Overcoming the Dilemma between Low Electrical Resistance and High Corrosion Resistance Using a Ta/(Ta,Ti)N/TiN/Ti Multilayer for Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Deposition of Coatings
2.2. Compositional and Structural Characterizations of Coatings
2.3. Corrosion Resistance Measurements
2.4. Electrochemical Impedance Spectroscopy (EIS)
2.5. Interfacial Contact Resistance (ICR)
3. Results and Discussion
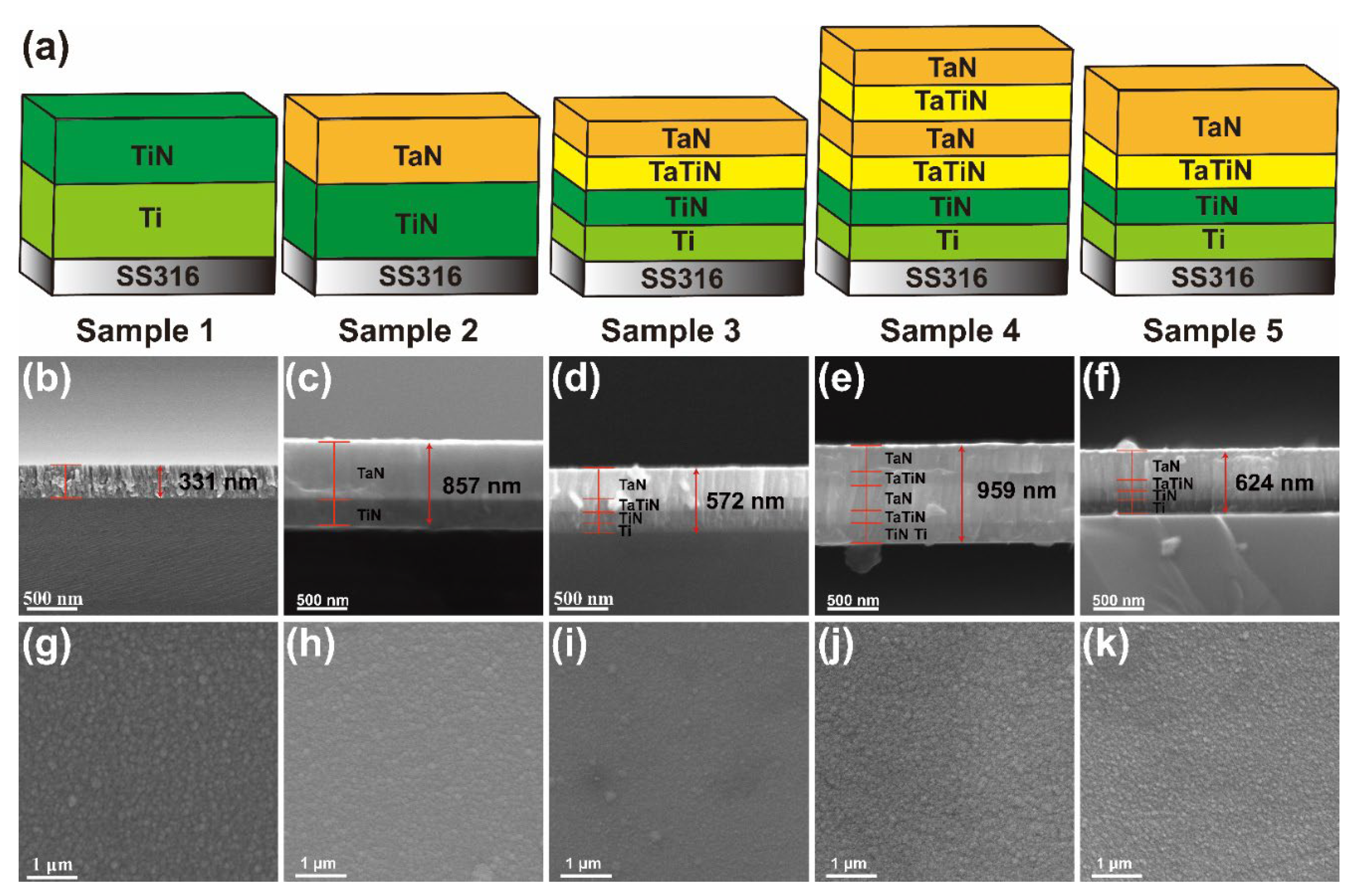
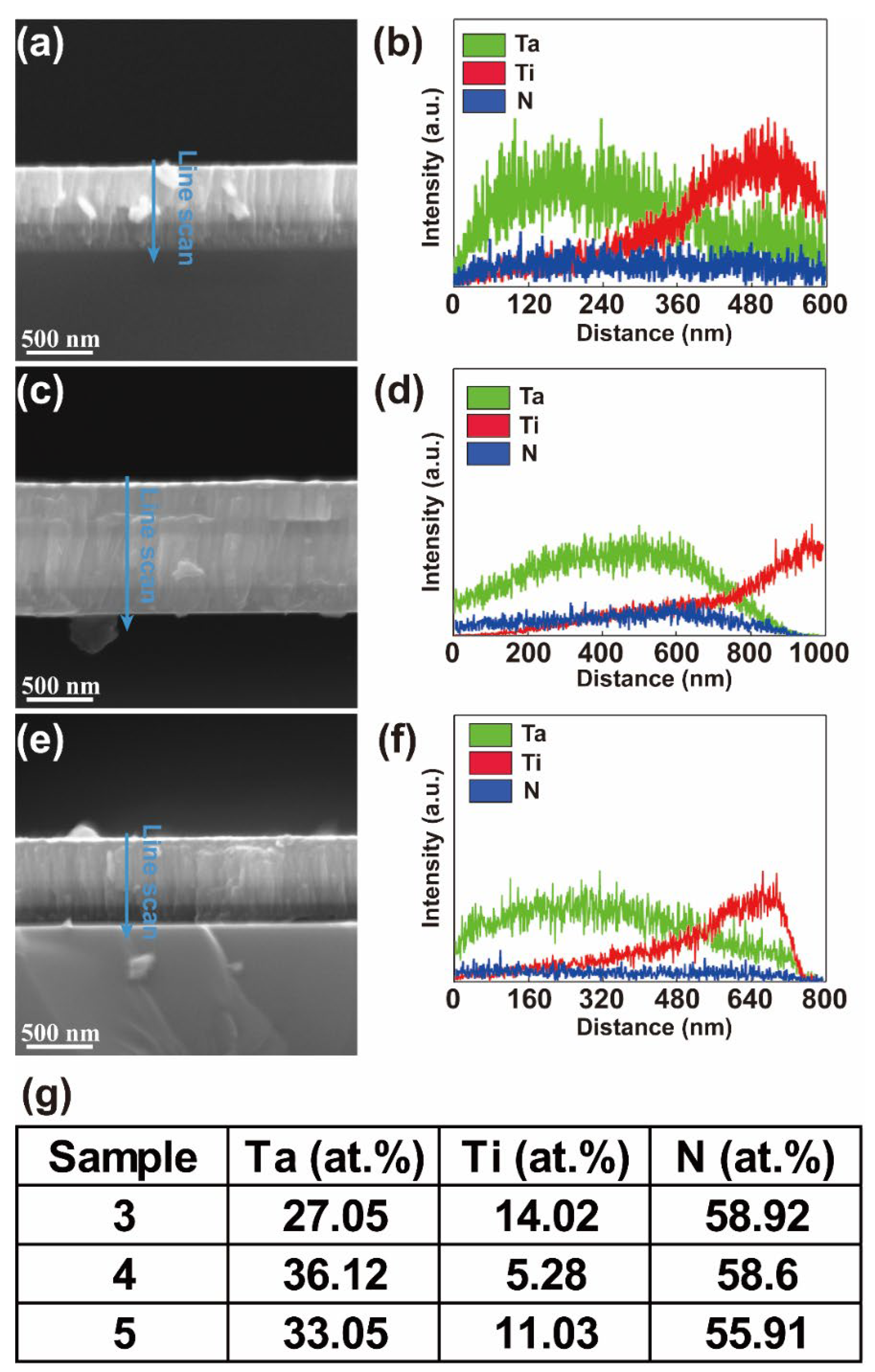
3.1. Morphology and Composition of the Coatings
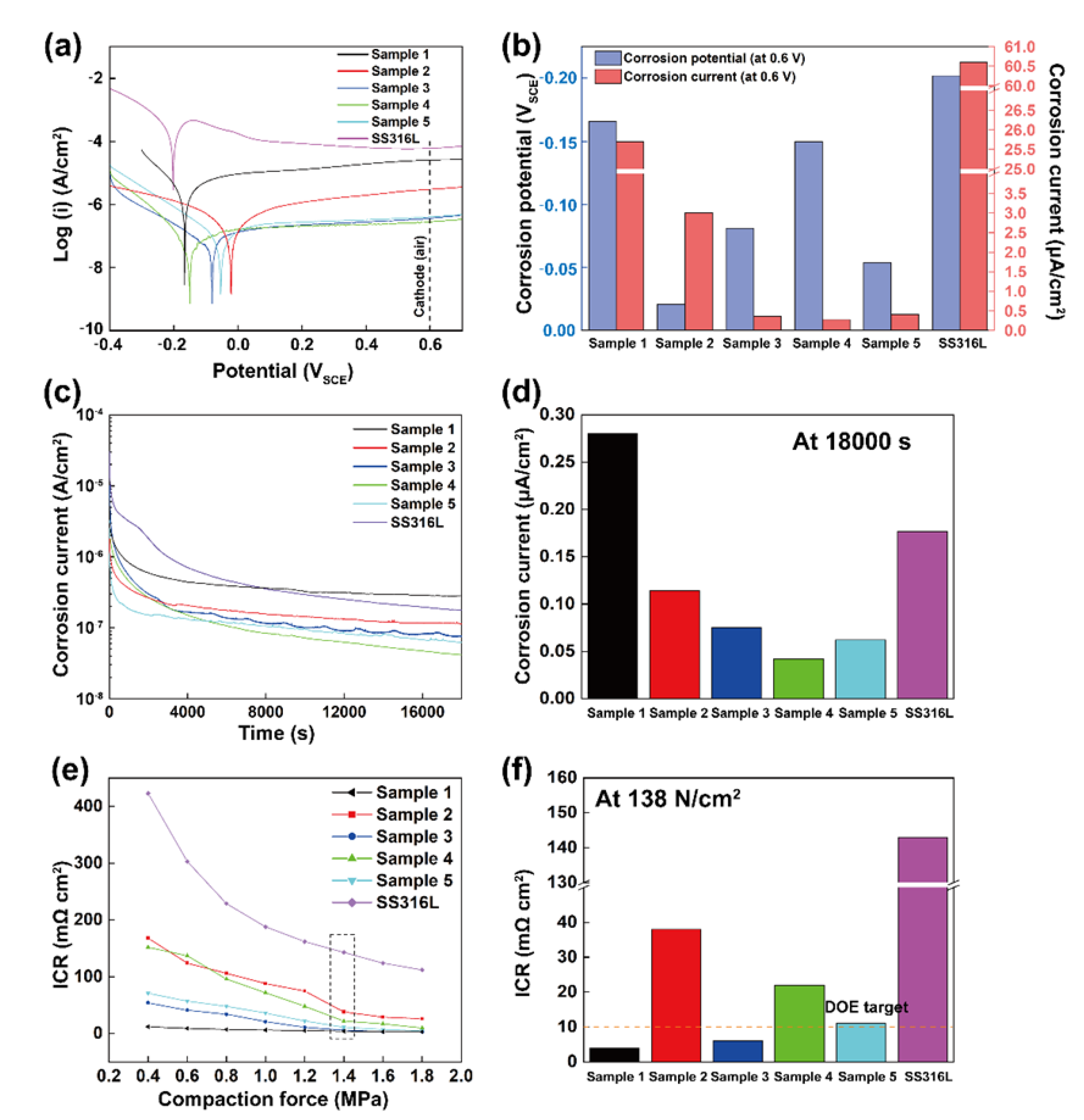
3.2. Corrosion Resistance and Interfacial Contact Resistance (ICR)
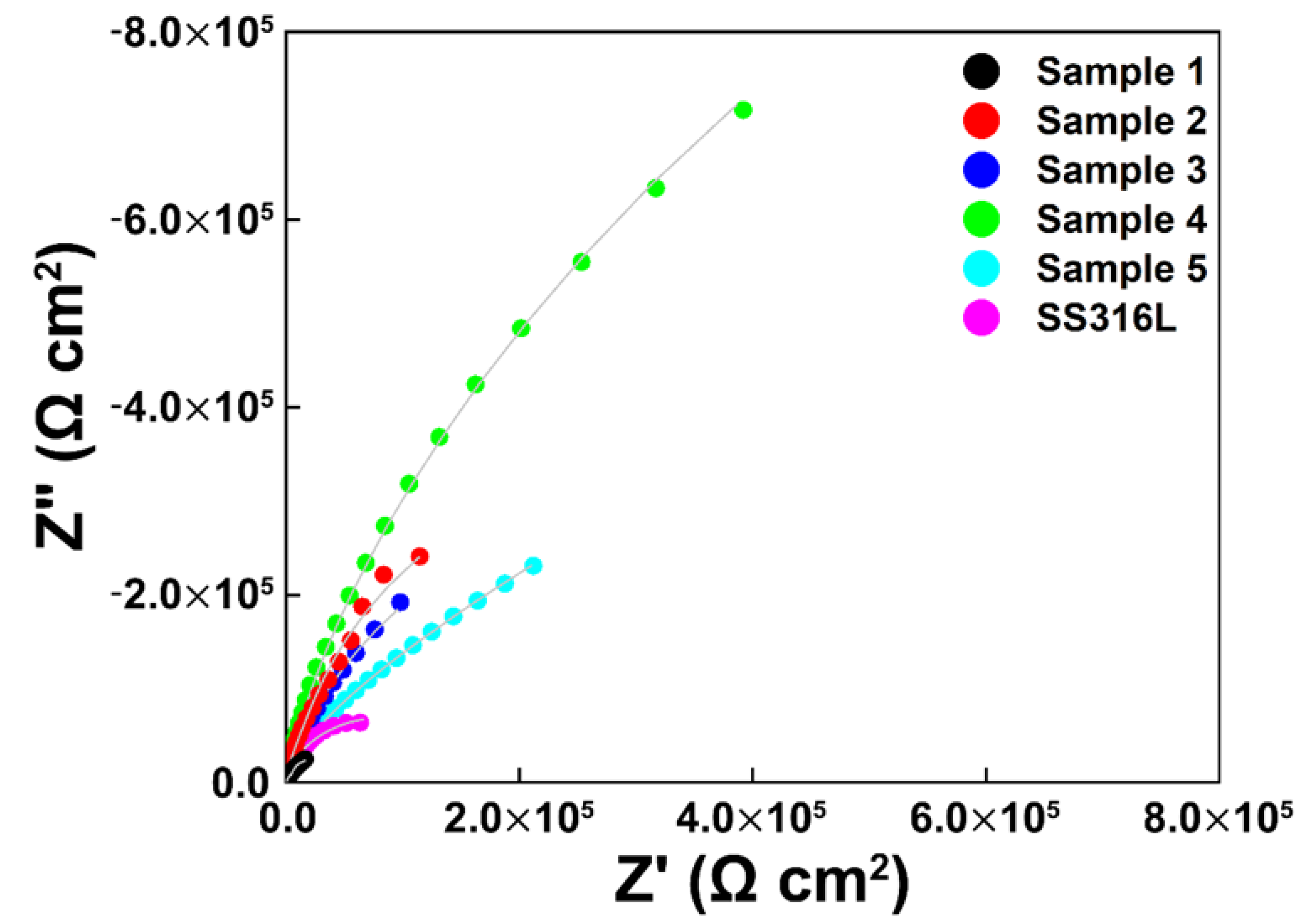
3.3. Electrochemical Impedance Spectroscopy (EIS)
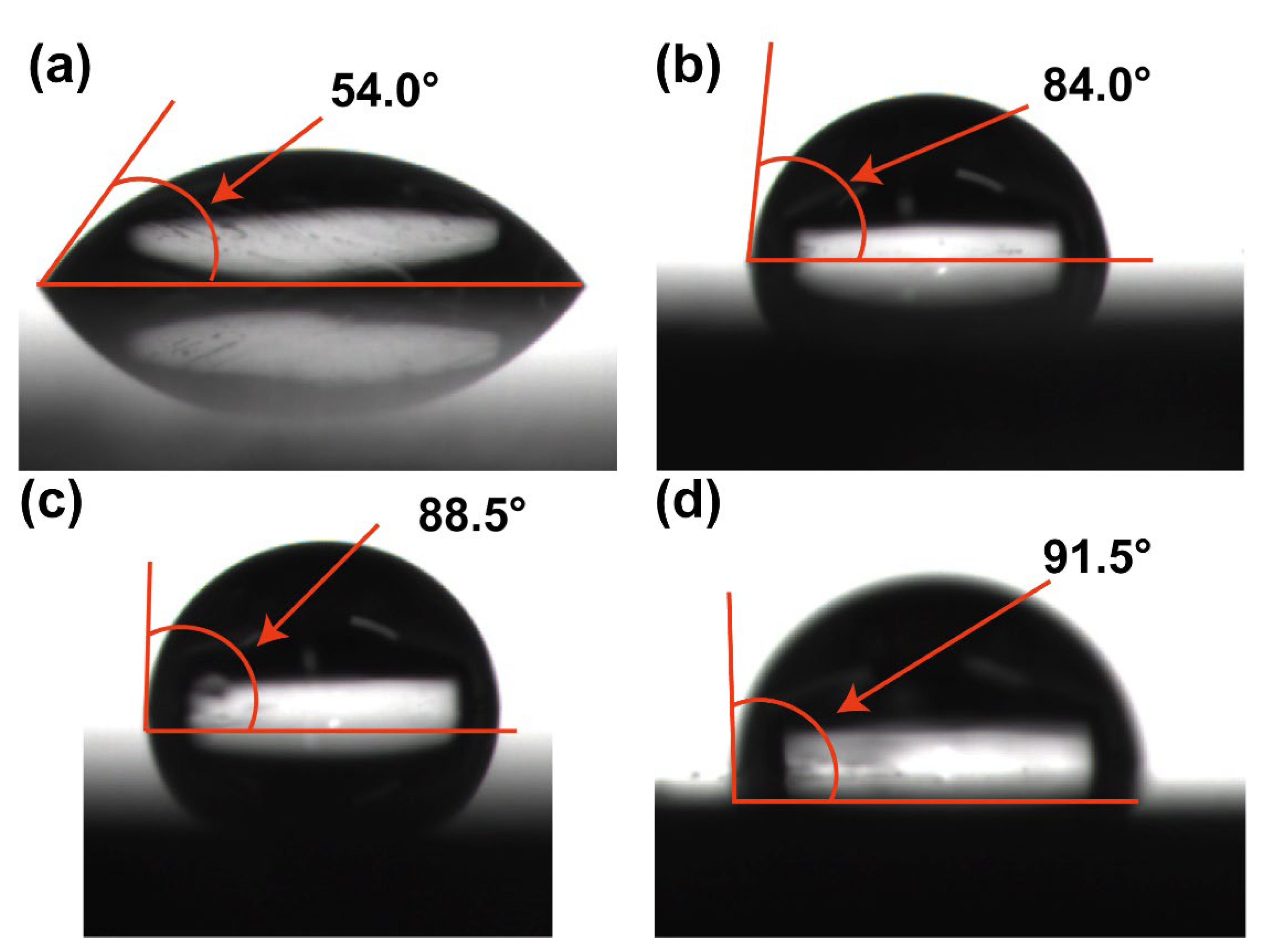
3.4. Contact Angle Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.C.; Zeng, C.L.; Luo, S.Z.; Shen, J.N.; Lin, H.C.; Cao, C.N. Electrochemical Corrosion Characteristics of Type 316 Stainless Steel in Simulated Anode Environment for PEMFC. Electrochim. Acta 2003, 48, 1735–1741. [Google Scholar] [CrossRef]
- Lee, S.J.; Lai, J.J.; Huang, C.H. Stainless Steel Bipolar Plates. J. Power Sources 2005, 145, 362–368. [Google Scholar] [CrossRef]
- Cho, E.A.; Jeon, U.S.; Hong, S.A.; Oh, I.H.; Kang, S.G. Performance of a 1 KW-Class PEMFC Stack Using TiN-Coated 316 Stainless Steel Bipolar Plates. J. Power Sources 2005, 142, 177–183. [Google Scholar] [CrossRef]
- Kim, M.; Lim, J.W.; Lee, D.G. Electrical Contact Resistance between Anode and Cathode Bipolar Plates with Respect to Surface Conditions. Compos. Struct. 2018, 189, 79–86. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar Plates for PEM Fuel Cells: A Review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Middelman, E.; Kout, W.; Vogelaar, B.; Lenssen, J.; de Waal, E. Bipolar Plates for PEM Fuel Cells. J. Power Sources 2003, 118, 44–46. [Google Scholar] [CrossRef]
- Cooper, J.S. Design Analysis of PEMFC Bipolar Plates Considering Stack Manufacturing and Environment Impact. J. Power Sources 2004, 129, 152–169. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Sulong, A.B.; Umer, A.; Yousaf, A.B.; Khan, M.A. Multi-Component MWCNT/NG/EP-Based Bipolar Plates with Enhanced Mechanical and Electrical Characteristics Fabricated by Compression Moulding. Ceram. Int. 2018, 44, 14457–14464. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Dong, H.; Du, S.; Lu, Y.; Ji, X.; Gu, D. Surface Modification of 316 Stainless Steel with Platinum for the Application of Bipolar Plates in High Performance Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 2338–2348. [Google Scholar] [CrossRef]
- Paulauskas, I.E.; Brady, M.P.; Meyer, H.M.; Buchanan, R.A.; Walker, L.R. Corrosion Behavior of CrN, Cr2N and π Phase Surfaces on Nitrided Ni-50Cr for Proton Exchange Membrane Fuel Cell Bipolar Plates. Corros. Sci. 2006, 48, 3157–3171. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.; Lozano, A.; Maza, M. Influence of CrN-Coating Thickness on the Corrosion Resistance Behaviour of Aluminium-Based Bipolar Plates. J. Power Sources 2011, 196, 4283–4289. [Google Scholar] [CrossRef]
- Haye, E.; Deschamps, F.; Caldarella, G.; Piedboeuf, M.L.; Lafort, A.; Cornil, H.; Colomer, J.F.; Pireaux, J.J.; Job, N. Formable Chromium Nitride Coatings for Proton Exchange Membrane Fuel Cell Stainless Steel Bipolar Plates. Int. J. Hydrogen Energy 2020, 45, 15358–15365. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, D. Effect of Surface Treatment on the Interfacial Contact Resistance and Corrosion Resistance of Fe-Ni-Cr Alloy as a Bipolar Plate for Polymer Electrolyte Membrane Fuel Cells. Energy 2014, 64, 242–247. [Google Scholar] [CrossRef]
- Yun, Y.H. Deposition of Gold-Titanium and Gold-Nickel Coatings on Electropolished 316L Stainless Steel Bipolar Plates for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 1713–1718. [Google Scholar] [CrossRef]
- Kumar, A.; Ricketts, M.; Hirano, S. Ex Situ Evaluation of Nanometer Range Gold Coating on Stainless Steel Substrate for Automotive Polymer Electrolyte Membrane Fuel Cell Bipolar Plate. J. Power Sources 2010, 195, 1401–1407. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of Stainless Steel and Titanium Bipolar Plates for Anticorrosion in PEMFC: A Review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlček, J.; Nam, K.H.; Han, J.G. Reactive Magnetron Sputtering of Thin Films: Present Status and Trends. Thin Solid Films 2005, 475, 208–218. [Google Scholar] [CrossRef]
- Firouzabadi, S.S.; Dehghani, K.; Naderi, M.; Mahboubi, F. Numerical Investigation of Sputtering Power Effect on Nano-Tribological Properties of Tantalum-Nitride Film Using Molecular Dynamics Simulation. Appl. Surf. Sci. 2016, 367, 197–204. [Google Scholar] [CrossRef]
- Riekkinen, T.; Molarius, J.; Laurila, T.; Nurmela, A.; Suni, I.; Kivilahti, J.K. Reactive Sputter Deposition and Properties of Ta N Thin Films. Microelectron. Eng. 2002, 64, 289–297. [Google Scholar] [CrossRef]
- Bi, F.; Hou, K.; Yi, P.; Peng, L.; Lai, X. Mechanisms of Growth, Properties and Degradation of Amorphous Carbon Films by Closed Field Unbalanced Magnetron Sputtering on Stainless Steel Bipolar Plates for PEMFCs. Appl. Surf. Sci. 2017, 422, 921–931. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Mai, J.; Liu, D.; Cai, X. An Investigation into Nickel Implanted 316L Stainless Steel as a Bipolar Plate for PEM Fuel Cell. J. Power Sources 2008, 182, 145–152. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Liu, H.; Wang, S.; Fang, H.; Gao, H.; Gao, K.; Zhang, Y.; Sun, J.; Yan, J. Polylaminate TaN/Ta Coating Modified Ferritic Stainless Steel Bipolar Plate for High Temperature Proton Exchange Membrane Fuel Cell. J. Power Sources 2018, 399, 343–349. [Google Scholar] [CrossRef]
- U.S. Department of Energy (2017) 3.4-18; 2017. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 27 March 2022).
- Bräuer, G.; Szyszka, B.; Vergöhl, M.; Bandorf, R. Magnetron Sputtering-Milestones of 30 Years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]
- Wang, H.; Sweikart, M.A.; Turner, J.A. Stainless Steel as Bipolar Plate Material for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Shen, J.; Gao, H.; Sun, J.; Wang, H.; Cao, Y. High Conductivity and Anti-Corrosive Tantalum Surface Modified Ferritic Stainless Steel Bipolar Plate for Direct Ethanol Fuel Cell. Results Phys. 2019, 14, 102394. [Google Scholar] [CrossRef]
- Choe, C.; Choi, H.; Hong, W.; Lee, J.J. Tantalum Nitride Coated AISI 316L as Bipolar Plate for Polymer Electrolyte Membrane Fuel Cell. Int. J. Hydrogen Energy 2012, 37, 405–411. [Google Scholar] [CrossRef]
- Manso, A.P.; Marzo, F.F.; Garicano, X.; Alegre, C.; Lozano, A.; Barreras, F. Corrosion Behavior of Tantalum Coatings on AISI 316L Stainless Steel Substrate for Bipolar Plates of PEM Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 20679–20691. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Y.; Xu, J.; Lin, G.; Hou, M. Chromium Nitride Films on Stainless Steel as Bipolar Plate for Proton Exchange Membrane Fuel Cell. J. Power Sources 2009, 194, 976–980. [Google Scholar] [CrossRef]
- Alishahi, M.; Mahboubi, F.; Mousavi Khoie, S.M.; Aparicio, M.; Hübner, R.; Soldera, F.; Gago, R. Electrochemical Behavior of Nanocrystalline Ta/TaN Multilayer on 316L Stainless Steel: Novel Bipolar Plates for Proton Exchange Membrane Fuel-Cells. J. Power Sources 2016, 322, 1–9. [Google Scholar] [CrossRef]
- Mendizabal, L.; Oedegaard, A.; Kongstein, O.E.; Lædre, S.; Walmsley, J.; Barriga, J.; Gonzalez, J.J. TaNX Coatings Deposited by HPPMS on SS316L Bipolar Plates for Polymer Electrolyte Membrane Fuel Cells: Correlation between Corrosion Current, Contact Resistance and Barrier Oxide Film Formation. Int. J. Hydrogen Energy 2017, 42, 3259–3270. [Google Scholar] [CrossRef]
- Fu, Y.; Hou, M.; Lin, G.; Hou, J.; Shao, Z.; Yi, B. Coated 316L Stainless Steel with CrxN Film as Bipolar Plate for PEMFC Prepared by Pulsed Bias Arc Ion Plating. J. Power Sources 2008, 176, 282–286. [Google Scholar] [CrossRef]
- Chen, G.S.; Chen, S.T.; Huang, S.C.; Lee, H.Y. Growth Mechanism of Sputter Deposited Ta and Ta ± Nthin Ælms Induced by an Underlying Titaniumlayer and Varying Nitrogen Flow Rates. Appl. Surf. Sci. 2001, 169, 353–357. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, L.; Yi, P.; Peng, L. Influence of Cr-C Film Composition on Electrical and Corrosion Properties of 316L Stainless Steel as Bipolar Plates for PEMFCs. Int. J. Hydrogen Energy 2016, 41, 1142–1150. [Google Scholar] [CrossRef]
- Jin, J.; Liu, H.; Zheng, D.; Zhu, Z. Effects of Mo Content on the Interfacial Contact Resistance and Corrosion Properties of CrN Coatings on SS316L as Bipolar Plates in Simulated PEMFCs Environment. Int. J. Hydrogen Energy 2018, 43, 10048–10060. [Google Scholar] [CrossRef]
- Xu, Z.; Qiu, D.; Yi, P.; Peng, L.; Lai, X. Towards Mass Applications: A Review on the Challenges and Developments in Metallic Bipolar Plates for PEMFC. Prog. Natl. Sci. Mater. Int. 2020, 30, 815–824. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Xu, S.; Munroe, P.; Xie, Z.H. A Nanocrystalline Zirconium Carbide Coating as a Functional Corrosion-Resistant Barrier for Polymer Electrolyte Membrane Fuel Cell Application. J. Power Sources 2015, 297, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Jin, N.; Li, Z.; Liu, X. First-Principles Calculations on the Electronic Structure and Bonding Nature of TaN(111)/TiN(111) Interface. J. Alloys Comp. 2017, 717, 326–332. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Liu, H. Influence of Fluoride Ions on Corrosion Performance of 316L Stainless Steel as Bipolar Plate Material in Simulated PEMFC Anode Environments. Int. J. Hydrogen Energy 2012, 37, 1875–1883. [Google Scholar] [CrossRef]
- Pugal Mani, S.; Rajendran, N. Corrosion and Interfacial Contact Resistance Behavior of Electrochemically Nitrided 316L SS Bipolar Plates for Proton Exchange Membrane Fuel Cells. Energy 2017, 133, 1050–1062. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Zeng, C.; Shen, J.; Lin, H.; Cao, C. Corrosion Behavior of TiN Coated Type 316 Stainless Steel in Simulated PEMFC Environments. Corros. Sci. 2004, 46, 1369–1380. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Ramadoss, A.; Kobayashi, A.; Muthirulandi, J. Nanocomposite Ti-Si-N Coatings Deposited by Reactive Dc Magnetron Sputtering for Biomedical Applications. J. Am. Ceram. Soc. 2012, 95, 2746–2752. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D.O. An Investigation of the Electrochemical Properties of PVD TiN-Coated SS410 in Simulated PEM Fuel Cell Environments. Int. J. Hydrogen Energy 2007, 32, 895–902. [Google Scholar] [CrossRef]
- Pugal Mani, S.; Srinivasan, A.; Rajendran, N. Effect of Nitrides on the Corrosion Behaviour of 316L SS Bipolar Plates for Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Hydrogen Energy 2015, 40, 3359–3369. [Google Scholar] [CrossRef]
- Er, D.; Taghavi Pourian Azar, G.; Kazmanlı, K.; Ürgen, M. The Corrosion Protection Ability of TiAlN Coatings Produced with CA-PVD under Superimposed Pulse Bias. Surf. Coat. Technol. 2018, 346, 1–8. [Google Scholar] [CrossRef]
- Ous, T.; Arcoumanis, C. Degradation Aspects of Water Formation and Transport in Proton Exchange Membrane Fuel Cell: A Review. J. Power Sources 2013, 240, 558–582. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Li, P.; Jing, B.; Li, S.; Wen, Z.; Ji, S. Niobized AISI 304 Stainless Steel Bipolar Plate for Proton Exchange Membrane Fuel Cell. J. Power Sources 2012, 208, 397–403. [Google Scholar] [CrossRef]
- Blanco, D.; Viesca, J.L.; Mallada, M.T.; Ramajo, B.; González, R.; Battez, A.H. Wettability and Corrosion of [NTf2] Anion-Based Ionic Liquids on Steel and PVD (TiN, CrN, ZrN) Coatings. Surf. Coat. Technol. 2016, 302, 13–21. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, R.; Min, R.; Yang, M.; Yuan, Y.; Zheng, L.; Li, Q.; Ji, B.; Zhang, S.; Yang, M.; Shi, J. Overcoming the Dilemma between Low Electrical Resistance and High Corrosion Resistance Using a Ta/(Ta,Ti)N/TiN/Ti Multilayer for Proton Exchange Membrane Fuel Cells. Coatings 2022, 12, 689. https://doi.org/10.3390/coatings12050689
Tu R, Min R, Yang M, Yuan Y, Zheng L, Li Q, Ji B, Zhang S, Yang M, Shi J. Overcoming the Dilemma between Low Electrical Resistance and High Corrosion Resistance Using a Ta/(Ta,Ti)N/TiN/Ti Multilayer for Proton Exchange Membrane Fuel Cells. Coatings. 2022; 12(5):689. https://doi.org/10.3390/coatings12050689
Chicago/Turabian StyleTu, Rong, Rui Min, Mai Yang, Yang Yuan, Long Zheng, Qizhong Li, Baifeng Ji, Song Zhang, Meijun Yang, and Ji Shi. 2022. "Overcoming the Dilemma between Low Electrical Resistance and High Corrosion Resistance Using a Ta/(Ta,Ti)N/TiN/Ti Multilayer for Proton Exchange Membrane Fuel Cells" Coatings 12, no. 5: 689. https://doi.org/10.3390/coatings12050689
APA StyleTu, R., Min, R., Yang, M., Yuan, Y., Zheng, L., Li, Q., Ji, B., Zhang, S., Yang, M., & Shi, J. (2022). Overcoming the Dilemma between Low Electrical Resistance and High Corrosion Resistance Using a Ta/(Ta,Ti)N/TiN/Ti Multilayer for Proton Exchange Membrane Fuel Cells. Coatings, 12(5), 689. https://doi.org/10.3390/coatings12050689







