Novel Micronized Mica Modified Casein–Aluminum Hydroxide as Fire Retardant Coatings for Wood Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Composite Preparation
2.3. Thermal Gravimetric Analysis
2.4. Cone Calorimetric Analysis
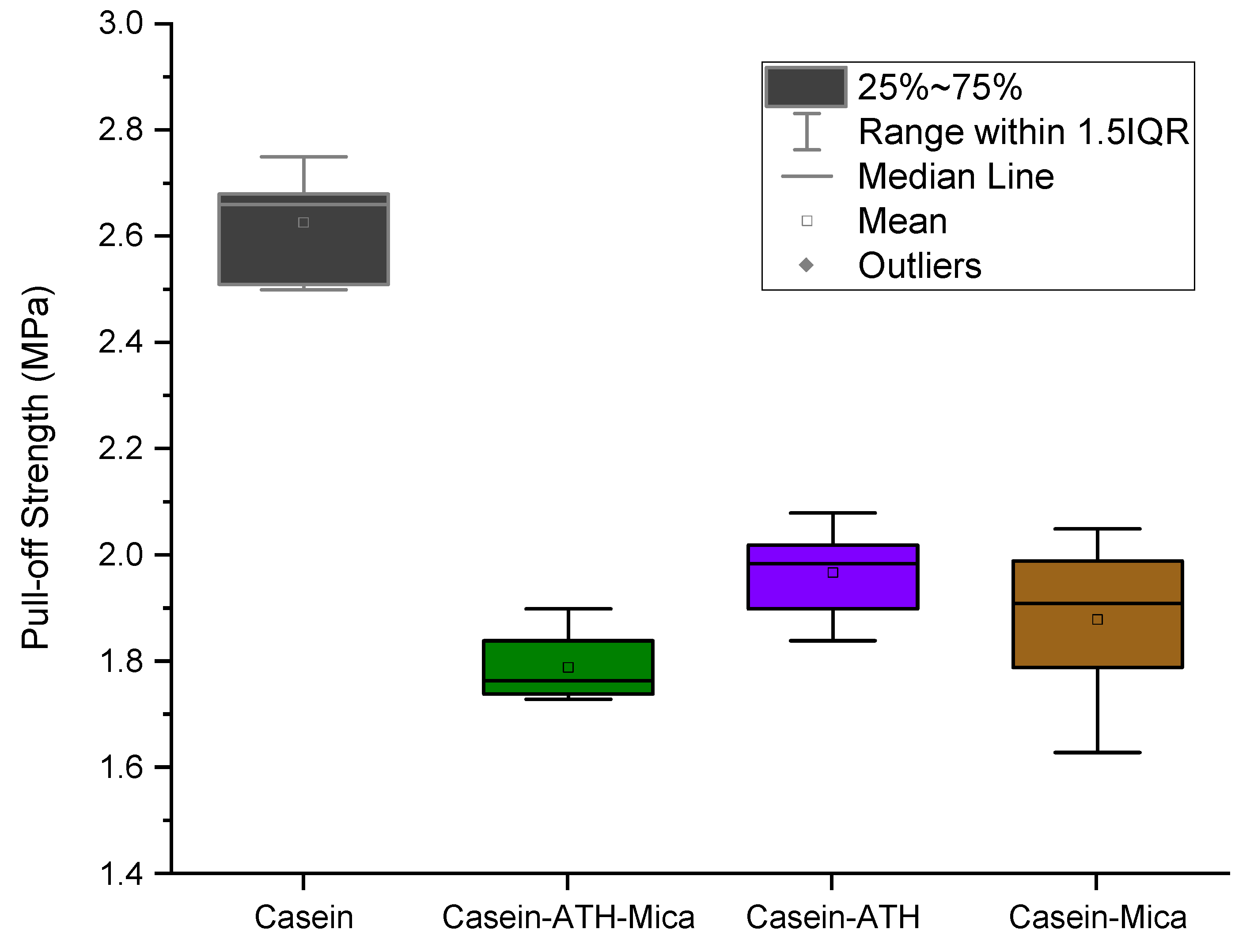
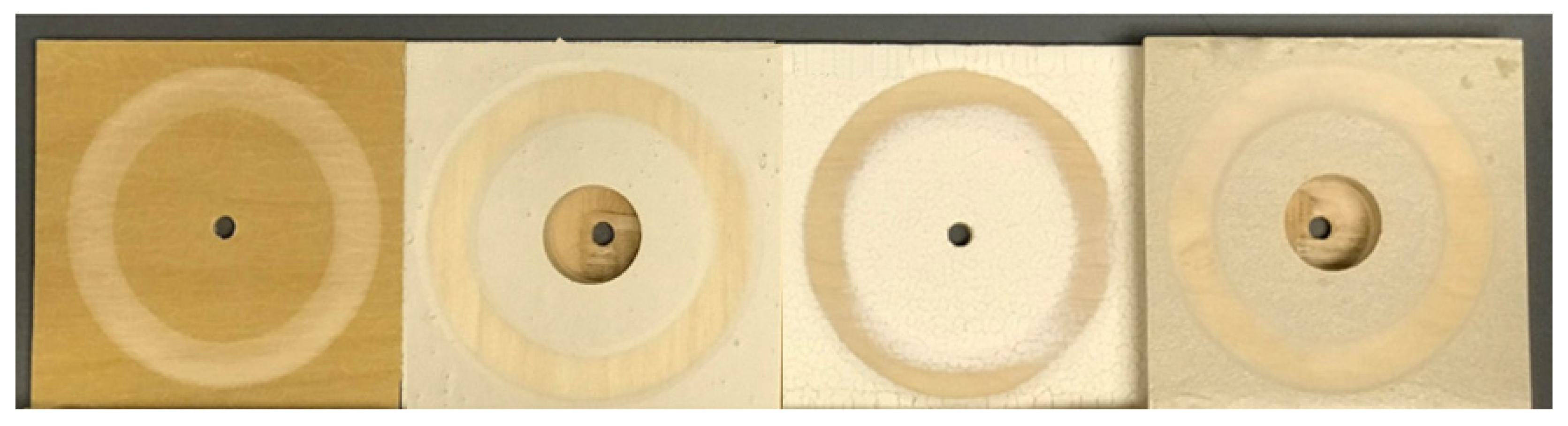
2.5. Pull-Off Adhesion Test
2.6. Abrasion Test
3. Results and Discussion
3.1. Thermogravimetric Analysis of Coatings
3.2. Fire Retardancy Assessment of Coatings
3.2.1. Time to Ignition
3.2.2. Peak Heat Release Rate
3.3. Pull-Off Strength of Coating Systems
3.4. Abrasion Resistance Performance of Coating Systems
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, A.H.; Levine, S. Wood-based building materials and atmospheric carbon emissions. Environ. Sci. Policy 1999, 2, 427–437. [Google Scholar] [CrossRef]
- Brandner, R.; Flatscher, G.; Ringhofer, A.; Schickhofer, G.; Thiel, A. Cross laminated timber (CLT): Overview and development. Eur. J. Wood Wood Prod. 2016, 74, 331–351. [Google Scholar] [CrossRef]
- Klippel, M.; Schmid, J.; Frangi, A. Fire design of CLT. In Proceedings of the Joint Conference of COST Actions FP1402 & FP1404 KTH Building Materials, Cross Laminated Timber—A Competitive Wood Product for Visionary and Fire Safe Buildings, Stockholm, Sweden, 10–11 March 2016; pp. 101–122. [Google Scholar]
- IFC, International Fire Code. 2018. Available online: https://codes.iccsafe.org/content/IFC2018 (accessed on 15 November 2021).
- Tsapko, Y.; Tsapko, A.; Bondarenko, O. Establishment of heat-exchange process regularities at inflammation of reed samples. East. Eur. J. Enterp. Technol. 2019, 1, 36–42. [Google Scholar]
- Chen, C.; Chien, S.; Ho, M. A study on fire spreading model for the safety distance between the neighborhood occupancies and historical buildings in Taiwan. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Tsapko, Y.; Tsapko, A. Establishment of the mechanism and fireproof efficiency of wood treated with an impregnating solution and coatings. East. Eur. J. Enterp. Technol. 2017, 3, 50–55. [Google Scholar] [CrossRef]
- Tsapko, Y. Effect of surface treatment of wood on the fire resistance of wooden structures. East. Eur. J. Enterp. Technol. 2013, 5, 11–14. [Google Scholar]
- Demir, H.; Arkış, E.; Balkose, D.; Ülkü, S. Synergistic effect of natural zeolites on flame retardant additives. Polym. Degrad. Stab. 2005, 89, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Tsapko, Y.; Kyrycyok, V.; Tsapko, A.; Bondarenko, O.; Guzii, S. Increase of fire resistance of coating wood with adding mineral fillers. MATEC Web Conf. 2018, 230, 02034. [Google Scholar] [CrossRef]
- Chang, W.; Pan, Y.; Chuang, C.; Guo, J.; Chen, S.; Wang, C.; Hsieh, K. Fabrication and characterization of waterborne polyurethane (WPU) with aluminum trihydroxide (ATH) and mica as flame retardants. J. Polym. Res. 2015, 22, 1–9. [Google Scholar] [CrossRef]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Casein/Apricot Filler in the Production of Flame-Retardant Polyurethane Composites. Materials 2021, 14, 3620. [Google Scholar] [CrossRef]
- Thomsen, C.; Lundanes, E.; Becher, G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J. Environ. Monit. 2001, 3, 366–370. [Google Scholar] [CrossRef]
- Thomsen, C.; Leknes, H.; Lundanes, E.; Becher, G. A new method for determination of halogenated flame retardants in human milk using solid-phase extraction. J. Anal. Toxicol. 2002, 26, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, I.; Sakai, S.-I. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003, 29, 665–682. [Google Scholar] [CrossRef]
- Osako, M.; Kim, Y.-J.; Sakai, S.-I. Leaching of brominated flame retardants in leachate from landfills in Japan. Chemosphere 2004, 57, 1571–1579. [Google Scholar] [CrossRef]
- Herat, S. Environmental impacts and use of brominated flame-retardants in electrical and electronic equipment. Environment 2008, 28, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Chen, S.; Luo, X.; Zhang, X.; Tang, C.; Luo, Y.; Mai, B. Occurrence of brominated flame-retardants other than polybrominated diphenyl ethers in environmental and biota samples from southern China. Chemosphere 2009, 74, 910–916. [Google Scholar] [CrossRef]
- Debenest, T.; Gagné, F.; Petit, A.; André, C.; Kohli, M.; Blaise, C. Ecotoxicity of a brominated flame retardant (tetrabromobisphenol A) and its derivatives to aquatic organisms. Comp. Biochem. Physiol. Part C Toxicol. 2010, 152, 407–412. [Google Scholar] [CrossRef]
- McKenna, S.T.; Hull, T.R. The fire toxicity of polyurethane foams. Fire Sci. Rev. 2016, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qian, M.; Song, P.; Huang, G.; Yu, Y.; Fu, S. Fabrication of Green Lignin-based Flame Retardants for Enhancing the Thermal and Fire Retardancy Properties of Polypropylene/Wood Composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Di Blasio, A.; Cuttica, F.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Merk, V.; Chanana, M.; Gaan, S.; Burgert, I. Mineralization of wood by calcium carbonate insertion for improved flame retardancy. Holzforschung 2016, 70, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Özparpucu, M.; Windeisen-Holzhauser, E.; Schlepütz, C.M.; Quadranti, E.; Gaan, S.; Dreimol, C.; Burgert, I. Struvite Mineralized Wood as Sustainable Building Material: Mechanical and Combustion Behavior. ACS Sustain. Chem. Eng. 2020, 8, 10402–10412. [Google Scholar] [CrossRef]
- Carosio, F.; Cuttica, F.; Medina, L.; Berglund, L. Clay nanopaper as multifunctional brick and mortar fire protection coating-wood case study. Mater. Des. 2016, 93, 357–363. [Google Scholar] [CrossRef]
- Carosio, F.; Kochumalayil, J.; Cuttica, F.; Camino, G.; Berglund, L. Oriented clay nanopaper from biobased components—Mechanisms for superior fire protection properties. ACS Appl. Mater. Interfaces 2015, 7, 5847–5856. [Google Scholar] [CrossRef]
- Kumar, S.P.; Takamori, S.; Araki, H.; Kuroda, S. Flame retardancy of clay-sodium silicate composite coatings on wood for construction purposes. RSC Adv. 2015, 5, 34109–34116. [Google Scholar] [CrossRef]
- Li, F.; Jianhuai, W.; Jiongtian, L.; Bingguo, O.W.; Shuojiang, S. Preparation and Fire Retardancy of Antimony Oxide Nanoparticles/Mica Composition. J. Compos. Mater. 2007, 41, 1487–1497. [Google Scholar] [CrossRef]
- He, Y.; Fan, Y.; Chen, C.; Zhong, F.; Qing, D. Synthesis of mica-multiwalled carbon nanotube (MWCNT) hybrid material and properties of mica-MWCNT/epoxy composites coating research. High Perform. Polym. 2014, 27, 191–199. [Google Scholar] [CrossRef]
- Robert, M.; Cousin, P.; Fam, A.; Benmokrane, B. Effect of the addition of modified mica on mechanical and durability properties of FRP composite materials for civil engineering. Polym. Compos. 2011, 32, 1202–1209. [Google Scholar] [CrossRef]
- Limparyoon, N.; Seetapan, N.; Kiatkamjornwong, S. Acrylamide/2-acrylamido-2-methylpropane sulfonic acid and associated sodium salt superabsorbent copolymer nanocomposites with mica as fire retardants. Polym. Degrad. Stab. 2011, 96, 1054–1063. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, L.; Zhang, C.; Wang, P.; Zhang, F.; Zhang, G. Novel High-Efficiency Casein-Based P–N-Containing Flame Retardants with Multiple Reactive Groups for Cotton Fabrics. ACS Sustain. Chem. Eng. 2019, 7, 13999–14008. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Mercero, J.M.; Fowler, J.E.; Ugalde, J.M. Aluminum (III) Interactions with the Acid Derivative Amino Acid Chains. J. Phys. Chem. A 2000, 104, 7053–70603. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Competition among Metal Ions for Protein Binding Sites: Determinants of Metal Ion Selectivity in Proteins. Chem. Rev. 2014, 114, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Rodzik, A.; Pomastowski, P.; Sagandykova, G.N.; Buszewski, B. Interactions of Whey Proteins with Metal Ions. Int. J. Mol. Sci. 2020, 21, 2156. [Google Scholar] [CrossRef] [Green Version]
- Motekaitis, R.J.; Martell, A.E. Complexes of aluminum(III) with hydroxy carboxylic acids. Inorg. Chem. 1984, 23, 18–23. [Google Scholar] [CrossRef]
- Mujika, J.I.; Torre, G.D.; Formoso, E.; Grande-Aztatzi, R.; Grabowski, S.J.; Exley, C.; Lopez, X. Aluminum’s preferential binding site in proteins: Sidechain of amino acids versus backbone interactions. J. Inorg. Biochem. 2018, 181, 111–116. [Google Scholar] [CrossRef]
- Liu, J.C.; Feldkamp, J.R.; White, J.L.; Hem, S.L. Adsorption of phosphate by aluminum hydroxycar bonate. J. Pharm. Sci. 1984, 73, 1355–1358. [Google Scholar] [CrossRef]
- Bleam, W.F.; Pfeffer, P.E.; Goldberg, S.; Taylor, R.W.; Dudley, R.A. 31P solid-state nuclear magnetic resonance study of phosphate adsorption at the boehmite/aqueous solution interface. Langmuir 1991, 7, 1702–1712. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rost, B.E.; Relyveld, E.; Siber, G.R. Adjuvant Properties of Aluminum and Calcium Compounds. In Vaccine Design: The Subunit and Adjuvant Approach; Powell, M.F., Newman, M.J., Burdman, J.R., Eds.; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Zheng, T.-T.; Sun, Z.-X.; Yang, X.-F.; Holmgren, A. Sorption of phosphate onto mesoporous γ-alumina studied with in-situ ATR-FTIR spectroscopy. Chem. Cent. J. 2012, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.; Kiviranta, K.; Suvanto, S.; Alvila, L.; Leskinen, J.; Lappalainen, R.; Haapala, A. Casein-magnesium composite as an intumescent fire retardant coating for wood. Fire Saf. J. 2019, 112, 102943. [Google Scholar] [CrossRef]
- ASTM D4541-17; Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4060-19; Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM International: West Conshohocken, PA, USA, 2019.
- Mocanu, A.M.; Moldoveanu, C.; Odochian, L.; Paius, C.M.; Apostolescu, N.; Neculau, R. Study on the thermal behavior of casein under nitrogen and air atmosphere by means of the TG-FTIR technique. Thermochim Acta 2012, 546, 120–126. [Google Scholar] [CrossRef]
- Troitzsch, J.H. Overview of flame retardants. Chim. Oggi 1998, 16, 18–24. [Google Scholar]
- Chen, G.; Chen, C.; Pei, Y.; He, S.; Liu, Y.; Jiang, B.; Jiao, M.; Gan, W.; Liu, D.; Yang, B.; et al. A Strong, Flame-retardant, and Thermally Insulating Wood Laminate. Chem. Eng. J. 2019, 383, 123109. [Google Scholar] [CrossRef]
- Wu, J.; Wang, M.; Guo, H. Synergistic Flame Retardant Effects of Different Zeolites on Intumescent Fire Retardant Coating for Wood. BioResources 2017, 12, 5369–5382. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.F.; Lu, Y.; Xia, Y.Z.; Yang, D.J.; Li, J.; Liu, Y.X. Flame retardancy of wood treated by TiO2/ZnO coating. Surf. Eng. 2012, 28, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.F.; Lu, Y.; Zhang, H.M.; Yang, D.J.; Xu, J.S.; Li, J.; Liu, Y.X.; Shi, J.T. Flame retardancy of wood coated by ZnO nanorod arrays via a hydrothermal method. Mater. Res. Innov. 2012, 16, 326–331. [Google Scholar] [CrossRef]
- Bourbigot, S.; Gilman, J.W.; Wilkie, C.A. Kinetic Analysis of the Thermal Degradation of Polystyrene-Montmorillonite Nanocomposite. Polym. Degrad. Stab. 2004, 84, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability Properties of Polymer−Layered-Silicate Nanocomposites. Polypropylene and Polystyrene Nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Foungfung, D.; Phattanarudee, S.; Seetapan, N.; Kiatkamjornwong, S. Acrylamide-itaconic acid superabsorbent polymers and superabsorbent polymer/mica nanocomposites. Polym. Adv. Technol. 2011, 22, 635–647. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, S.; Zhao, C.; Hu, G.; Yang, M. Flame retardant mechanism of polymer/clay nanocomposites based on polypropylene. Polymer 2005, 46, 8386–8395. [Google Scholar] [CrossRef]
- Tian, C.; Guo, H.; Zhang, H.; Xu, J.; Shi, J. Study on the thermal degradation of cotton cellulose ammonium phosphate and its metal complexes. Thermochim. Acta 1995, 253, 243–251. [Google Scholar] [CrossRef]
- Soares, S.; Camino, G.; Levchik, S. Effect of metal carboxylates on the thermal decomposition of cellulose. Polym. Degrad. Stab. 1998, 62, 25–31. [Google Scholar] [CrossRef]
- Minelli, M.; De Angelis, M.; Doghieri, F.; Marini, M.; Toselli, M.; Pilati, F. Oxygen permeability of novel organic–inorganic coatings: I. Effects of organic–inorganic ratio and molecular weight of the organic component. Eur. Polym. J. 2008, 44, 2581–2588. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym. Adv. Technol. 2006, 17, 772–777. [Google Scholar] [CrossRef]
- Mostapha, M.; Jahar, N.A.; Chin, S.X.; Jaafar, S.N.S.; Zakaria, S.; Aizat, W.M.; Azizan, K.A. Effect of zeolite catalyst on sugar dehydration for 5-hydroxymethylfurfural synthesis. In Proceedings of the AIP Conference 1784, Penang, Malaysia, 10–12 April 2016. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Recio, M.; Morales, I.J.; Arias, P.L.; Santamaría-González, J.; Maireles-Torres, P. The Key Role of Textural Properties of Aluminosilicates in the Acid-Catalysed Dehydration of Glucose into 5-Hydroxymethylfurfural. ChemistrySelect 2017, 2, 2444–2451. [Google Scholar] [CrossRef]
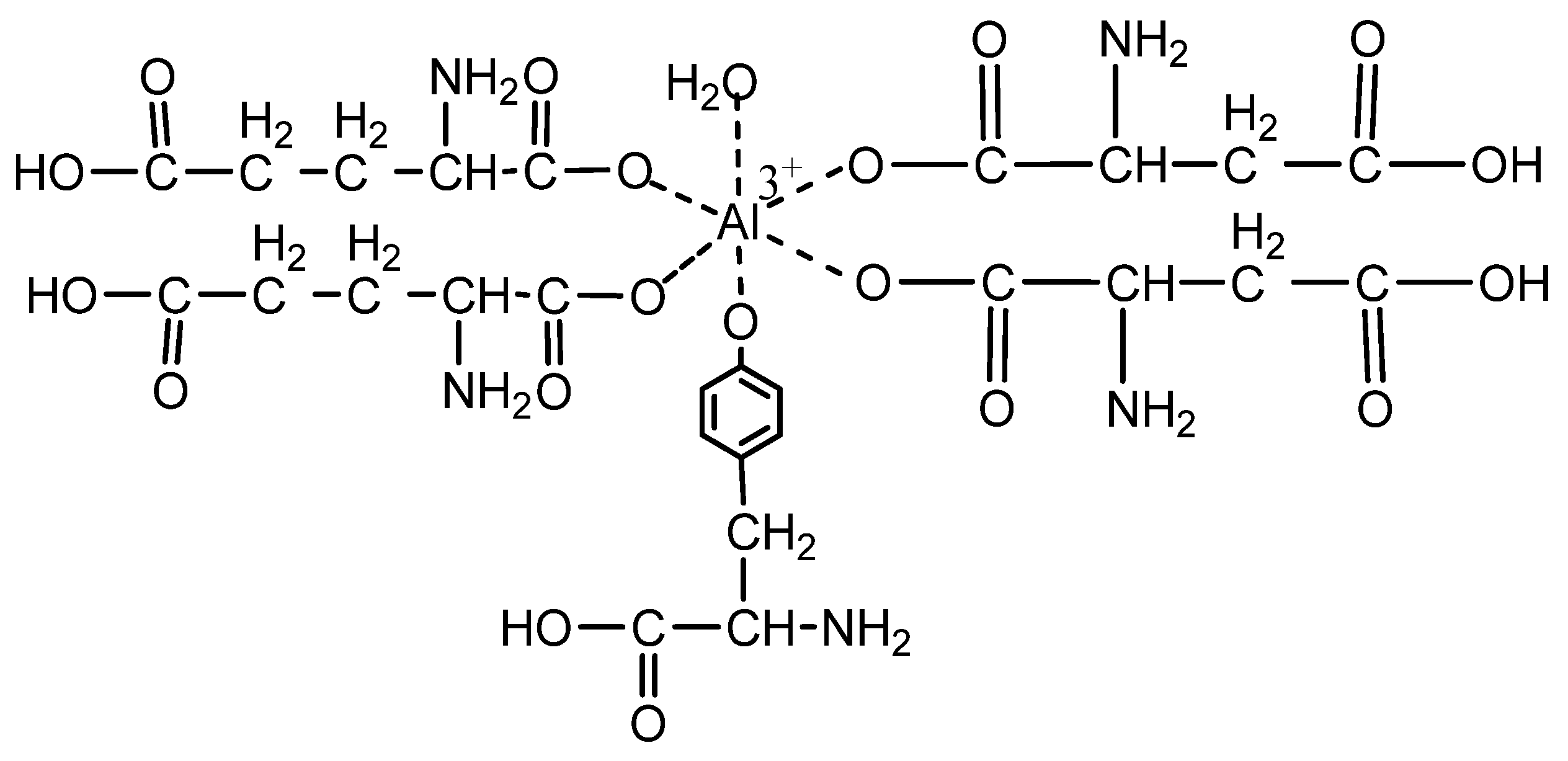
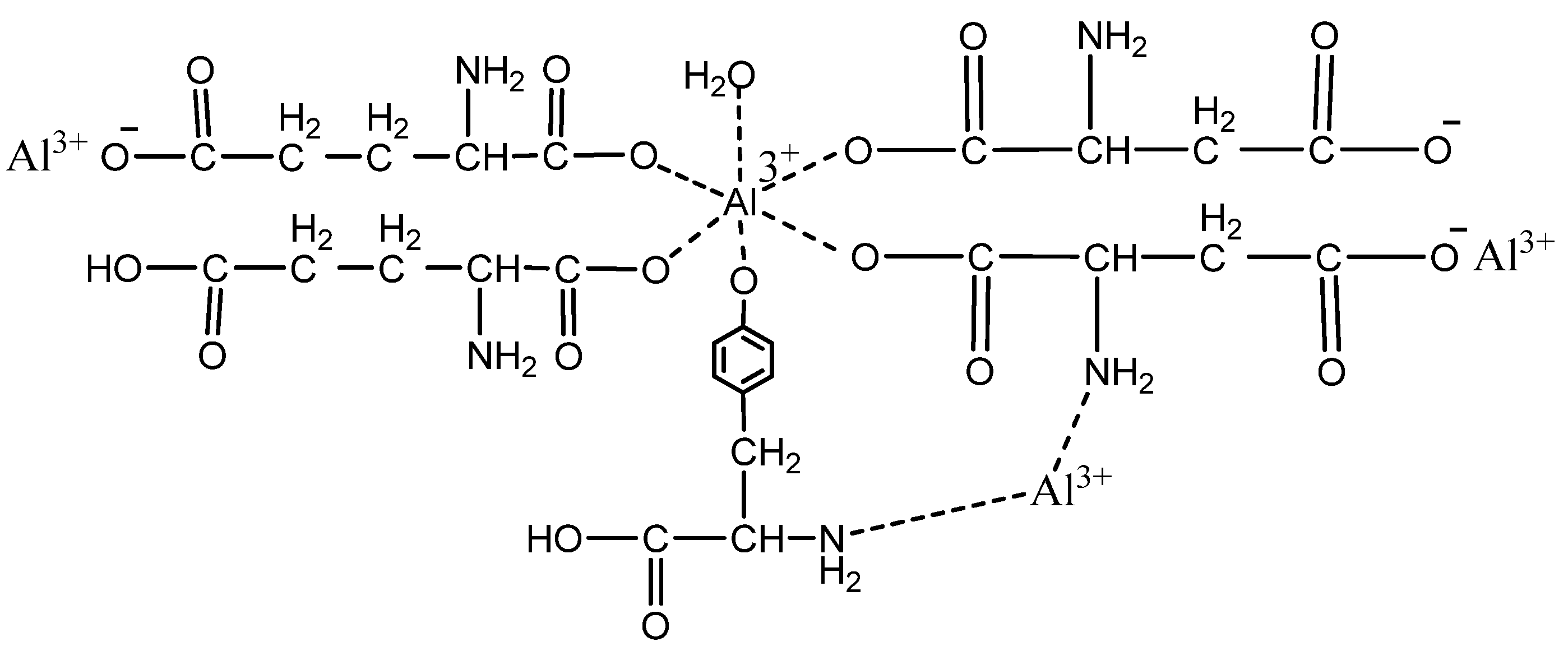
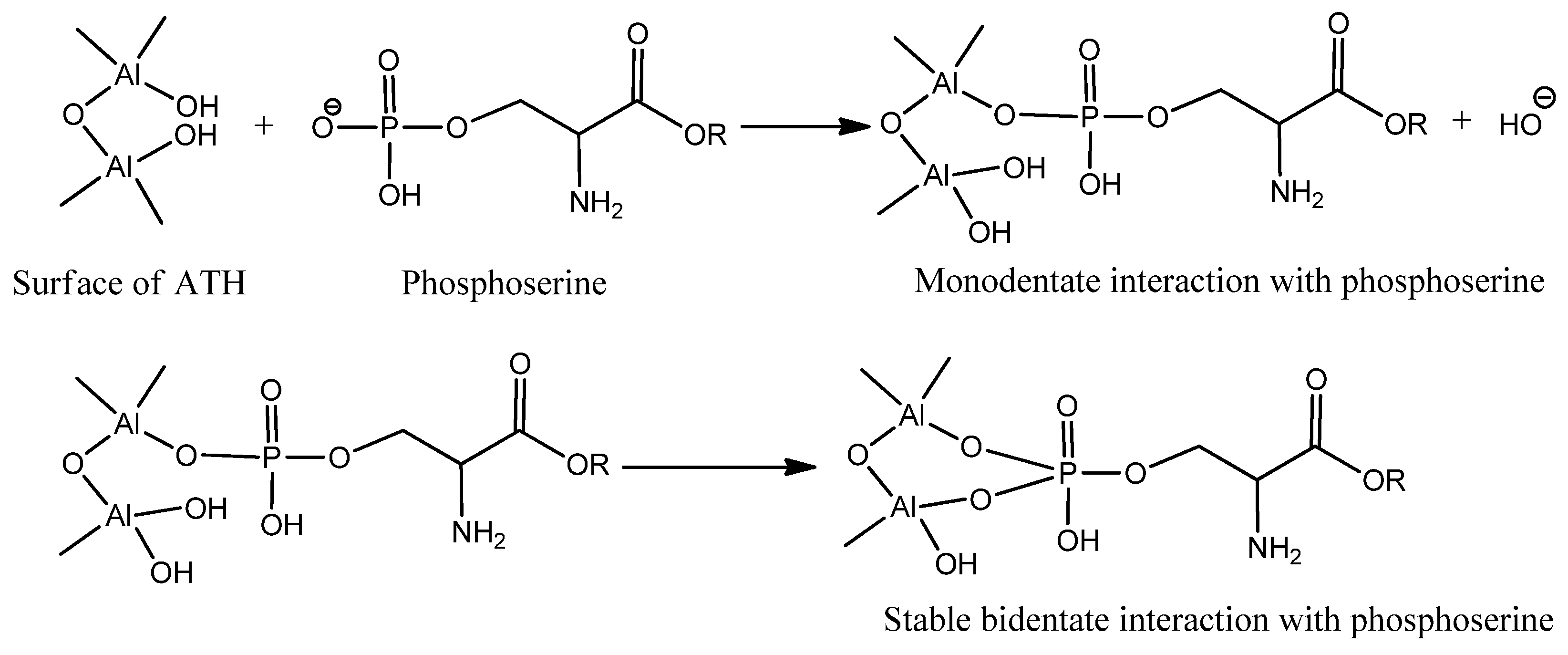

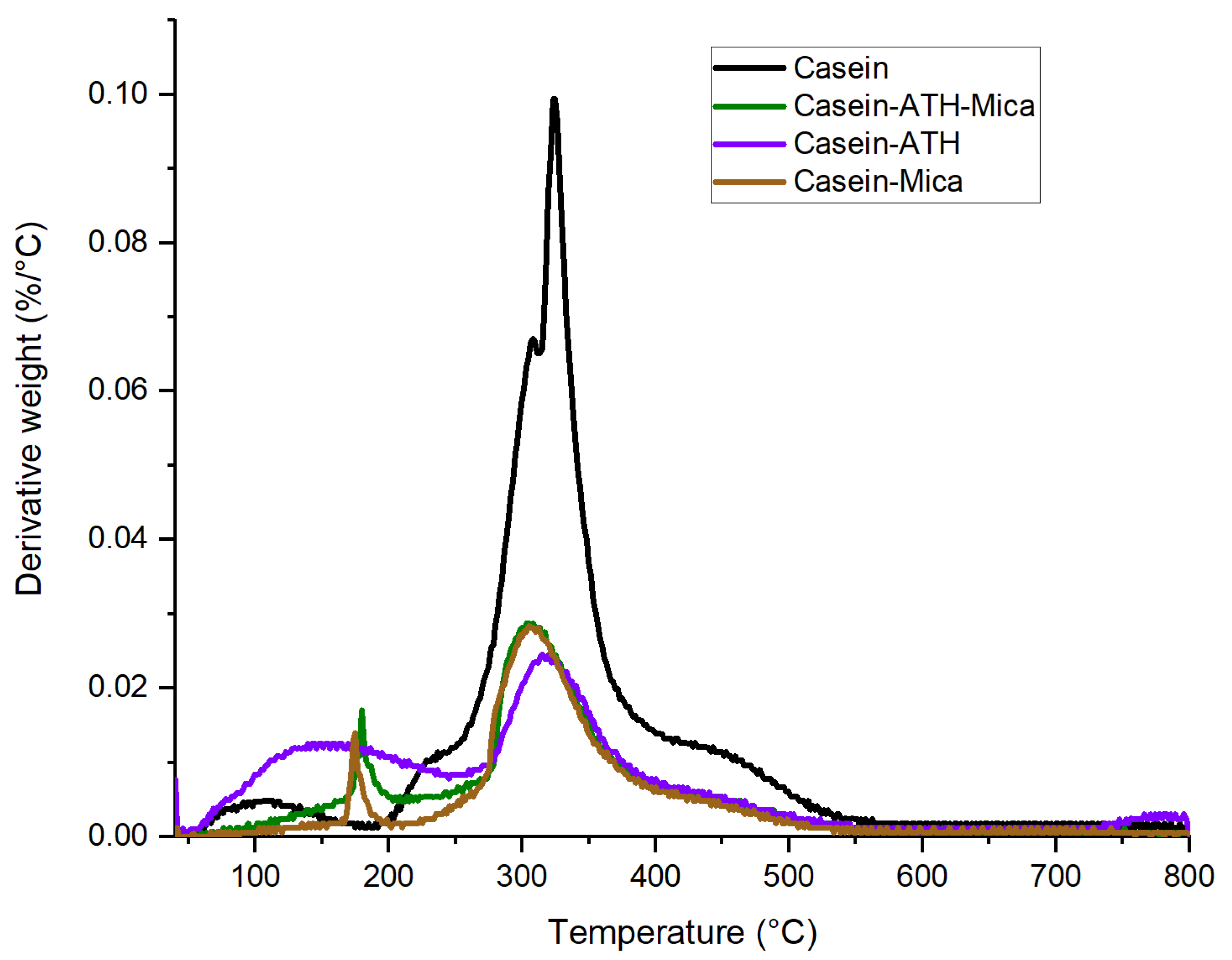


| Sample Code | Coating Composition | Wt of Wood (g) | Wt after Coating (g) | Coating Wt (g) | Coating Wt (g/m2) |
|---|---|---|---|---|---|
| Reference | Uncoated pinewood | 80.50 | - | - | - |
| Casein | 2.5 g C suspension | 99.50 | 100.95 | 1.45 | 145.00 |
| Casein–ATH | 2.5 g C + 4 g ATH | 100.95 | 105.98 | 5.03 | 502.60 |
| Casein–Mica | 2.5 g C + 4 g mica | 110.39 | 116.86 | 6.47 | 647.05 |
| Casein–ATH–Mica | 2.5 g C + 1 g ATH + 3 g mica | 121.18 | 126.57 | 5.39 | 538.66 |
| Sample Code | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | TML (g) |
|---|---|---|---|---|
| Reference | 12 | 216 | 80 | 47 |
| Casein | 7 | 215 | 86 | 52 |
| Casein–ATH–Mica | 31 | 150 | 58 | 41 |
| Casein–ATH | 42 | 203 | 70 | 46 |
| Casein–Mica | 17 | 97 | 50 | 37 |
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between Groups | 2.607945833 | 3 | 0.869315278 | 77.82009201 | 3.3362 × 10−11 | 3.098391212 |
| Within Groups | 0.223416667 | 20 | 0.011170833 | |||
| Total | 2.8313625 | 23 |
| Sample | Average Weight Loss (g) | Average No. of Cycles | Average Wear Index (g/Cycles) |
|---|---|---|---|
| Casein | 0.420 | 180 | 2.333 |
| Casein–ATH–Mica | 2.018 | 407 | 4.957 |
| Casein–ATH | 1.650 | 3 | 3.811 |
| Casein–Mica | 1.970 | 528 | 3.731 |
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between Groups | 14.20487 | 3 | 4.734958 | 89.43616 | 1.77 × 10−8 | 3.490295 |
| Within Groups | 0.635308 | 12 | 0.052942 | |||
| Total | 14.84018 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, M.; Alabbad, M.; Li, L.; Orell, O.; Sarlin, E.; Haapala, A. Novel Micronized Mica Modified Casein–Aluminum Hydroxide as Fire Retardant Coatings for Wood Products. Coatings 2022, 12, 673. https://doi.org/10.3390/coatings12050673
Uddin M, Alabbad M, Li L, Orell O, Sarlin E, Haapala A. Novel Micronized Mica Modified Casein–Aluminum Hydroxide as Fire Retardant Coatings for Wood Products. Coatings. 2022; 12(5):673. https://doi.org/10.3390/coatings12050673
Chicago/Turabian StyleUddin, Mezbah, Maitham Alabbad, Ling Li, Olli Orell, Essi Sarlin, and Antti Haapala. 2022. "Novel Micronized Mica Modified Casein–Aluminum Hydroxide as Fire Retardant Coatings for Wood Products" Coatings 12, no. 5: 673. https://doi.org/10.3390/coatings12050673






