Effects of the Deposition Mode and Heat Treatment on the Microstructure and Wettability of Y2O3 Coatings Prepared by Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
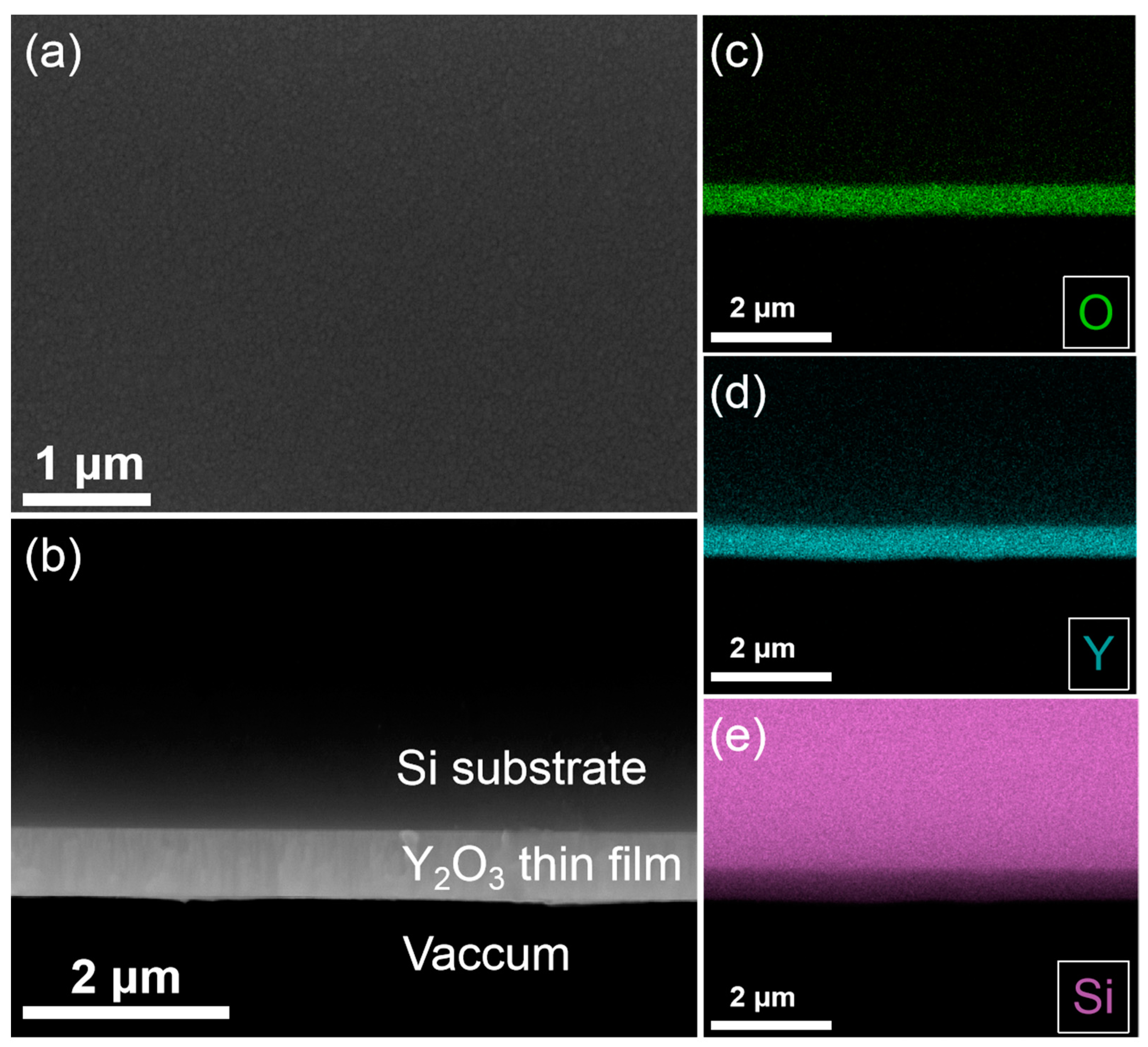
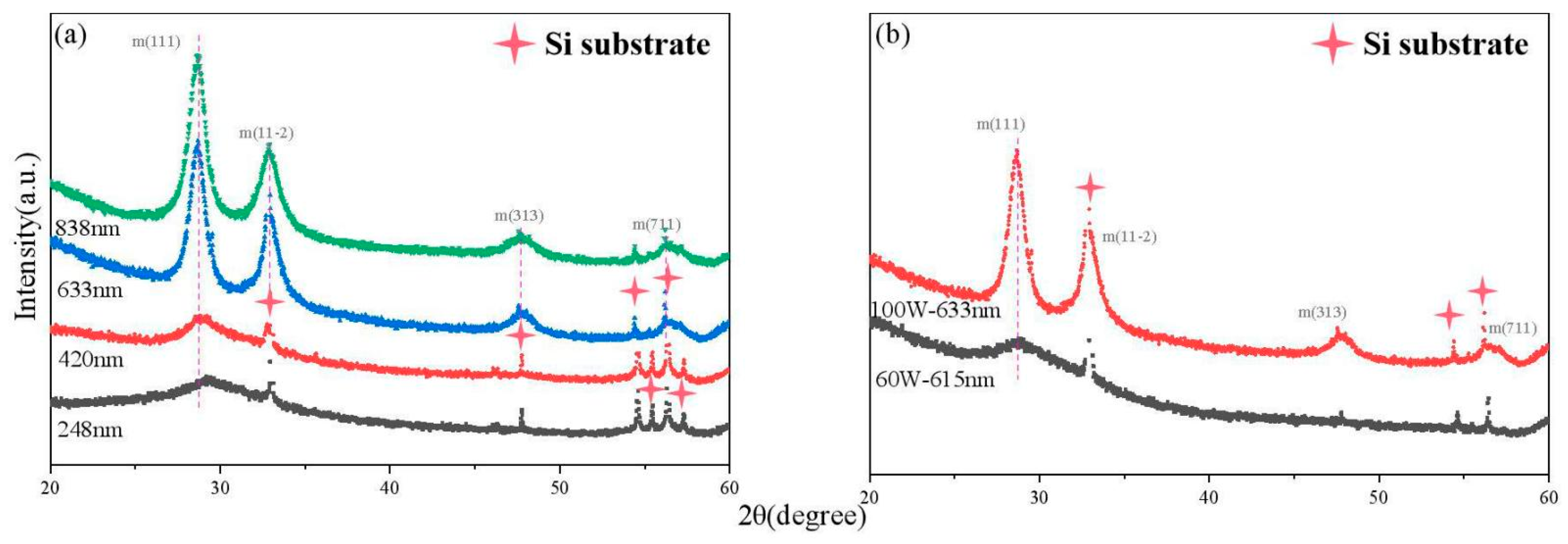
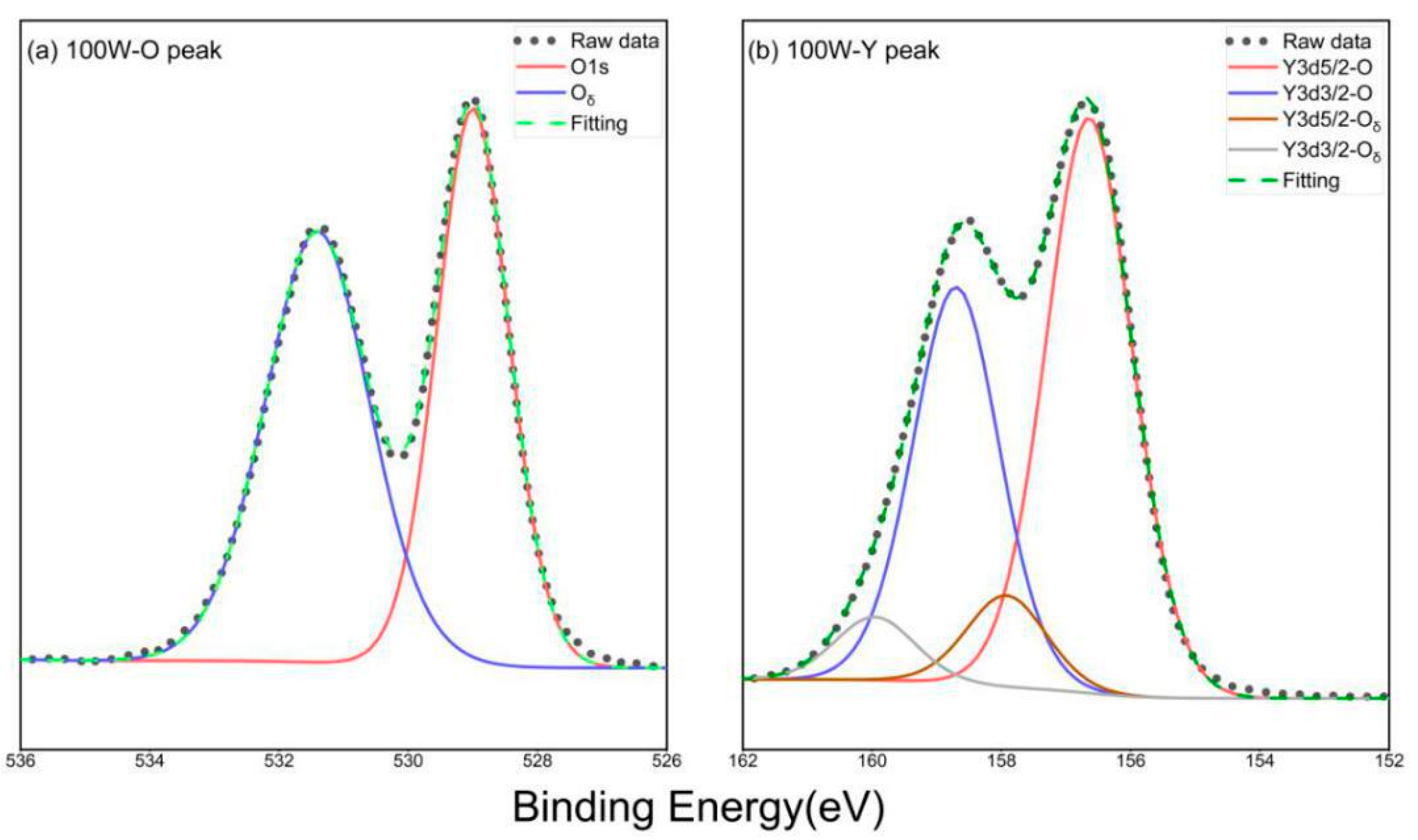
3.1. The Crystalline Structure and Chemical Bonding of the Y2O3 Films
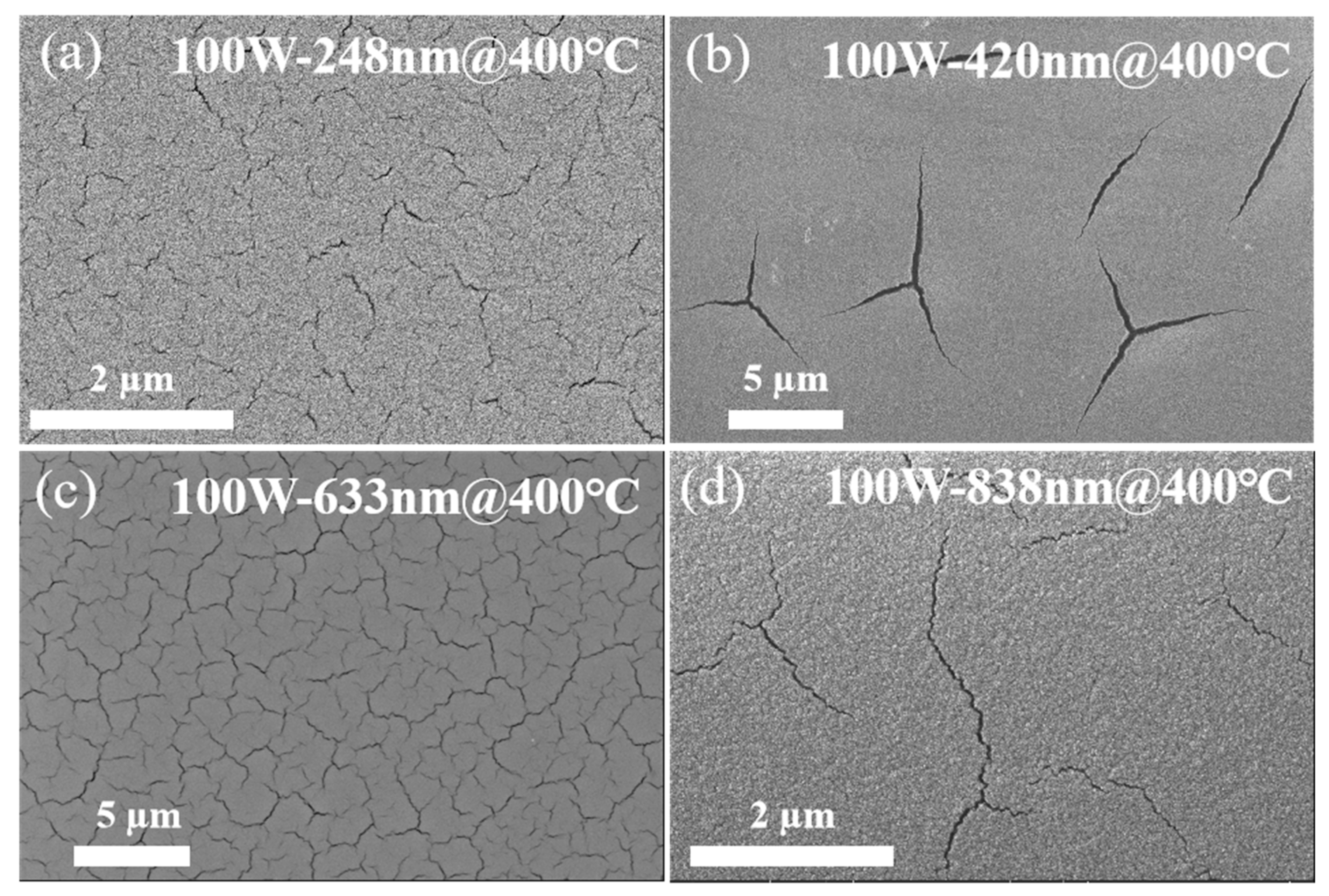
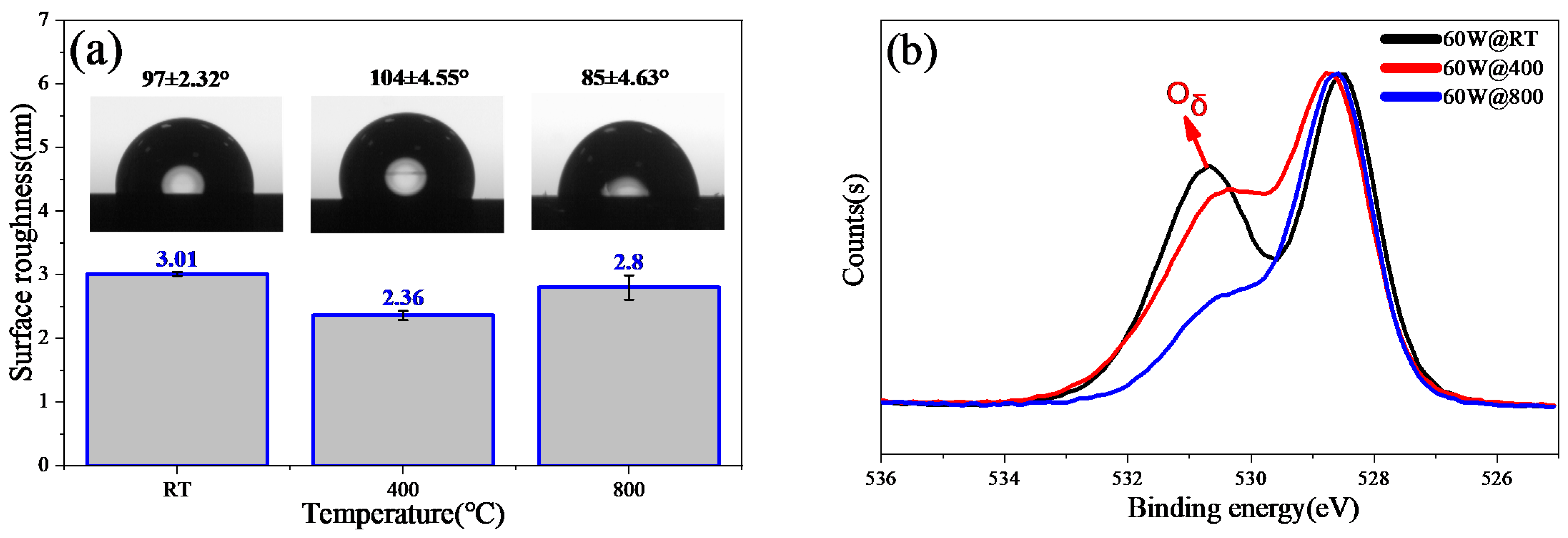
3.2. Post-Annealing Treatment of the Film
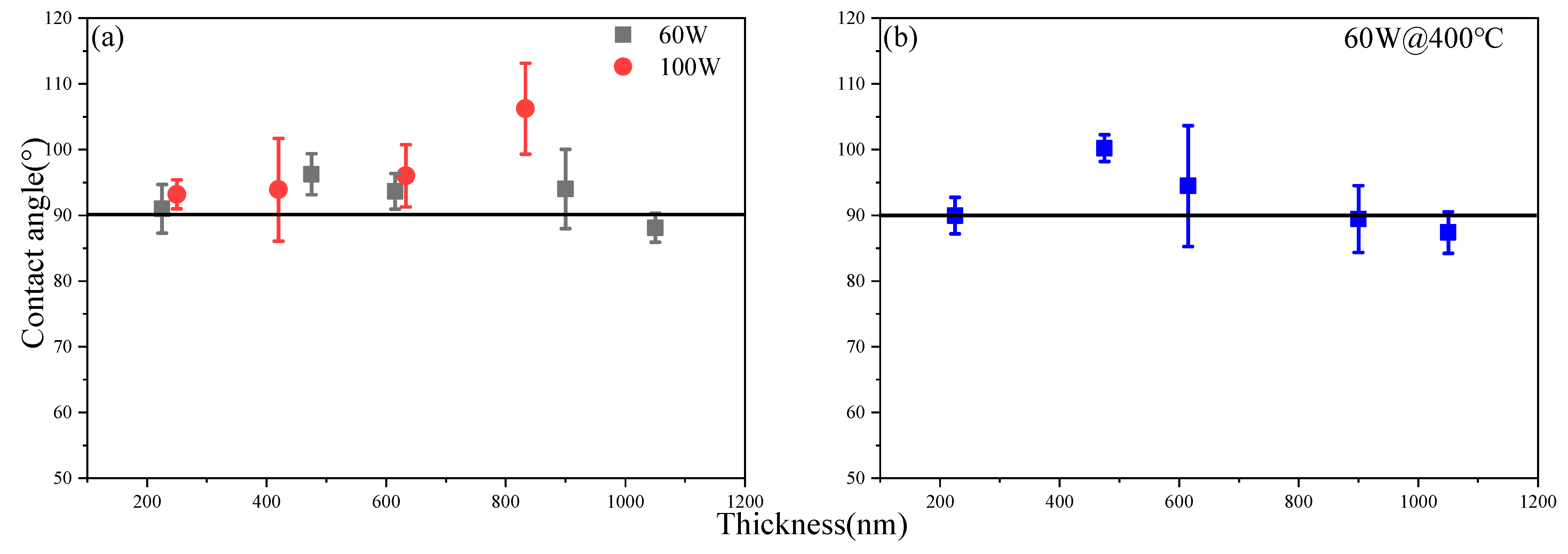
3.3. The Wetting Properties of the Y2O3 Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, P.; Zhang, K.; Huang, H.; Wen, M.; Li, Q.; Zhang, W.; Hu, C.; Zheng, W. Oxygen vacancies dependent phase transition of Y2O3 films. Appl. Surf. Sci. 2017, 410, 470–478. [Google Scholar] [CrossRef]
- Hua, C.; Li, C.; Guo, J.; Yan, X.; Liu, J.; Chen, L.; Wei, J.; Hei, L. Optical properties of cubic and monoclinic Y2O3 films prepared through radio frequency magnetron sputtering. Surf. Coat. Technol. 2017, 320, 279–283. [Google Scholar] [CrossRef]
- Hua, C.; Chen, L.; Li, C.; Wang, M.; Guo, J.; Liu, J.; Yan, X.; Zhao, Y.; An, K.; Wei, J.; et al. Effects of oxygen-to-argon ratio on crystalline structure and properties of Y2O3 anti-reflection films for freestanding CVD diamond. J. Alloy. Compd. 2017, 693, 468–473. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, J.H. Structure and Photoluminescence of Optically Transparent Y2O3:Eu Thin Films Prepared on Sapphire Substrates by RF Magnetron Sputtering. Appl. Sci. Converg. Technol. 2021, 30, 34–37. [Google Scholar] [CrossRef]
- Lei, P.; Chen, X.; Yan, Y.; Zhu, J. The tunable dielectric properties of sputtered yttrium oxide films. Appl. Phys. A 2021, 127, 99. [Google Scholar] [CrossRef]
- Lizarraga-Medina, E.G.; Caballero-Espitia, D.L.; Jurado-Gonzalez, J.; López, J.; Marquez, H.; Contreras-López, O.E.; Tiznado, H. Al2O3-Y2O3 nanolaminated slab optical waveguides by atomic layer deposition. Opt. Mater. 2020, 103, 109822. [Google Scholar] [CrossRef]
- Quah, H.J.; Cheong, K.Y. Effects of post-deposition annealing ambient on Y2O3 gate deposited on silicon by RF magnetron sputtering. J. Alloy. Compd. 2012, 529, 73–83. [Google Scholar] [CrossRef]
- Chopade, S.S.; Barve, S.A.; Thulasi Raman, K.H.; Chand, N.; Deo, M.N.; Biswas, A.; Rai, S.; Lodha, G.S.; Rao, G.M.; Patil, D.S. RF plasma MOCVD of Y2O3 thin films: Effect of RF self-bias on the substrates during deposition. Appl. Surf. Sci. 2013, 285, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Lei, P.; Dai, B.; Zhu, J.; Chen, X.; Liu, G.; Zhu, Y.; Han, J. Controllable phase formation and physical properties of yttrium oxide films governed by substrate heating and bias voltage. Ceram. Int. 2015, 41, 8921–8930. [Google Scholar] [CrossRef]
- Gischkat, T.; Döbeli, M.; Bächli, A.; Botha, R.; Balogh-Michels, Z. Influence of Ar-impurities on the wettability of IBS-deposited Y2O3 thin films. Appl. Surf. Sci. 2021, 568, 150880. [Google Scholar] [CrossRef]
- Zhao, B.; Mattelaer, F.; Rampelberg, G.; Dendooven, J.; Detavernier, C. Thermal and Plasma-Enhanced Atomic Layer Deposition of Yttrium Oxide Films and the Properties of Water Wettability. ACS Appl. Mater. Interfaces 2020, 12, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-K.; Kim, K.; Lee, Z.; Ko, K.Y.; Lee, C.-W.; Lee, S.J.; Myung, J.M.; Lansalot-Matras, C.; Noh, W.; Dussarrat, C.; et al. Hydrophobicity of Rare Earth Oxides Grown by Atomic Layer Deposition. Chem. Mater. 2015, 27, 148–156. [Google Scholar] [CrossRef]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of rare-earth oxide ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Barshilia, H.C.; Chaudhary, A.; Kumar, P.; Manikandanath, N.T. Wettability of Y2O3: A Relative Analysis of Thermally Oxidized, Reactively Sputtered and Template Assisted Nanostructured Coatings. Nanomaterials 2012, 2, 65. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Liu, J.; Chen, L.; Wei, J.; Li, C. Research Progress of Rare Earth Infrared Anti-reflection Films. Chin. J. Rare Met. 2019, 43, 1346–1356. [Google Scholar]
- Ellmer, K.; Welzel, T. Reactive magnetron sputtering of transparent conductive oxide thin films: Role of energetic particle (ion) bombardment. J. Mater. Res. 2012, 27, 765–779. [Google Scholar] [CrossRef]
- Mao, Y.; Engels, J.; Houben, A.; Rasinski, M.; Steffens, J.; Terra, A.; Linsmeier, C.; Coenen, J.W. The influence of annealing on yttrium oxide thin film deposited by reactive magnetron sputtering: Process and microstructure. Nucl. Mater. Energy 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Lei, P.; Zhu, J.; Zhu, Y.; Jiang, C.; Yin, X. Yttrium oxide thin films prepared under different oxygen-content atmospheres: Microstructure and optical properties. Appl. Phys. A 2012, 108, 621–628. [Google Scholar] [CrossRef]
- Endoh, R.; Nakamura, K.; Fujita, H.; Matsunaga, M.; Kimura, K.; Riesch, J.; Hishinuma, Y.; Chikada, T. Deuterium permeation behavior through yttria-stabilized zirconia coating fabricated by magnetron sputtering. Fusion Eng. Des. 2020, 157, 111769. [Google Scholar] [CrossRef]
- Xia, J.; Liang, W.; Miao, Q.; Depla, D. On the influence of local oxygen addition on the growth of sputter deposited yttrium oxide thin films. Surf. Coat. Technol. 2019, 357, 768–773. [Google Scholar] [CrossRef]
- Lei, P.; Zhu, J.; Zhu, Y.; Jiang, C.; Yin, X. Evolution of composition, microstructure and optical properties of yttrium oxide thin films with substrate temperature. Surf. Coat. Technol. 2013, 229, 226–230. [Google Scholar] [CrossRef]
- Xu, S.; Yao, Z.; Zhou, J.; Du, M. Effects of post-deposition annealing on the chemical composition, microstructure, optical and mechanical properties of Y2O3 film. Surf. Coat. Technol. 2018, 344, 636–643. [Google Scholar] [CrossRef]
- Abubakar, S.; Kaya, S.; Aktag, A.; Yilmaz, E. Yttrium oxide nanostructured thin films deposited by radio frequency sputtering: The annealing optimizations and correlations between structural, morphological, optical and electrical properties. J. Mater. Sci. Mater. Electron. 2017, 28, 13920–13927. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Z.T.; Liu, W.T. The properties of the Y2O3 films exposed at elevated temperature. Phys. B Condens. Matter 2011, 406, 2827–2833. [Google Scholar] [CrossRef]
- Yu, Z.; Liang, L.Y.; Liu, Z.M.; Xu, W.Y.; Sun, X.L.; Cao, H.T. Effects of sputtering pressure and post-metallization annealing on the physical properties of rf-sputtered Y2O3 films. J. Alloy. Compd. 2011, 509, 5810–5815. [Google Scholar] [CrossRef]
- Lei, P.; Leroy, W.; Dai, B.; Zhu, J.; Chen, X.; Han, J.; Depla, D. Study on reactive sputtering of yttrium oxide: Process and thin film properties. Surf. Coat. Technol. 2015, 276, 39–46. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, L.; Liu, J.; Liu, Z.; Chen, Y. Effect of sputtering power on the properties of SiO2 films grown by radio frequency magnetron sputtering at room temperature. Opt. Quantum Electron. 2021, 53, 15. [Google Scholar] [CrossRef]
- Mudavakkat, V.H.; Atuchin, V.V.; Kruchinin, V.N.; Kayani, A.; Ramana, C.V. Structure, morphology and optical properties of nanocrystalline yttrium oxide (Y2O3) thin films. Opt. Mater. 2012, 34, 893–900. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Z.T.; Liu, W.T. Structural and optical properties of yttrium trioxide thin films prepared by RF magnetron sputtering. Vacuum 2011, 86, 72–77. [Google Scholar] [CrossRef]
- Cho, M.H.; Ko, D.H.; Jeong, K.; Whangbo, S.W.; Whang, C.N.; Choi, S.C.; Cho, S.J. Structural transition of crystalline Y2O3 film on Si(111) with substrate temperature. Thin Solid Film. 1999, 349, 266–269. [Google Scholar] [CrossRef]
- Mahieu, S.; Leroy, W.P.; Van Aeken, K.; Depla, D. Modeling the flux of high energy negative ions during reactive magnetron sputtering. J. Appl. Phys. 2009, 106, 093302. [Google Scholar] [CrossRef]
- Gaboriaud, R.J.; Pailloux, F.; Perriere, J. Pulsed laser deposition of Y2O3 thin films on MgO. Appl. Surf. Sci. 2002, 186, 477–482. [Google Scholar] [CrossRef]
- Gaboriaud, R.J.; Pailloux, F.; Paumier, F. Characterisation of Y2O3 thin films deposited by laser ablation on MgO: Why a biaxial epitaxy. Appl. Surf. Sci. 2002, 188, 29–35. [Google Scholar] [CrossRef]
- Gąszowski, D.; Ilczyszyn, M. Hydrogen bonding to xenon: A comparison with neon, argon and krypton complexes. Chem. Phys. Lett. 2013, 556, 59–64. [Google Scholar] [CrossRef]


| Sample | Deposition Power (W) | Thickness (nm) |
|---|---|---|
| 1-1 | 100 | 248 |
| 1-2 | 100 | 420 |
| 1-3 | 100 | 633 |
| 1-4 | 100 | 838 |
| 1-5 | 100 | 1010 |
| 2-1 | 60 | 225 |
| 2-2 | 60 | 476 |
| 2-3 | 60 | 615 |
| 2-4 | 60 | 900 |
| 2-5 | 60 | 1015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Huang, Z.; Feng, L. Effects of the Deposition Mode and Heat Treatment on the Microstructure and Wettability of Y2O3 Coatings Prepared by Reactive Magnetron Sputtering. Coatings 2022, 12, 790. https://doi.org/10.3390/coatings12060790
Ma X, Huang Z, Feng L. Effects of the Deposition Mode and Heat Treatment on the Microstructure and Wettability of Y2O3 Coatings Prepared by Reactive Magnetron Sputtering. Coatings. 2022; 12(6):790. https://doi.org/10.3390/coatings12060790
Chicago/Turabian StyleMa, Xiaorui, Zeyi Huang, and Lin Feng. 2022. "Effects of the Deposition Mode and Heat Treatment on the Microstructure and Wettability of Y2O3 Coatings Prepared by Reactive Magnetron Sputtering" Coatings 12, no. 6: 790. https://doi.org/10.3390/coatings12060790






