Facile and Eco-Friendly Preparation of Mild Steel Based Superhydrophobic Surfaces without Chemical Modifications
Abstract
:1. Introduction
2. Materials and Methods
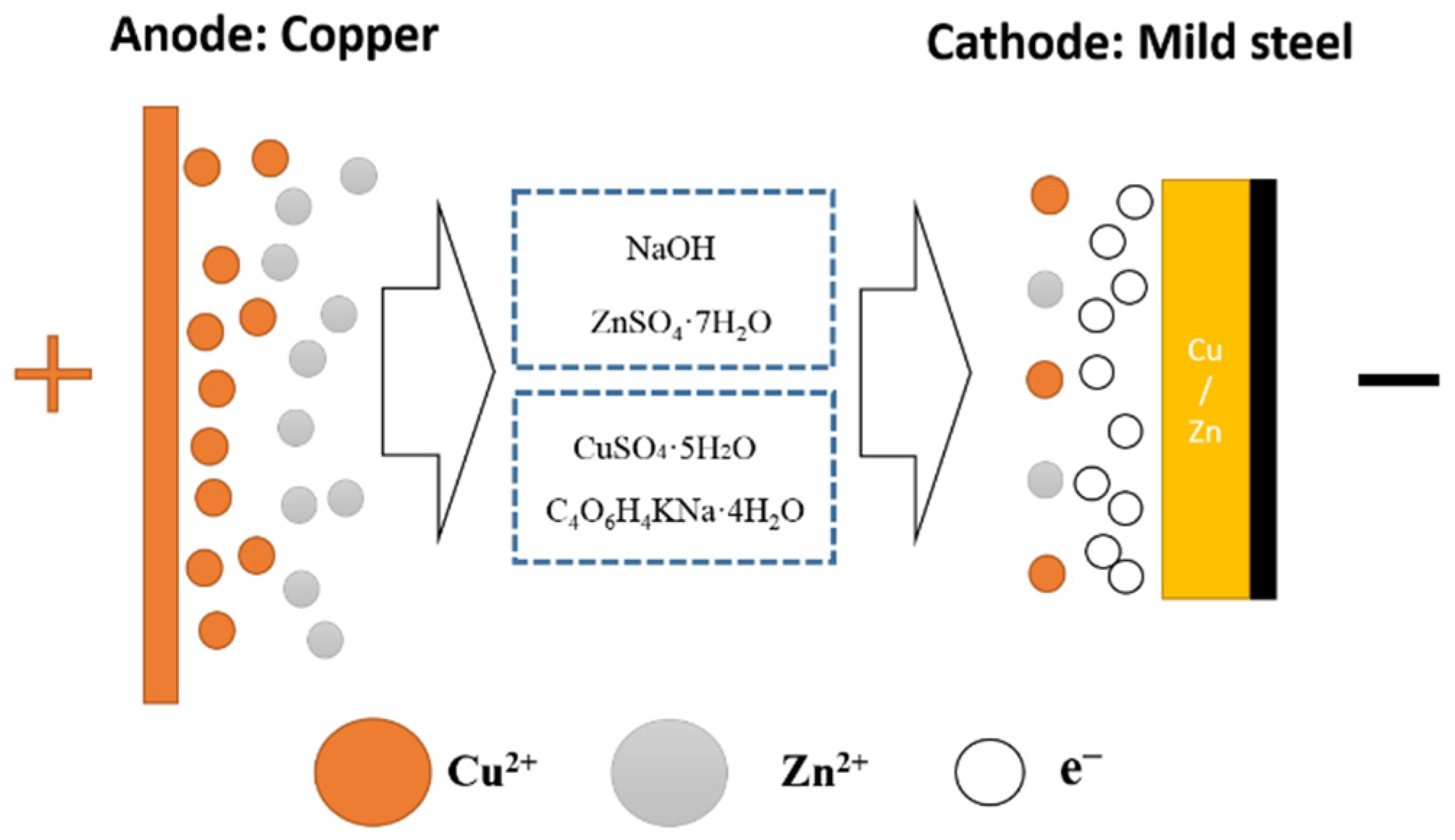
2.1. Materials
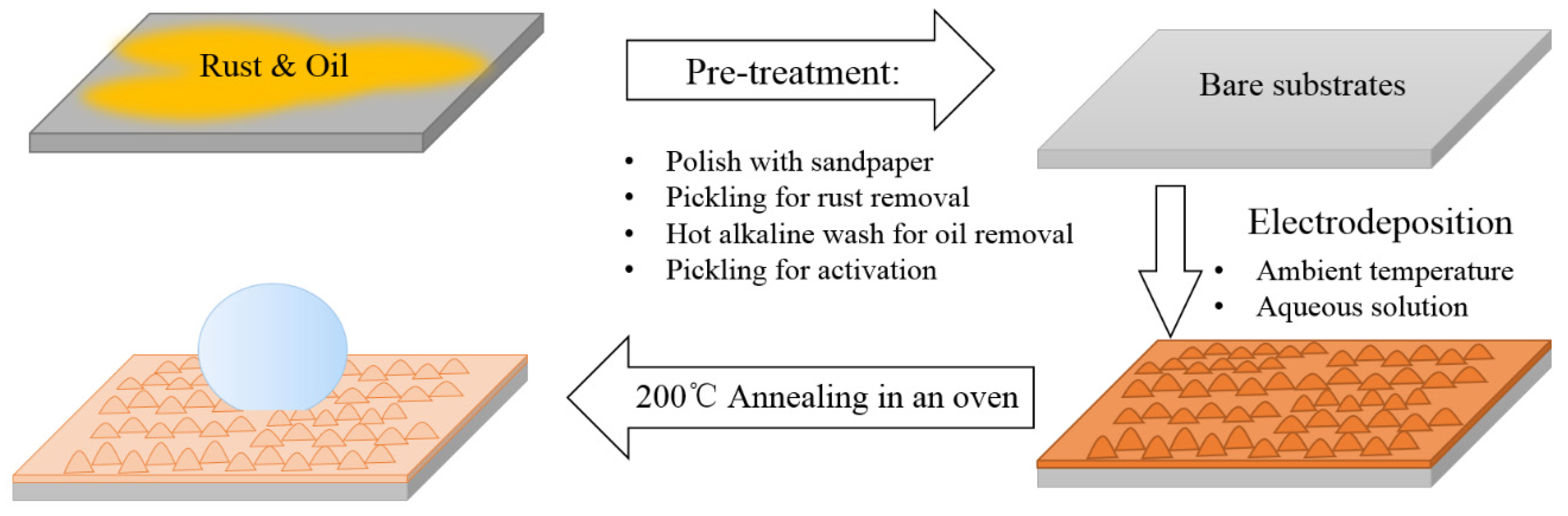
2.2. Preparation Process
2.3. Characterization
2.3.1. Morphological Characterization
2.3.2. Surface Composition Characterization
2.3.3. Wettability Characterization
2.3.4. Electrochemical Analysis
3. Results and Discussion
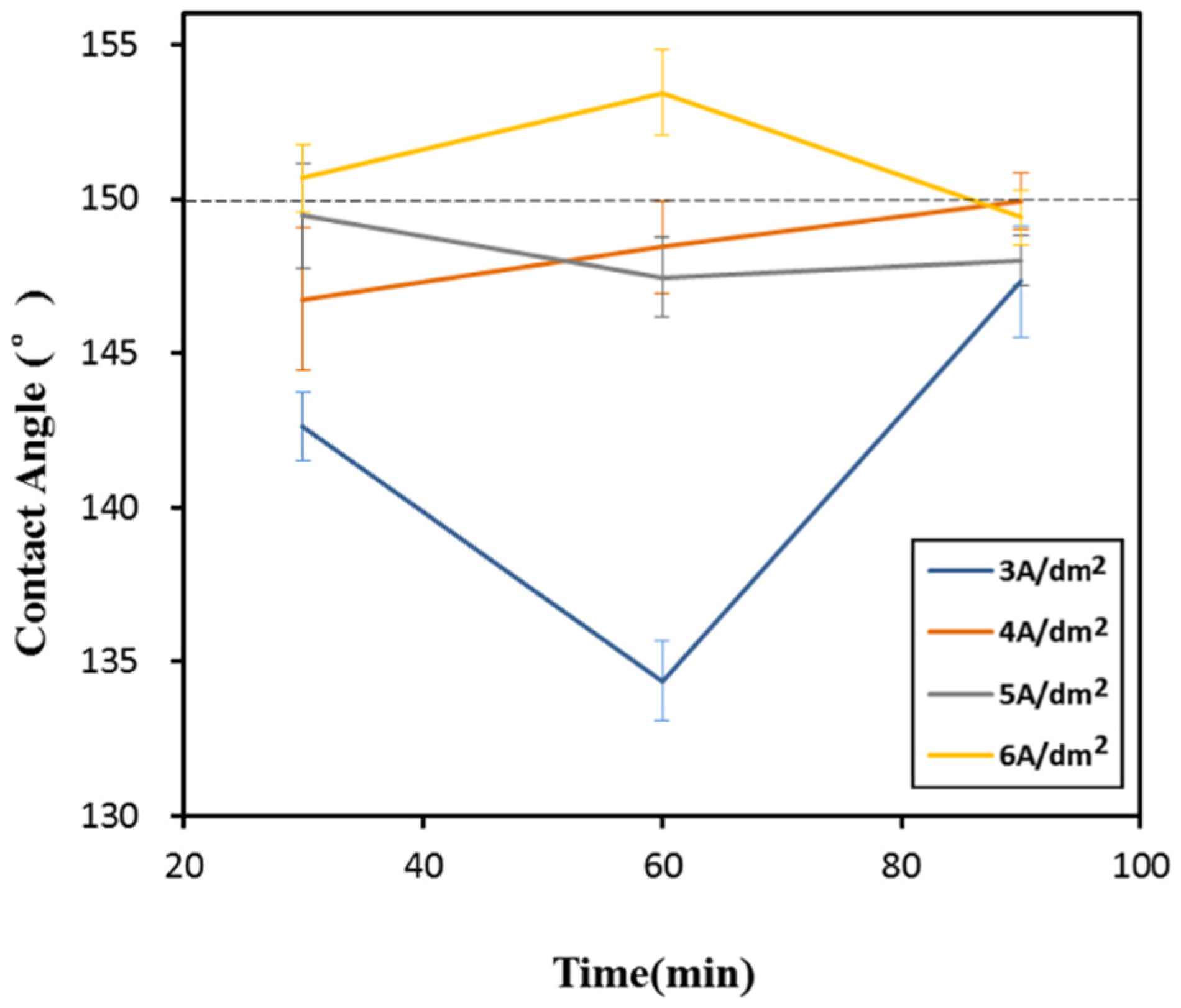
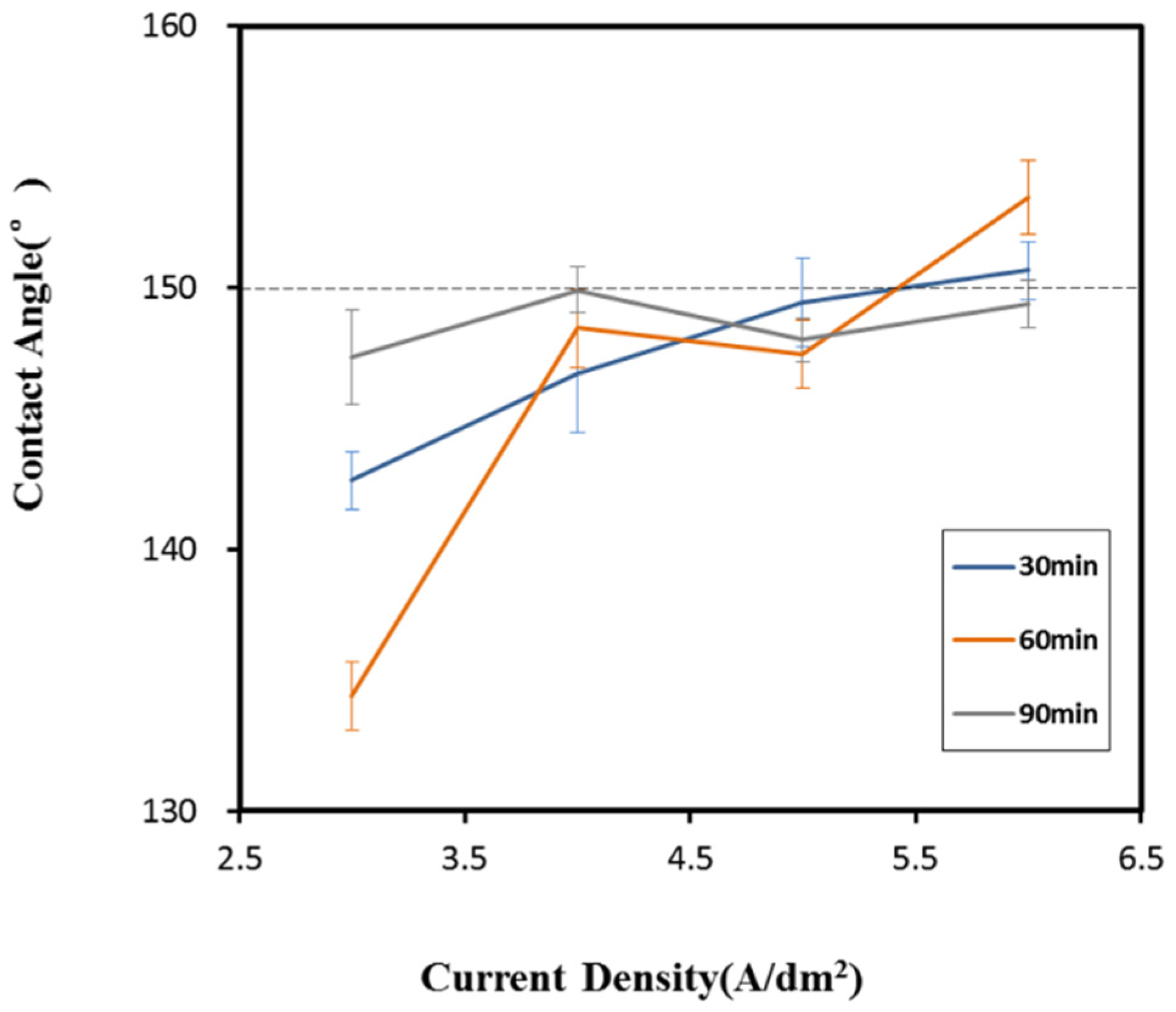
3.1. To Determine the Annealing Time Parameters
3.2. Morphology Analysis
3.3. Surface Wettability Analysis
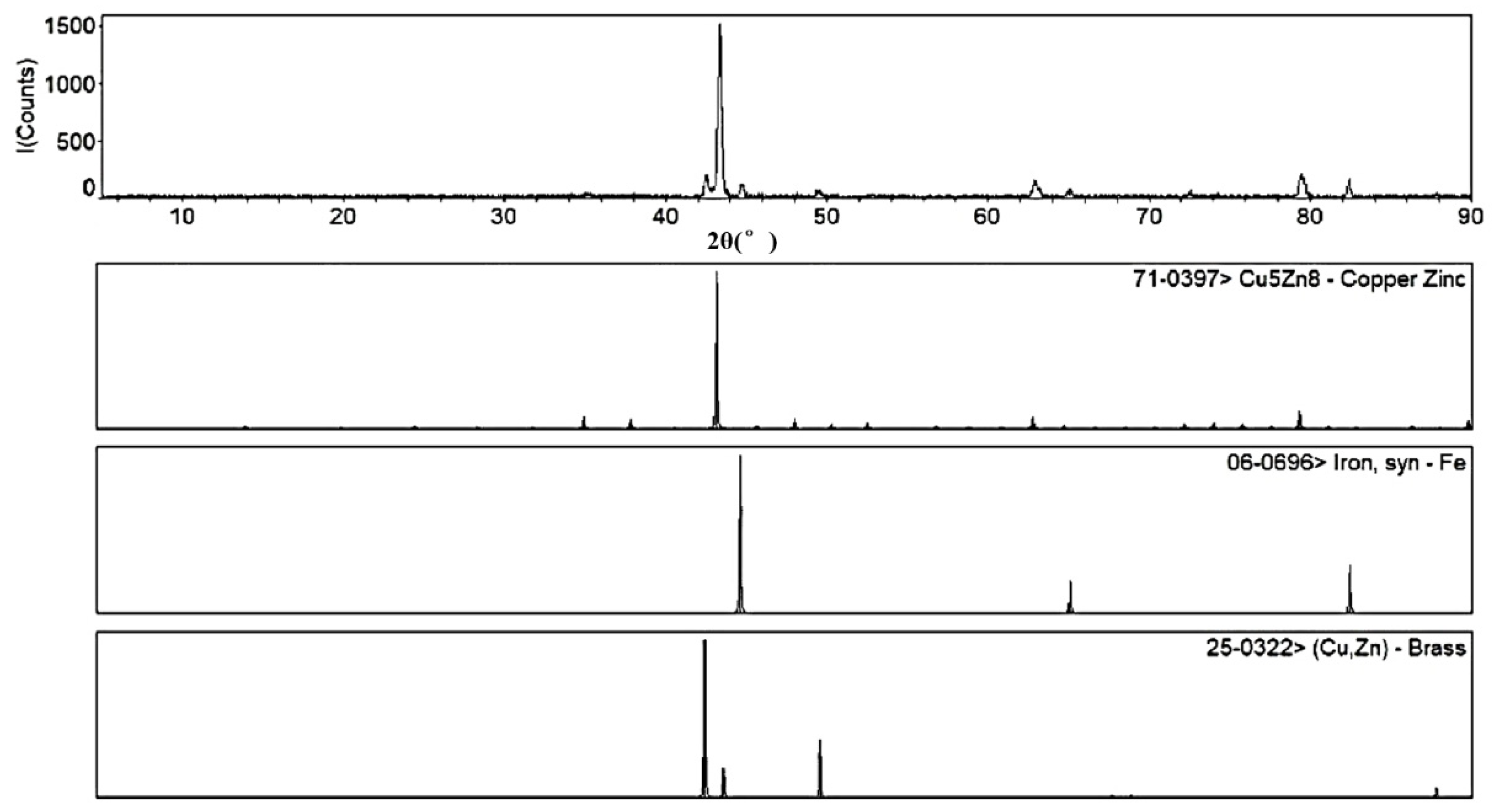
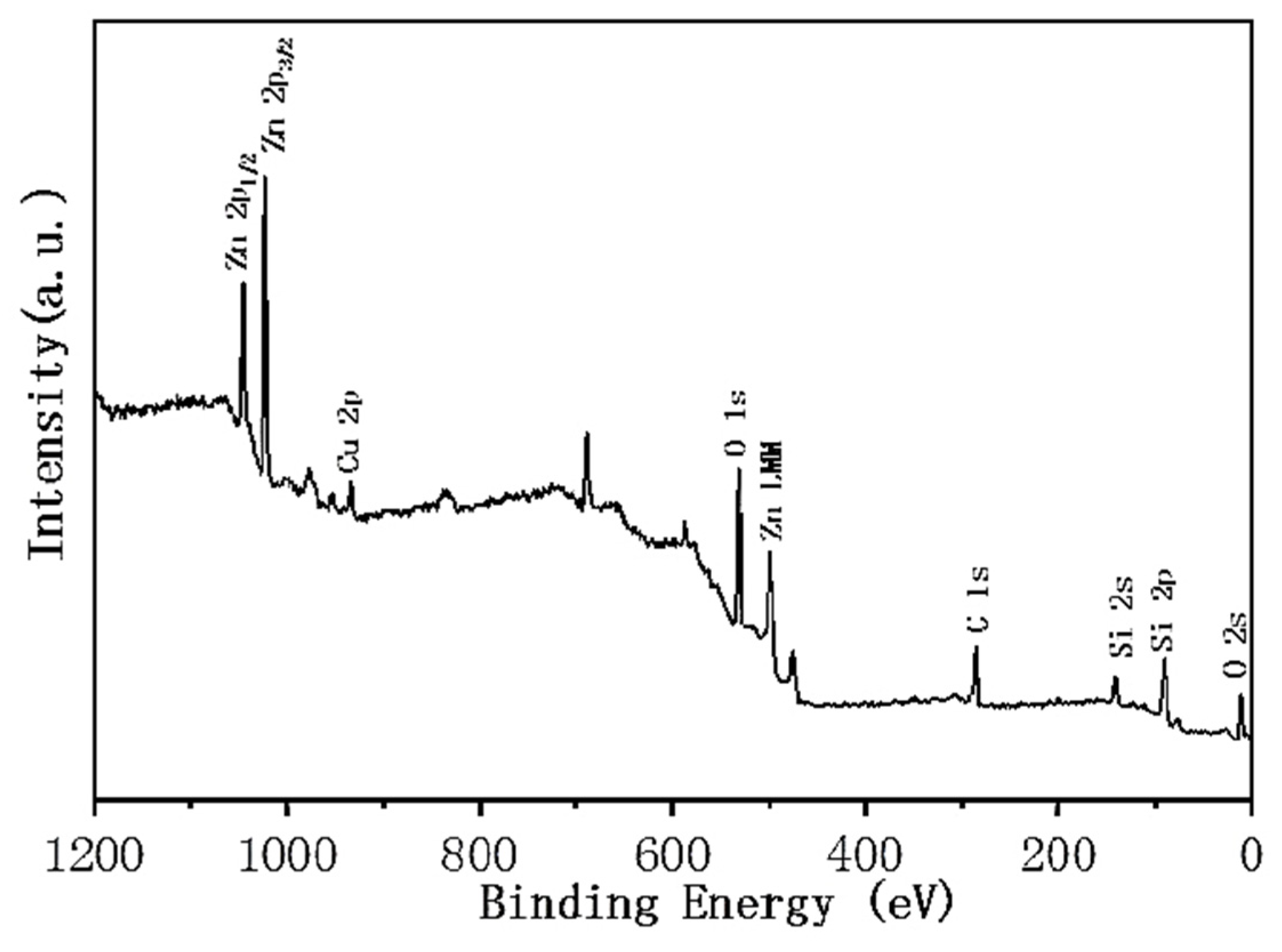
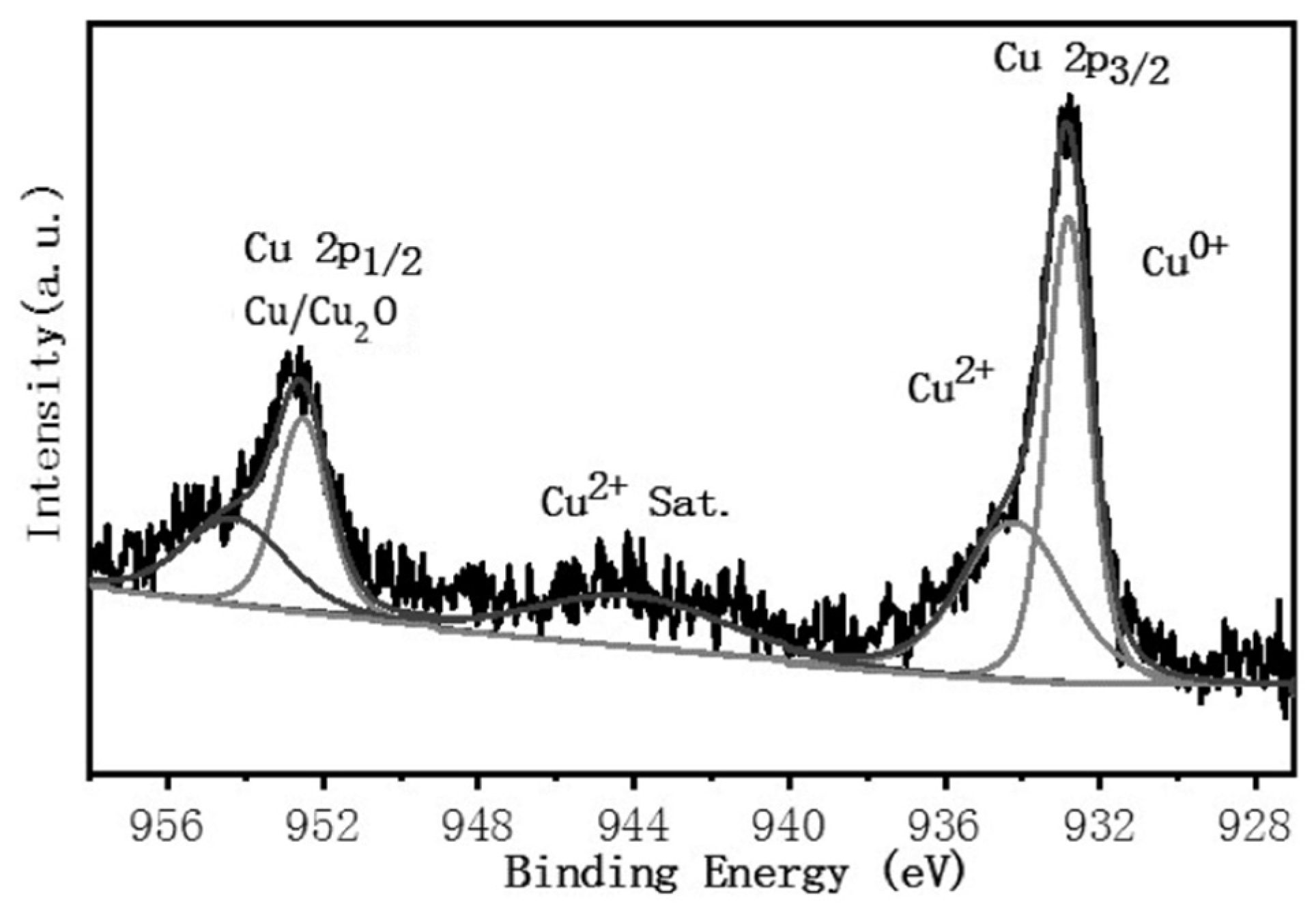
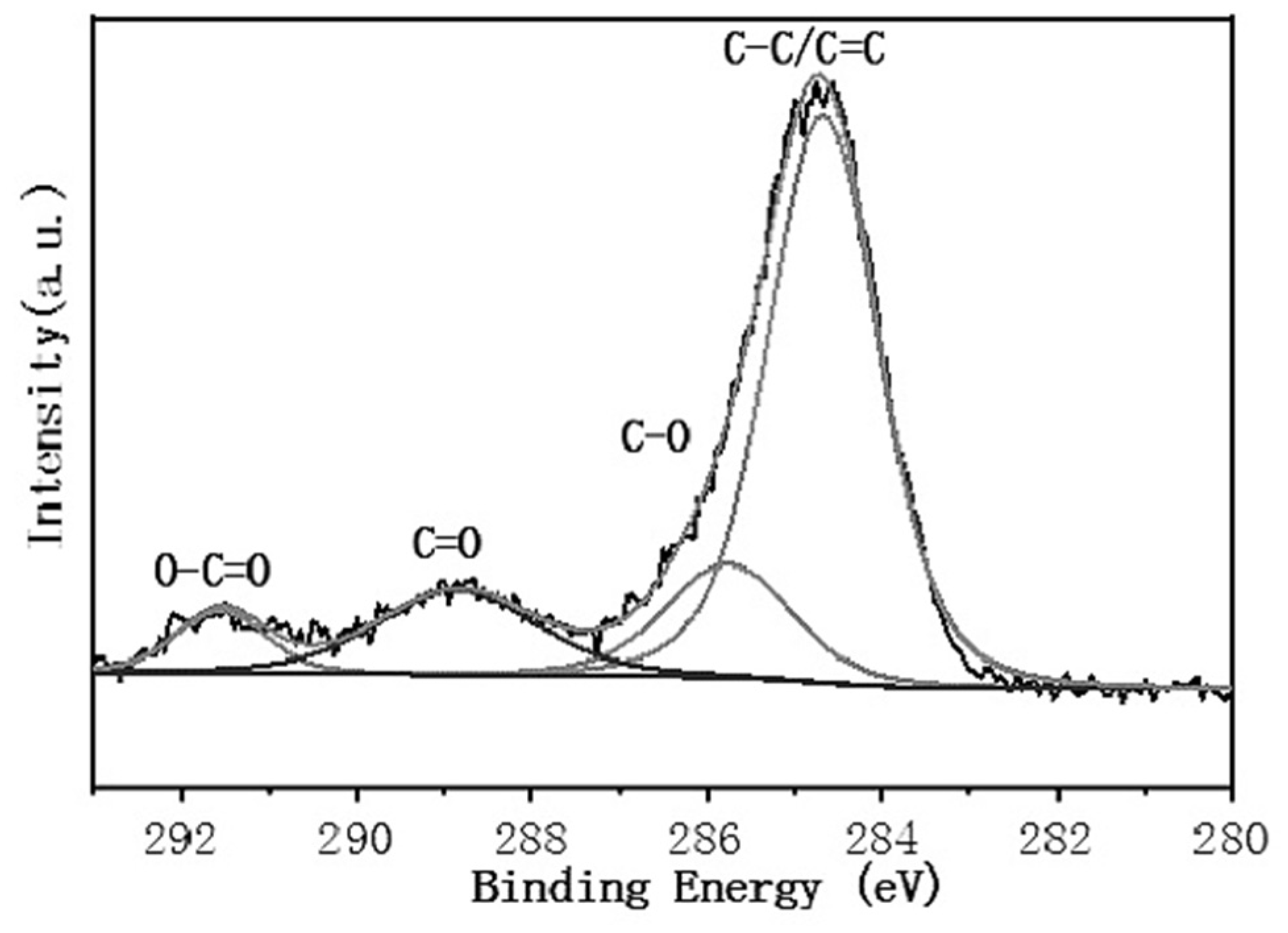
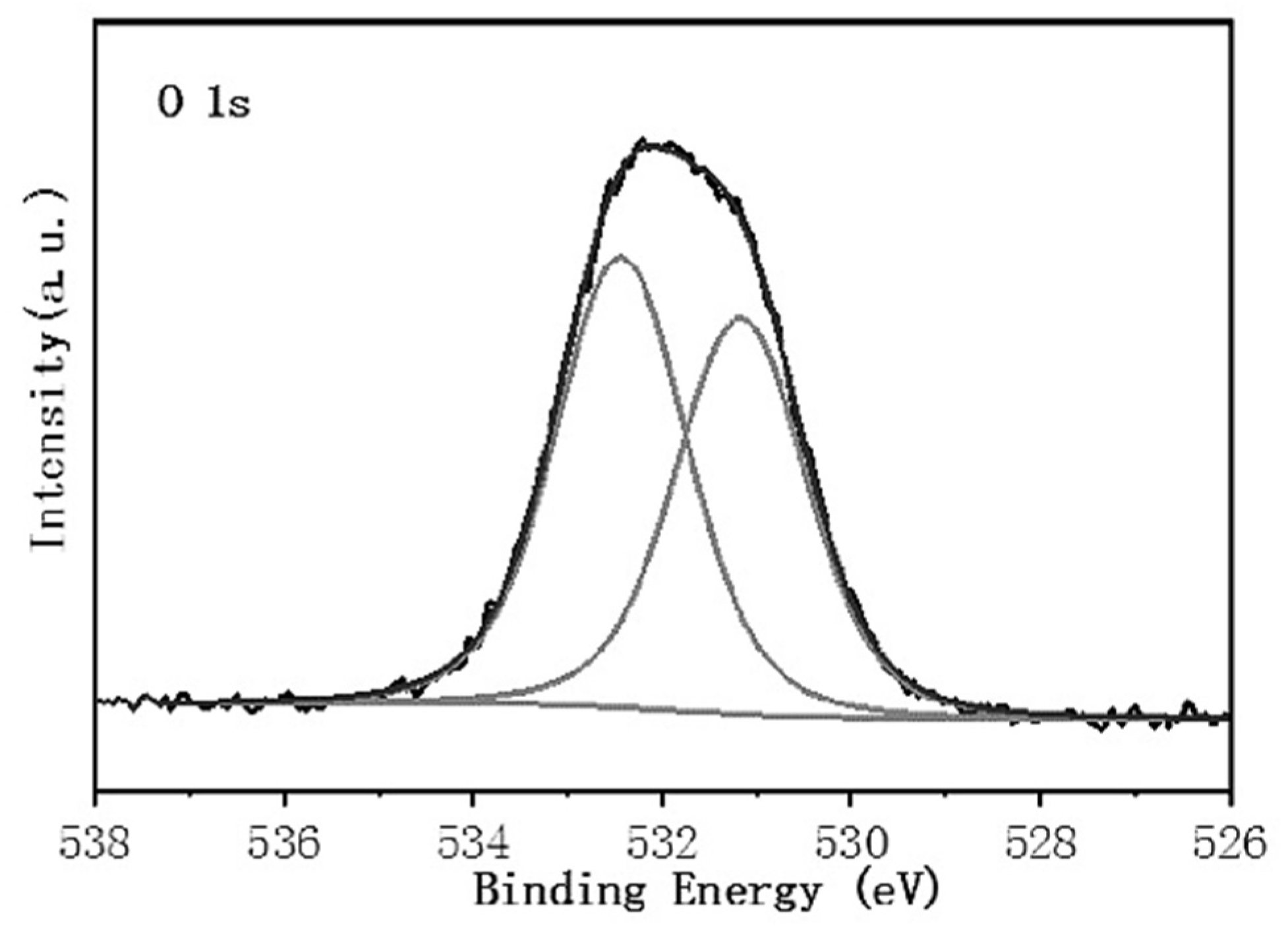
3.4. Superhydrophobic Surface Chemical Composition Analysis
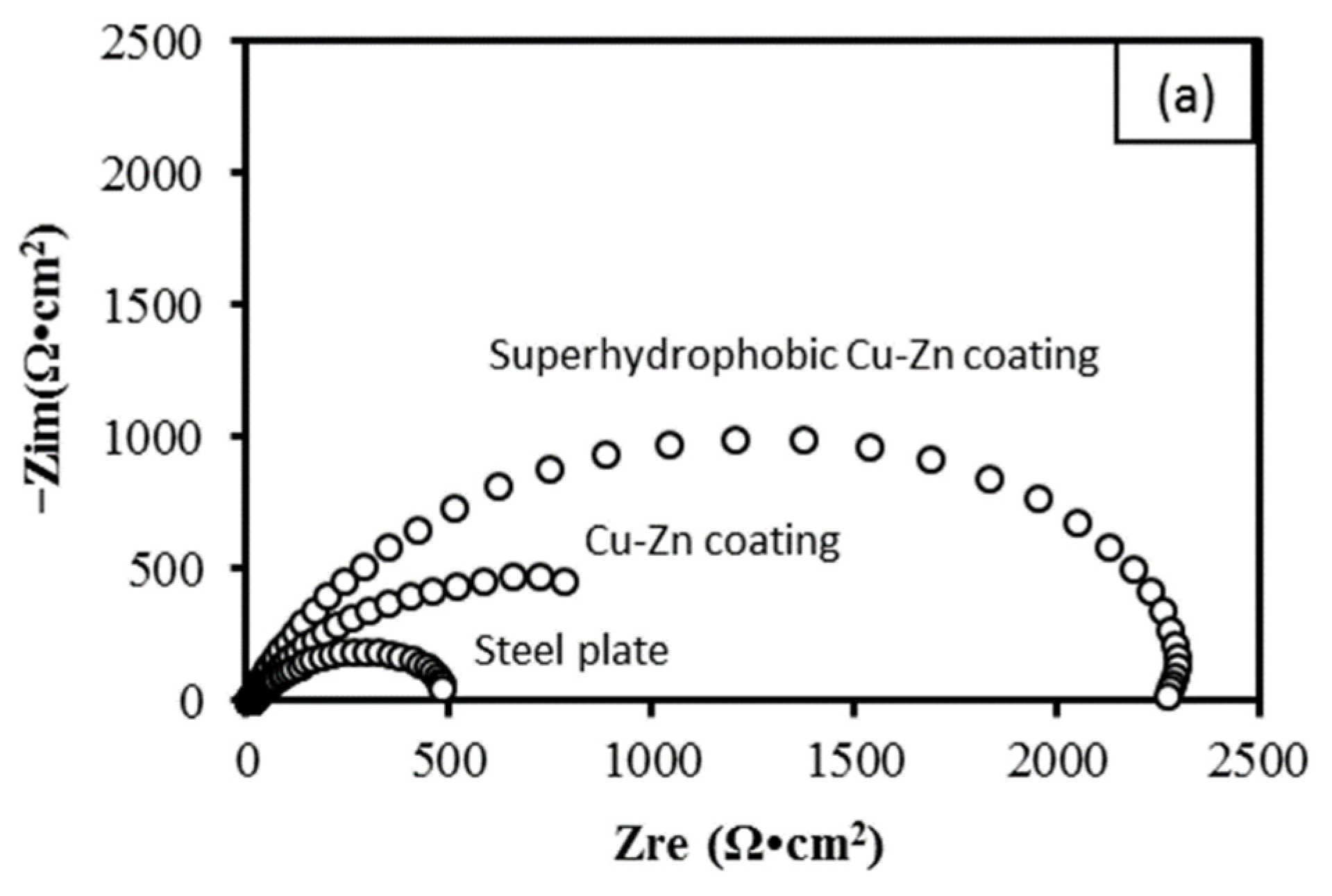
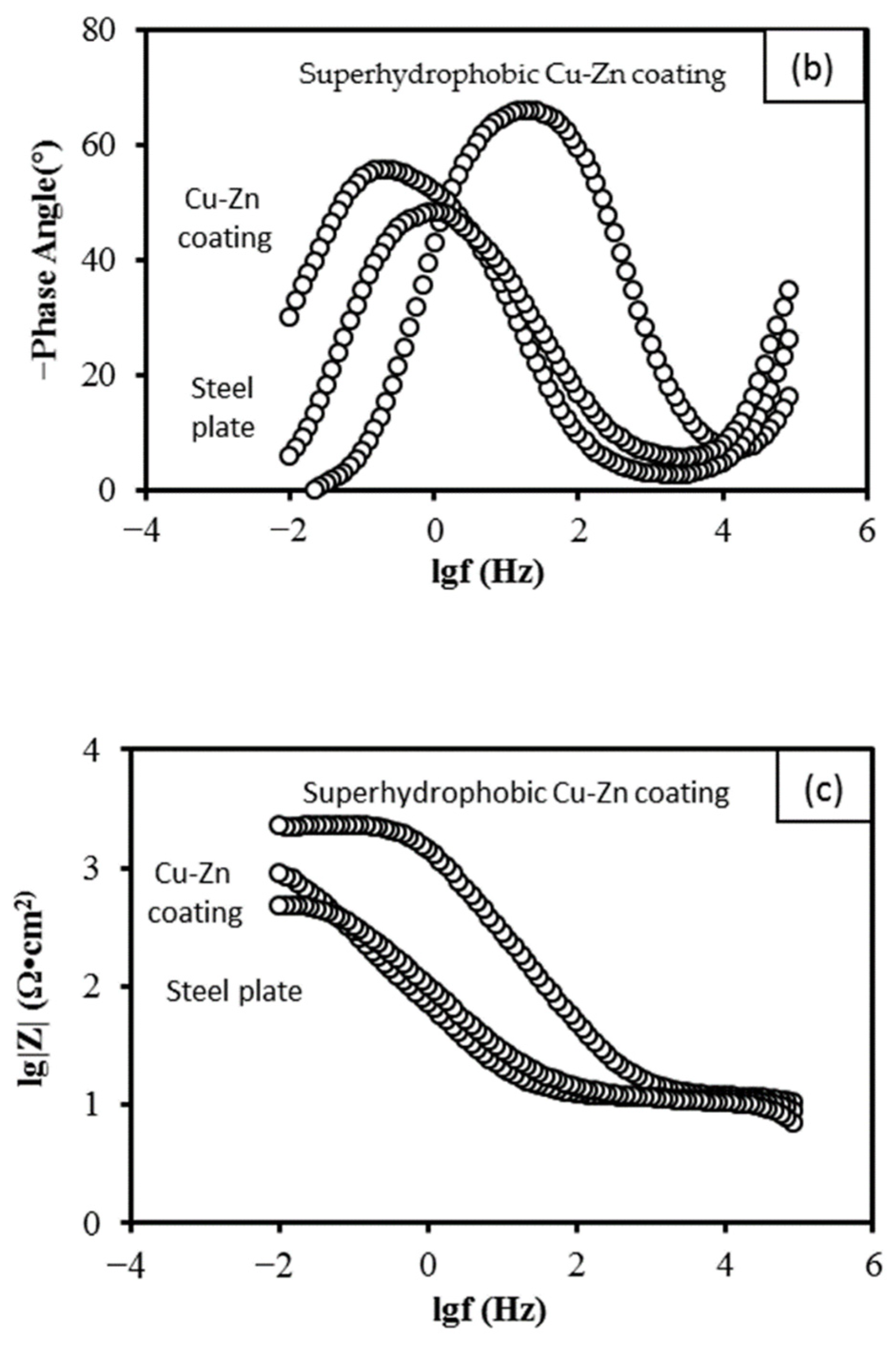
3.5. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Hou, L.; Du, H.; Wei, H.; Liu, B.; Wei, Y. A Study on the Interaction between Chloride Ions and CO2 towards Carbon Steel Corrosion. Corros. Sci. 2020, 167, 108531. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, Z.; Zhang, X.; Wu, S.; Wang, R.; Zhu, Y. A Novel Electrodeposition Route for Fabrication of the Superhydrophobic Surface with Unique Self-Cleaning, Mechanical Abrasion and Corrosion Resistance Properties. Chem. Eng. J. 2016, 303, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Oguzie, E.E.; Enenebeaku, C.K.; Akalezi, C.O.; Okoro, S.C.; Ayuk, A.A.; Ejike, E.N. Adsorption and Corrosion-Inhibiting Effect of Daayodis Edulis Extract on Low-Carbon-Steel Corrosion in Acidic Media. J. Colloid Interface Sci. 2010, 349, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Guo, L.; Yang, H.; Zhang, F.; El Bakri, Y. Synergistic Effect of Potassium Iodide and Sodium Dodecyl Sulfonate on the Corrosion Inhibition of Carbon Steel in HCl Medium: A Combined Experimental and Theoretical Investigation. RSC Adv. 2020, 10, 15163–15170. [Google Scholar] [CrossRef] [Green Version]
- Hayatdavoudi, H.; Rahsepar, M. A Mechanistic Study of the Enhanced Cathodic Protection Performance of Graphene-Reinforced Zinc Rich Nanocomposite Coating for Corrosion Protection of Carbon Steel Substrate. J. Alloys Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Huang, J.; Lou, C.; Xu, D.; Lu, X.; Xin, Z.; Zhou, C. Cardanol-Based Polybenzoxazine Superhydrophobic Coating with Improved Corrosion Resistance on Mild Steel. Prog. Org. Coat. 2019, 136, 105191. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Xu, W.; Yu, T.; Chen, Z.; Duan, J. Eco-Friendly Anticorrosion Superhydrophobic Al2O3@PDMS Coating with Salt Deliquescence Self-Coalescence Behaviors Under High Atmospheric Humidity. Front. Mater. 2022, 9, 839948. [Google Scholar] [CrossRef]
- Binyaseen, A.M.; Bayazeed, A.; Al-nami, S.Y.; Abu Al-Ola, K.; Alqarni, S.A.; Abdel-Hafez, S.H.; El-Metwaly, N.M. Development of Epoxy/Rice Straw-Based Cellulose Nanowhiskers Composite Smart Coating Immobilized with Rare-Earth Doped Aluminate: Photoluminescence and Anticorrosion Properties for Sustainability. Ceram. Int. 2022, 48, 4841–4850. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Snari, R.M.; Alkhamis, K.; Alatawi, N.M.; Alhasani, M.; Al-Nami, S.Y.; El-Metwaly, N.M. Development of Silica-Coated Rare-Earth Doped Strontium Aluminate toward Superhydrophobic, Anti-Corrosive and Long-Persistent Photoluminescent Epoxy Coating. Luminescence 2022, 37, 479–489. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Muellen, K.; Li, C.; Butt, H.-J.; Vollmer, D. Transparent, Thermally Stable and Mechanically Robust Superhydrophobic Surfaces Made from Porous Silica Capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Bioinspired Surfaces with Superamphiphobic Properties: Concepts, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1707415. [Google Scholar] [CrossRef]
- Cao, X.; Pan, J.; Cai, G.; Xiao, S.; Ma, X.; Zhang, X.; Dong, Z. A Chemically Robust and Self-Healing Superhydrophobic Polybenzoxazine Coating without Fluorocarbon Resin Modification: Fabrication and Failure Mechanism. Prog. Org. Coat. 2022, 163, 106630. [Google Scholar] [CrossRef]
- Lou, C.; Zhang, R.; Lu, X.; Zhou, C.; Xin, Z. Facile Fabrication of Epoxy/Polybenzoxazine Based Superhydrophobic Coating with Enhanced Corrosion Resistance and High Thermal Stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 8–15. [Google Scholar] [CrossRef]
- Vigdorovich, V.I.; Tsygankova, L.E.; Uryadnikova, M.N.; Emel’yanenko, K.A.; Chulkova, E.V.; Uryadnikov, A.A. Protective Effect of Superhydrophobic Coatings on Carbon Steel in Different Environments. Int. J. Corros. Scale Inhib. 2021, 10, 1157–1167. [Google Scholar] [CrossRef]
- Tsygankova, L.E.; Vigdorovich, V.; Uryadnikov, A.A.; Vigdorowitsch, M.V.; Shel, E.Y.; Uryadnikova, M.N. Protection of Carbon Steel with Superhydrophobic Coating against Corrosion in NaCl Solution. Surf. Interface Anal. 2020, 52, 855–858. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Hu, W.; Zheng, H.; Dan, Y.; Hu, J.; Chen, Z. Preparation of Superhydrophobic Nanoplate Iron Oxide Surface on a Carbon Steel for Anti-Wetting Applications. Mater. Des. 2021, 211, 110169. [Google Scholar] [CrossRef]
- Bandi, P.; Muralidhar, K.V.; Kausley, S.; Rai, B. Development of Superhydrophobic and Corrosion Resistant Coatings on Mild Steel-A Greener Approach. Mater. Today Commun. 2020, 25, 101625. [Google Scholar] [CrossRef]
- Du, C.; He, X.; Tian, F.; Bai, X.; Yuan, C. Preparation of Superhydrophobic Steel Surfaces with Chemical Stability and Corrosion. Coatings 2019, 9, 398. [Google Scholar] [CrossRef] [Green Version]
- Leoni, G.B.; de Freitas, D.S.; Gomes, J.A.P.C.; Brasil, S.L.D.C. Evaluation of Structure, Heterogeneities, Thickness and Corrosion Protection of Electrodeposited Sol-Gel Superhydrophobic Coatings. Mater. Res. 2022, 25, e20210460. [Google Scholar] [CrossRef]
- El-Din, M.R.N.; Hashem, A.; Morsi, R.E.; Abd El-Azeim, A. New Superhydrophobic Nanocomposites as Anti-Corrosion Coating Films. Part I: Synthesis and Characterization of Poly (Styrene/Vinyl Acetate)-SiO2 Nanocomposites as a Water-Repelling Surface via Nanoemulsion Polymerization Technique. J. Mol. Liq. 2021, 322, 114885. [Google Scholar] [CrossRef]
- Leoni, G.B.; de Freitas, D.S.; Ponciano Gomes, J.A.C.; Brasil, S.L.D.C. Multivariable Analysis of Electrodeposited Silane Based Superhydrophobic Coatings for Corrosion Protection of Carbon Steel. J. Sol.-Gel. Sci. Technol. 2020, 94, 695–707. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Y.; Ban, C.; Xue, Y.; Taleb, A.; Jin, Y. Fabrication of Robust Tungsten Carbide Particles Reinforced Co-Ni Superhydrophobic Composite Coating by Electrochemical Deposition. Surf. Coat. Technol. 2020, 385, 125390. [Google Scholar] [CrossRef]
- Luo, S.; Zheng, L.; Luo, H.; Luo, C. A Ceramic Coating on Carbon Steel and Its Superhydrophobicity. Appl. Surf. Sci. 2019, 486, 371–375. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Maurya, R.; Katiyar, P.K.; Balani, K. Superhydrophobic, Self-Cleaning Carbon Nanofiber CVD Coating for Corrosion Protection of AISI 1020 Steel and AZ31 Magnesium Alloys. Surf. Coat. Technol. 2020, 404, 126421. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Li, X.; Yin, H.; Tang, J.; Hu, C.; Ma, B.; Li, T. An Effective Approach to Fabricate the Corrosion Resistance of Superhydrophobic ZnO/Ni Composite Coating on Carbon Steel Substrate. J. Adhes. Sci. Technol. 2021, 1–18. [Google Scholar] [CrossRef]
- Hu, C.; Xie, X.; Zheng, H.; Qing, Y.; Ren, K. Facile Fabrication of Superhydrophobic Zinc Coatings with Corrosion Resistance via an Electrodeposition Process. New J. Chem. 2020, 44, 8890–8901. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Zhao, G.; Taleb, A.; Jin, Y. Fabrication of Ni-Co Coating by Electrochemical Deposition with High Super-Hydrophobic Properties for Corrosion Protection. Surf. Coat. Technol. 2019, 363, 352–361. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Bi, P.; Zhao, G.; Jin, Y. Super-Hydrophobic Co-Ni Coating with High Abrasion Resistance Prepared by Electrodeposition. Coatings 2019, 9, 232. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Xie, X.; Ren, K. A Facile Method to Prepare Stearic Acid-TiO2/Zinc Composite Coating with Multipronged Robustness, Self-Cleaning Property, and Corrosion Resistance. J. Alloys Compd. 2021, 882, 160636. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, M.; Zhao, Y.; Li, J.; Wang, H.; Zhang, X.; Yan, H. High Corrosion Protection Performance of a Novel Nonfluorinated Biomimetic Superhydrophobic Zn-Fe Coating with Echinopsis Multiplex-like Structure. ACS Appl. Mater. Interfaces 2019, 11, 38205–38217. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Vanithakumari, S.C.; George, R.P.; Philip, J. Template-Free One-Step Electrodeposition Method for Fabrication of Robust Superhydrophobic Coating on Ferritic Steel with Self-Cleaning Ability and Superior Corrosion Resistance. Langmuir 2019, 35, 12665–12679. [Google Scholar] [CrossRef]
- Chen, C.; Fan, Y.; He, Y.; Chen, X.; Yang, Q. Fabrication of Superhydrophobic Zirconium Surface with a Facile Electrodeposition Process. Surf. Innov. 2018, 6, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Heidari, G.; Hosseini, S. Electrochemical Fabrication of Superhydrophobic and Superoleophilic Coating: Applications in Corrosion-Resistant Surfaces and Oil Cleanup. Bull. Mater. Sci. 2021, 44, 253. [Google Scholar] [CrossRef]
- Prado, L.H.; Virtanen, S. Cu-MoS2 Superhydrophobic Coating by Composite Electrodeposition. Coatings 2020, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, T.-Y. Oxygen Adsorption Induced Superhydrophilic-to-Superhydrophobic Transition on Hierarchical Nanostructured CuO Surface. J. Colloid Interface Sci. 2012, 377, 438–441. [Google Scholar] [CrossRef]
- Liu, P.; Cao, L.; Zhao, W.; Xia, Y.; Huang, W.; Li, Z. Insights into the Superhydrophobicity of Metallic Surfaces Prepared by Electrodeposition Involving Spontaneous Adsorption of Airborne Hydrocarbons. Appl. Surf. Sci. 2015, 324, 576–583. [Google Scholar] [CrossRef]
- Li, H.; Yu, S. Three-Level Hierarchical Superhydrophobic Cu–Zn Coating on a Steel Substrate without Chemical Modification for Self-Cleaning Property. New J. Chem. 2017, 41, 5436–5444. [Google Scholar] [CrossRef]
- Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Superhydrophobic Surfaces by Electrochemical Processes. Adv. Mater. 2013, 25, 1378–1394. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Effect of ZnO Pore-Sealing Layer on Anti-Corrosion and in-Vitro Bioactivity Behavior of Plasma Electrolytic Oxidized AZ91 Magnesium Alloy. Mater. Lett. 2020, 258, 126779. [Google Scholar] [CrossRef]
- Watanabe, T. Nano-Plating: Microstructure Control Theory of Plated Film and Data Base of Plated Film Microstructure; Elsevier Science: Amsterdam, The Netherlands, 2004; ISBN 978-0-08-044375-1. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Jamali, R.; Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M.; Kolahi, A. Effects of Co-Incorporated Ternary Elements on Biocorrosion Stability, Antibacterial Efficacy, and Cytotoxicity of Plasma Electrolytic Oxidized Titanium for Implant Dentistry. Mater. Chem. Phys. 2022, 276, 125436. [Google Scholar] [CrossRef]




| Item | Parameter | Unit |
|---|---|---|
| Anode (Copper) | 60 × 80 × 5 | mm |
| Cathode (Mild steel) | 40 × 60 × 1 | mm |
| Current density | 3, 4, 5, 6 | A/dm2 |
| Time | 30, 60, 90 | min |
| Power supply mode | Constant current | |
| Plating temperature | Ambient temperature | |
| Annealing temperature | 200 | °C |
| Elements | Weight | Atom |
|---|---|---|
| % | % | |
| C K | 11.78 | 40.32 |
| O K | 1.56 | 4.02 |
| Fe K | 2.57 | 1.89 |
| Cu K | 48.10 | 31.13 |
| Zn K | 36.00 | 22.64 |
| Total | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Liu, Y.; Wang, J.; Li, R.; Liu, X.; Ai, S. Facile and Eco-Friendly Preparation of Mild Steel Based Superhydrophobic Surfaces without Chemical Modifications. Coatings 2022, 12, 737. https://doi.org/10.3390/coatings12060737
Jiang W, Liu Y, Wang J, Li R, Liu X, Ai S. Facile and Eco-Friendly Preparation of Mild Steel Based Superhydrophobic Surfaces without Chemical Modifications. Coatings. 2022; 12(6):737. https://doi.org/10.3390/coatings12060737
Chicago/Turabian StyleJiang, Wenxuan, Yujun Liu, Ji Wang, Rui Li, Xiao Liu, and Shaohua Ai. 2022. "Facile and Eco-Friendly Preparation of Mild Steel Based Superhydrophobic Surfaces without Chemical Modifications" Coatings 12, no. 6: 737. https://doi.org/10.3390/coatings12060737
APA StyleJiang, W., Liu, Y., Wang, J., Li, R., Liu, X., & Ai, S. (2022). Facile and Eco-Friendly Preparation of Mild Steel Based Superhydrophobic Surfaces without Chemical Modifications. Coatings, 12(6), 737. https://doi.org/10.3390/coatings12060737





