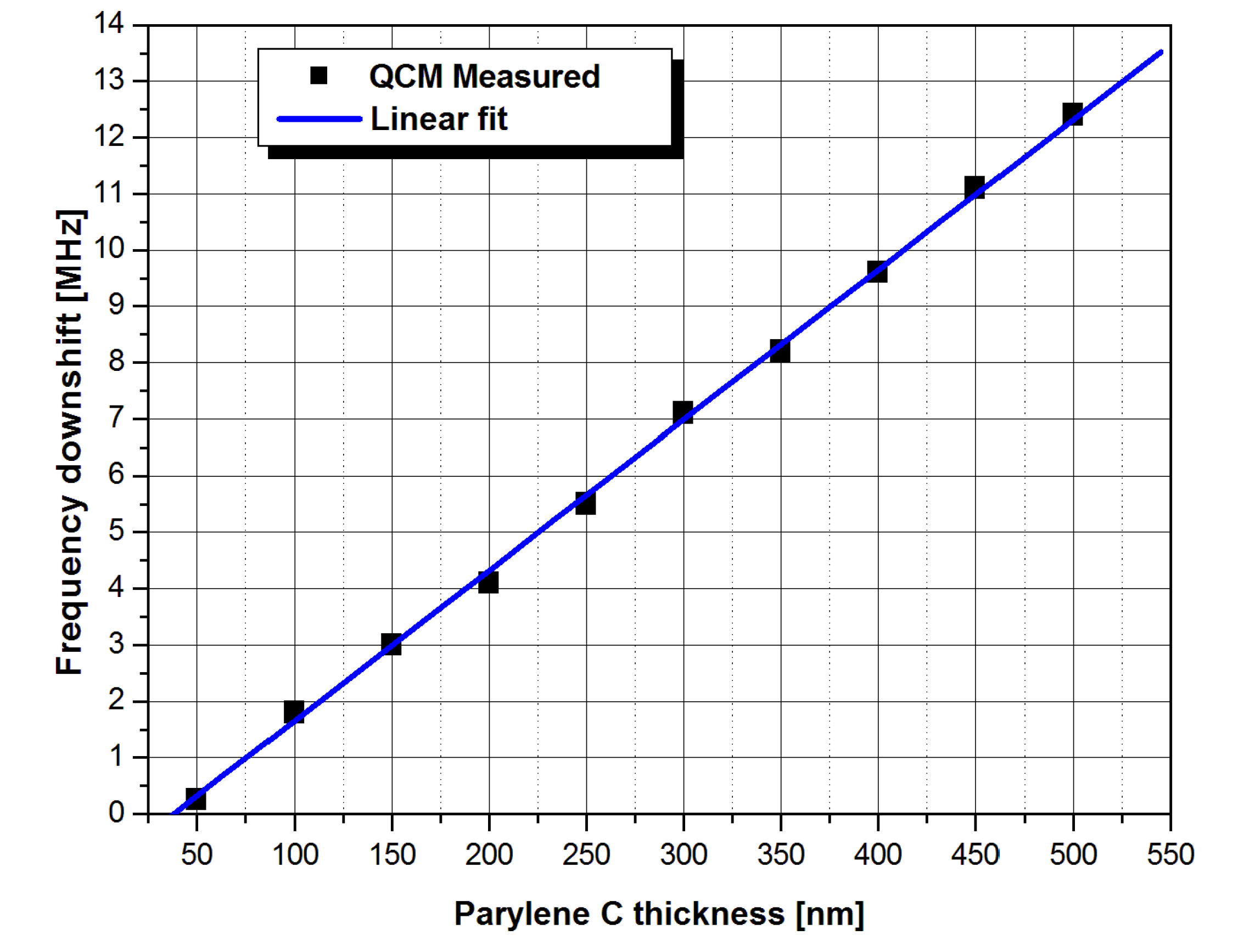
2.5. Mass Calibration of the QSM Device
2.6. Performance Degradation of the QSM Device with LB Film Coating
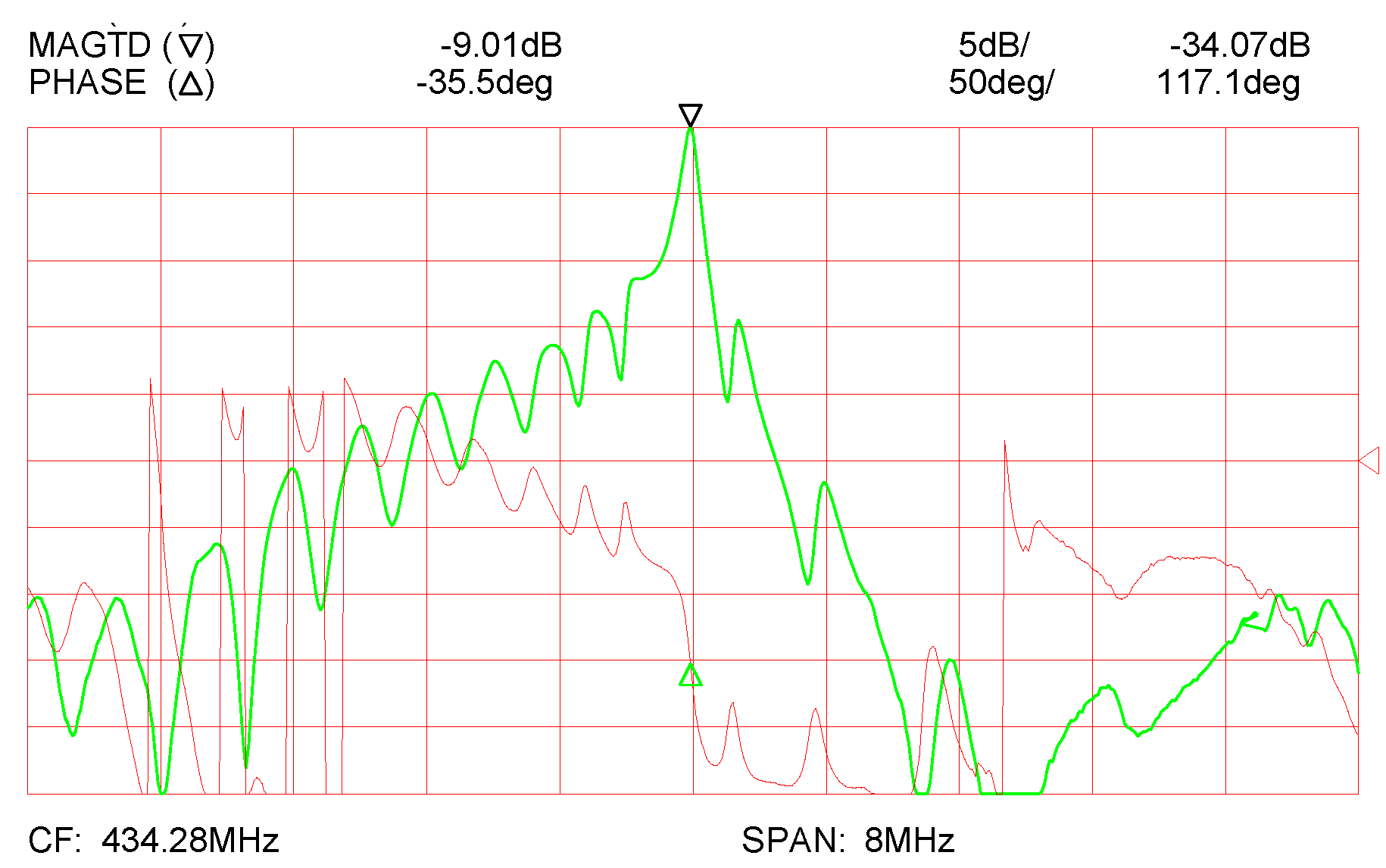
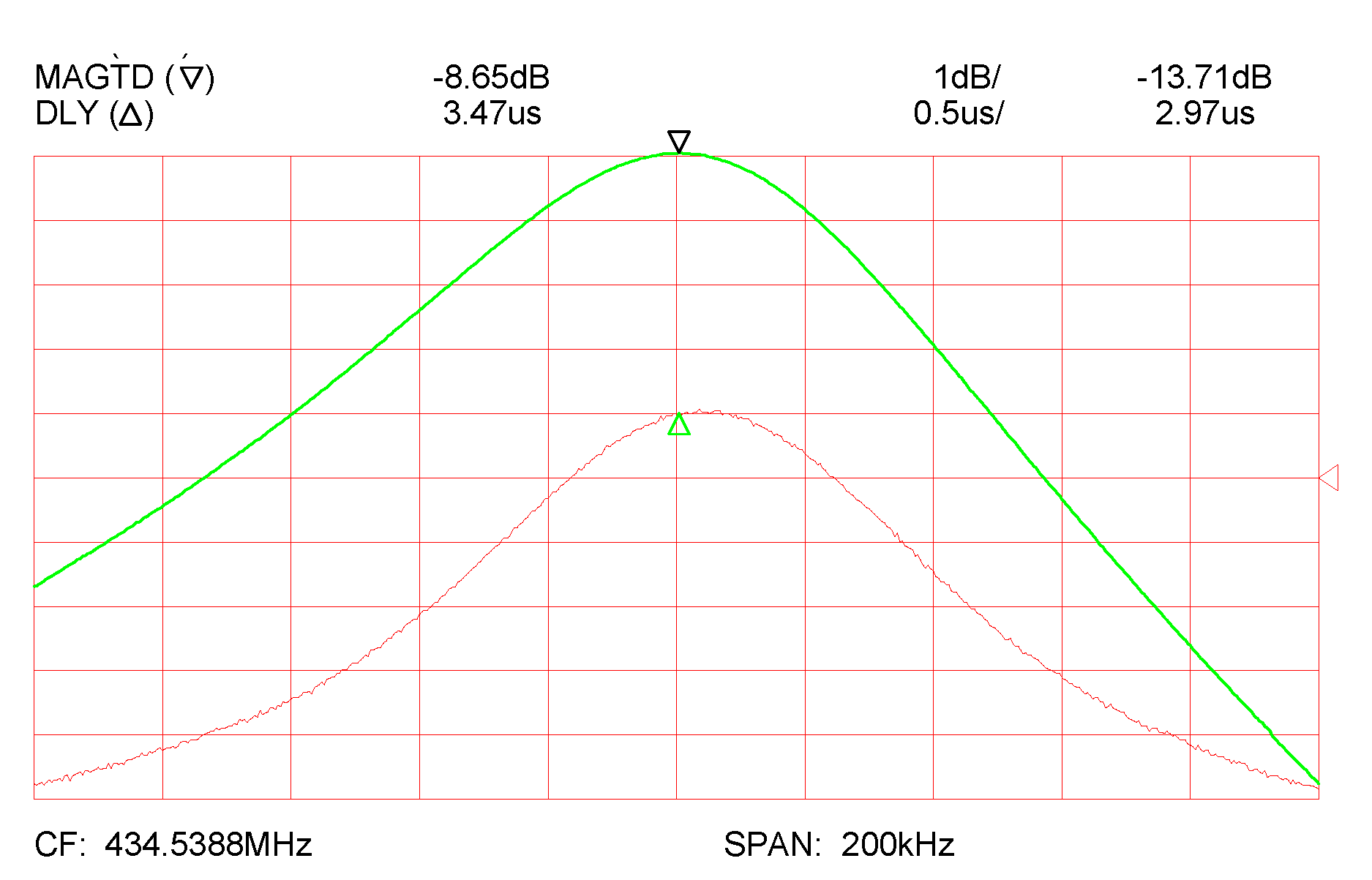
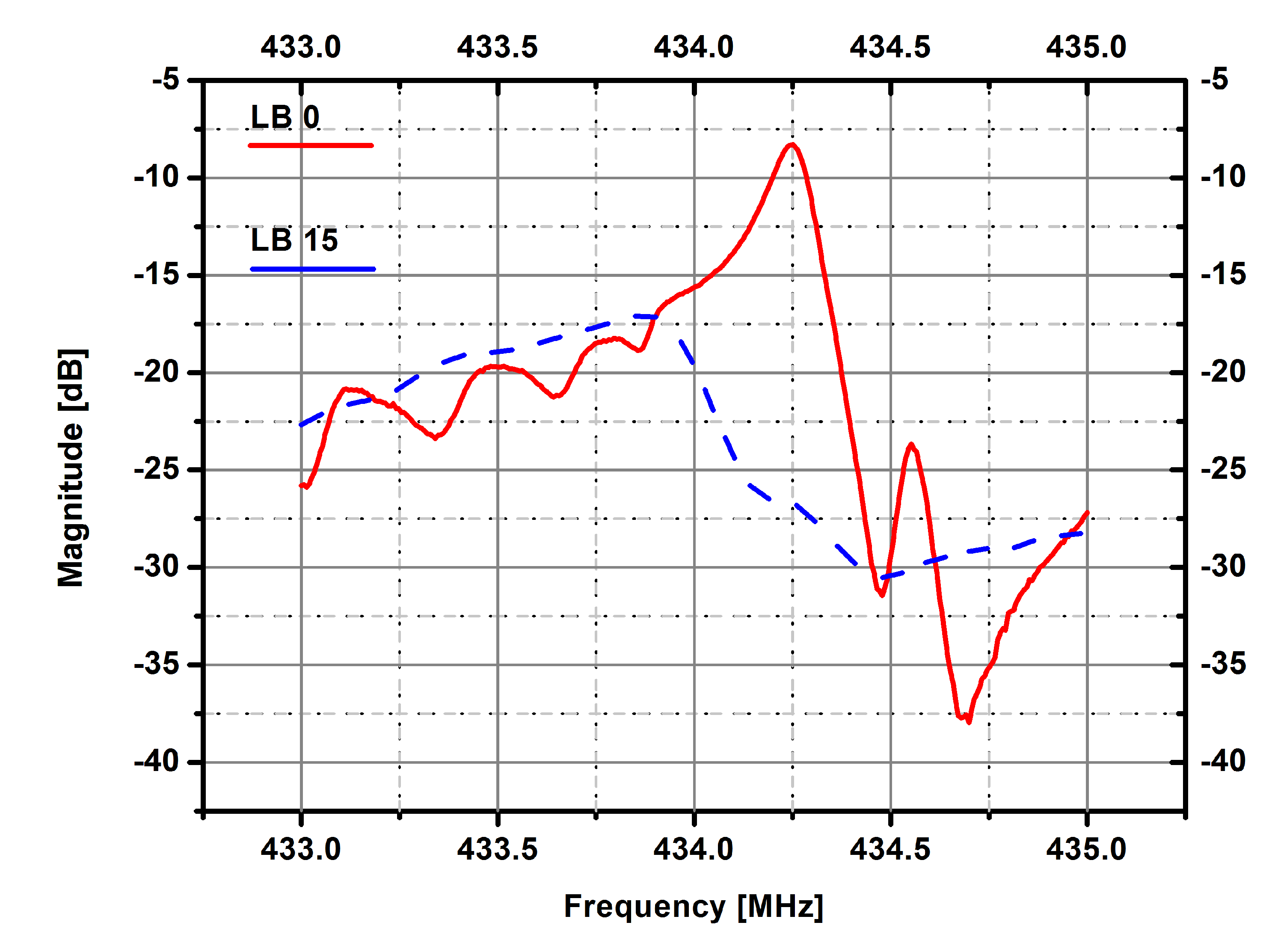
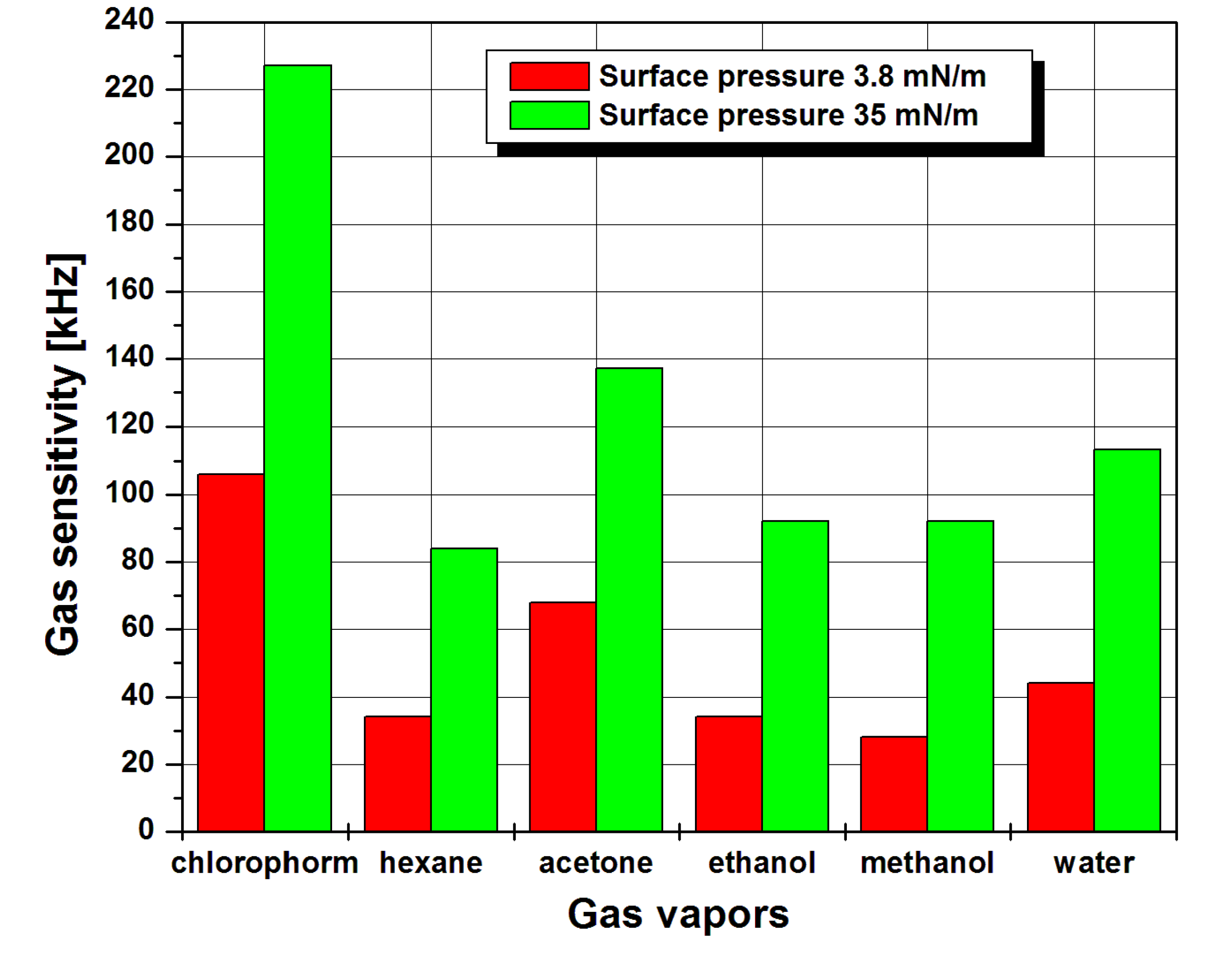
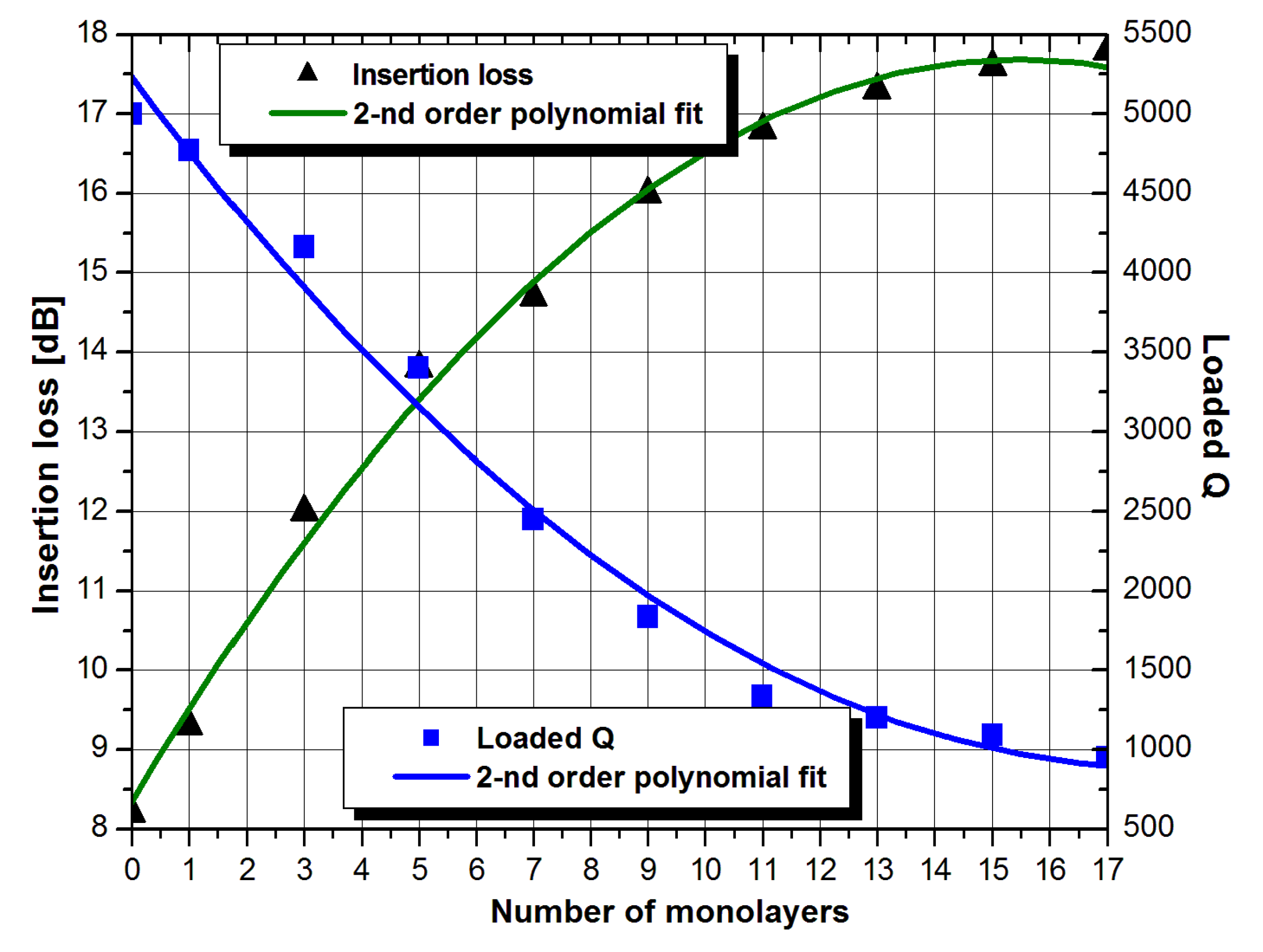
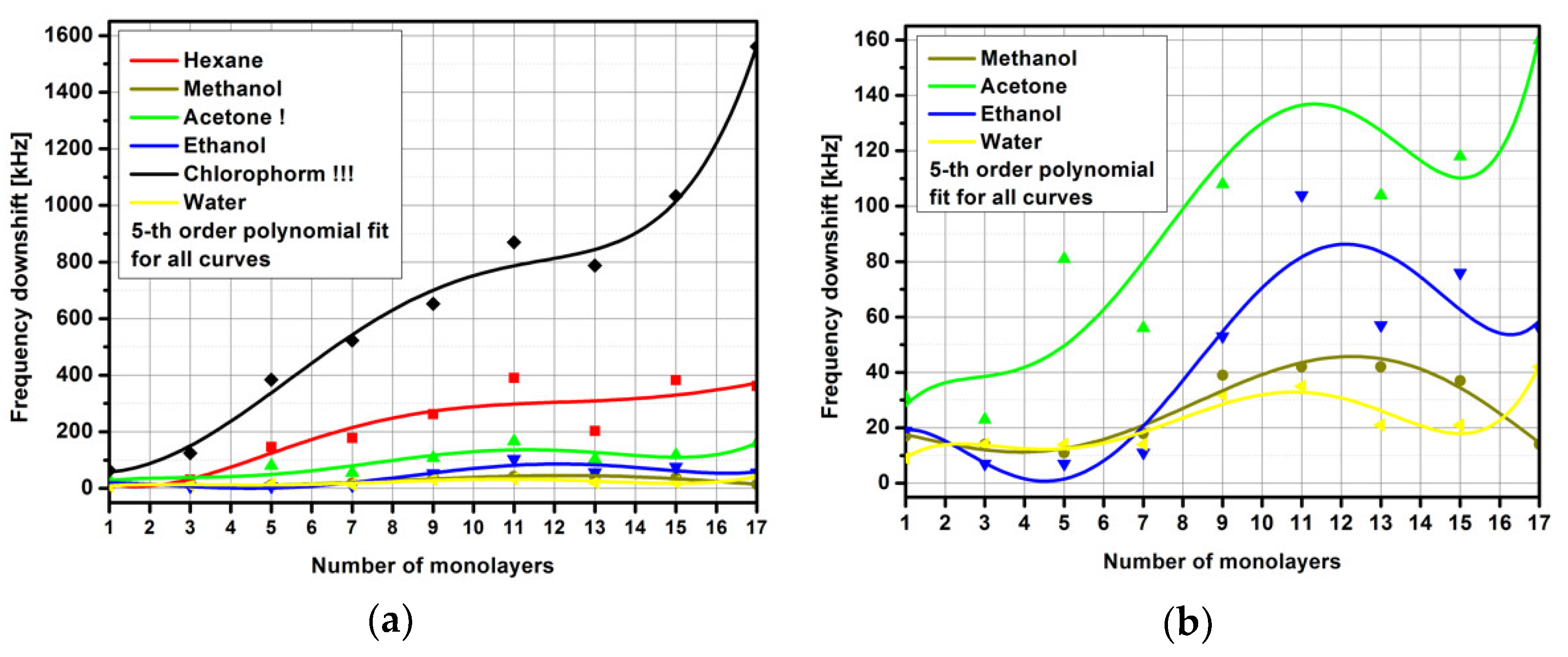
3. Results and Discussion
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- What Are Volatile Organic Compounds ()? Available online: https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs (accessed on 2 April 2022).
- Koppmann, R. (Ed.) Volatile Organic Compounds in the Atmosphere; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; ISBN 9780470988657. [Google Scholar] [CrossRef]
- Salthammer, T. Very volatile organic compounds: An understudied class of indoor air pollutants. Indoor Air 2016, 26, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.M.; Lawal, O.; Nijsen, T.M.; Goodacre, R.; Fowler, S.J. Exhaled Volatile Organic Compounds of Infection: A Systematic Review. ACS Infect. Dis. 2017, 3, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Grzywinski, D.; Ligor, T.; Stacewicz, T.; Bielecki, Z.; Wojtas, J. Detection of volatile organic compounds as biomarkers in breath analysis by different analytical techniques. Bioanalysis 2013, 5, 2287–2306. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, C. Volatile organic compounds gas sensor based on quartz crystal microbalance for fruit freshness detection: A review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.M. Seeing SAW Potential. Anal. Chem. 2003, 75, 355–358. [Google Scholar] [CrossRef][Green Version]
- Dewulf, J.; Langenhove, H.V. Analysis of volatile organic compounds using gas chromatography. Trends Anal. Chem. 2002, 21, 637–646. [Google Scholar] [CrossRef]
- Randon, J.; Maret, M.; Ferronato, C. Gas chromatography–mass spectroscopy optimization by computer simulation, application to the analysis of 93 volatile organic compounds in workplace ambient air. Anal. Chim. Acta 2014, 812, 258–264. [Google Scholar] [CrossRef]
- Natale, C.D.; Paolesse, R.; Macagnano, A.; Troitsky, V.I.; Berzina, T.S.; D’Amico, A. Pattern recognition approach to the study of the interactions between metalloporphyrin Langmuir-Blodgett films and volatile organic compounds. Anal. Chim. Acta 1999, 384, 249–259. [Google Scholar] [CrossRef]
- Andre, R.S.; Sanfelice, R.C.; Pavinatto, A.; Mattoso, L.H.C.; Correa, D.S. Hybrid nanomaterials designed for volatile organic compounds sensors: A review. Mater. Des. 2018, 156, 154–166. [Google Scholar] [CrossRef]
- McGinn, C.K.; Lamport, Z.A.; Kymissis, I. Review of Gravimetric Sensing of Volatile Organic Compounds. ACS Sens. 2020, 5, 1514–1534. [Google Scholar] [CrossRef]
- Avramov, I.D. Polymer Coated Rayleigh SAW and STW Resonators for Gas Sensor Applications. In Acoustic Waves—From Microdevices to Helioseismology; Beghi, M.G., Ed.; InTech International: London, UK, 2011; ISBN 978-953-307-572-3. [Google Scholar] [CrossRef]
- Afzal, A.; Iqbal, N.; Mujahid, A.; Schirhagl, R. Advanced vapor recognition materials for selective and fast responsive surface acoustic wave sensors: A review. Anal. Chim. Acta 2013, 787, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.J.R.; Borro, M.S.; Jesus, L.R.M.; Braunger, M.L.; Olivati, C.A. Using Langmuir-Schaefer deposition technique to improve the gas sensing performance of regiorandom polythiophene films. Sens. Actuators Rep. 2022, 4, 100094. [Google Scholar] [CrossRef]
- Roberts, G.G. (Ed.) Langmuir-Blodgett Films; Springer: Boston, MA, USA, 1990. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Polevska, Z. First observation of 3D aggregates in a single-component Langmuir film below the equilibrium spreading pressure. MATEC Web Conf. 2017, 98, 01003. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Kostadinov, K.G.; Song, Z. Formation of Nanosized Needle Structures in Ultra-Thin Organic Film for Biosensor Application. In Proceedings of the 2019 IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Zhenjiang, China, 4–8 August 2019; pp. 350–353. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of Volatile Organic Compounds by Self-assembled Monolayer Coated Sensor Array with Concentration-independent Fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef]
- Hanley, C.M.; Quinn, J.A.; Vanderlick, T.K. Transport properties of water and pentane in Langmuir–Blodgett films. AlChE J. 1996, 42, 1234–1243. [Google Scholar] [CrossRef]
- Girard, K.P.; Hanley, C.M.; Vanderlick, T.K.; Quinn, J.A. Effect of the Skeletonization Process on Vapor Sorption into Langmuir−Blodgett Multilayers. Ind. Eng. Chem. Res. 2001, 40, 4283–4287. [Google Scholar] [CrossRef]
- Girard, K.P.; Quinn, J.A.; Vanderlick, T.K. Vapor Sorption into Pure and Mixed Fatty Acid Multilayers. Langmuir 2002, 18, 5830–5834. [Google Scholar] [CrossRef]
- Chi, L.F.; Eng, L.M.; Graf, K.; Fuchs, H. Structure and Stability of Langmuir-Blodgett Films Investigated by Scanning Force Microscopy. Langmuir 1992, 8, 2255–2261. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Avramov, I.D. Langmuir-Blodgett Films from Fluorescently Labelled Phospholipids Deposited on Surface Acoustic Wave Devices. J. Phys. Conf. Ser. 2019, 1186, 012007. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Polevska, Z. Computerized System for Investigation of Organic Monolayers at the Air-water Interface and for Deposition of Nano Thin Layers Following the Langmuir and Blodgett Method. In Proceedings of the 27th International Scientific Symposium Metrology and Metrology Assurance, Sozopol, Bulgaria, 7–11 September 2017; pp. 260–265. Available online: http://metrology-bg.org/fulltextpapers/370.pdf (accessed on 2 April 2022).
- Ivanov, G.R.; Georgiev, G.; Lalchev, Z. Fluorescently Labeled Phospholipids—New Class of Materials for Chemical Sensors for Environmental Monitoring. In Relevant Perspectives in Global Environmental Change; Agboola, J.A., Ed.; InTech International: London, UK, 2011; Chapter 6; pp. 89–112. [Google Scholar] [CrossRef]
- Claesson, P.M.; Berg, J.M. The Stability of Carboxylic Acid Langmuir-Blodgett Films as Studied by the Surface Force Technique. Thin Solid Films 1989, 176, 157–164. [Google Scholar] [CrossRef]
- Avramov, I.D.; Voigt, A.; Rapp, M. Rayleigh SAW resonators using gold electrode structure for gas sensor applications in chemically reactive environments. Electron. Lett. 2005, 41, 450–452. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung Dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Avramov, I.D.; Stahl, U. On the Mass Sensitivity of Rayleigh Surface Acoustic Wave (RSAW) Resonators. In Proceedings of the 2017 40th International Spring Seminar on Electronics Technology, Sofia, Bulgaria, 10–14 May 2017. [Google Scholar] [CrossRef]
- Avramov, I.D.; Länge, K.; Rupp, S.; Rapp, B.; Rapp, M. Polymer coating behavior of Rayleigh-SAW resonators with gold electrode structure for gas sensor applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 54, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Avramov, I.D. The Quartz Surface Microbalance—A Possible Candidate for Rapid Respiratory Virus Detection. In Proceedings of the 2021 IEEE International Symposium on Applications of Ferroelectrics (ISAF), Sydney, Australia, 16–21 May 2021. [Google Scholar] [CrossRef]
- Group and Phase Delay Measurements with Vector Network Analyzer ZVR. Available online: https://scdn.rohde-schwarz.com/ur/pws/dl_downloads/dl_application/application_notes/1ez35/1ez35_1e.pdf (accessed on 2 April 2022).
- Parker, T.E.; Montress, G.K. Precision surface-acoustic-wave (SAW) oscillators. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1988, 35, 342–364. [Google Scholar] [CrossRef]
- Garnaes, J.; Schwartz, D.K.; Visvanathan, R.; Zasadzinski, J.A.N. Nano Scale Defects in Langmuir-Blodgett Film Observed by Atomic Force Microscopy. Synth. Met. 1993, 55–57, 3795–3800. [Google Scholar] [CrossRef]
- Avramov, I.D.; Radeva, E.; Lazarov, Y.; Grakov, T.; Vergov, L. Sensitivity Enhancement in Plasma Polymer Films for Surface AcousticWave Based Sensor Applications. Coatings 2021, 11, 1193. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramov, I.D.; Ivanov, G.R. Layer by Layer Optimization of Langmuir–Blodgett Films for Surface Acoustic Wave (SAW) Based Sensors for Volatile Organic Compounds (VOC) Detection. Coatings 2022, 12, 669. https://doi.org/10.3390/coatings12050669
Avramov ID, Ivanov GR. Layer by Layer Optimization of Langmuir–Blodgett Films for Surface Acoustic Wave (SAW) Based Sensors for Volatile Organic Compounds (VOC) Detection. Coatings. 2022; 12(5):669. https://doi.org/10.3390/coatings12050669
Chicago/Turabian StyleAvramov, Ivan D., and George R. Ivanov. 2022. "Layer by Layer Optimization of Langmuir–Blodgett Films for Surface Acoustic Wave (SAW) Based Sensors for Volatile Organic Compounds (VOC) Detection" Coatings 12, no. 5: 669. https://doi.org/10.3390/coatings12050669
APA StyleAvramov, I. D., & Ivanov, G. R. (2022). Layer by Layer Optimization of Langmuir–Blodgett Films for Surface Acoustic Wave (SAW) Based Sensors for Volatile Organic Compounds (VOC) Detection. Coatings, 12(5), 669. https://doi.org/10.3390/coatings12050669





