Electrochemical Corrosion Behavior of Ni–TiO2 Composite Coatings Electrodeposited from a Deep Eutectic Solvent-Based Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Electrodeposition of Composite Nickel–Titania Coatings
3.2. Corrosion Performance of Composite Nickel–Titania Coatings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet., P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting—A review. J. Energy Chem. 2019, 34, 111–160. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Jamesh, M.I. Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Source 2016, 333, 213–236. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.; An, M. Electrodeposition: Synthesis of advanced transition metal-based catalyst for hydrogen production via electrolysis of water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.Y.; Chen, C.C.; Hwang, B.J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Fruehwald, H.M.; Moghaddam, R.B.; Melino, P.D.; Ebralidze, I.I.; Zenkina, O.V.; Easton, E.B. Ni on graphene oxide: A highly active and stable alkaline oxygen evolution catalyst. Catal. Sci. Technol. 2021, 11, 4026–4033. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazyte, J.; Vilkauskaite, R.; Jasulaitiene, V. Nickel surface anodic oxidation and electrocatalysis of oxygen evolution. J. Solid State Electrochem. 2008, 12, 1469–1479. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Edler, K.J.; Page, A.J. Deep eutectic solvents—The vital link between ionic liquids and ionic solutions. J. Chem. Phys. 2021, 155, 150401. [Google Scholar] [CrossRef]
- Wang, S.; Zou, X.; Lu, Y.; Rao, S.; Xie, X.; Pang, Z.; Lu, X.; Xu, Q.; Zhou, Z. Electrodeposition of nano-nickel in deep eutectic solvents for hydrogen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2018, 43, 15673–15686. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Baskevich, A.S.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 24604–24616. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Bobrova, L.S.; Danilov, F.I. Preparation and characterization of Ni–TiO2 composites electrodeposited from an ethylene glycol-based deep eutectic solvent. Mater. Today Proc. 2022, in press. [CrossRef]
- Thiemig, D.; Bund, A. Characterization of electrodeposited Ni–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Chen, W.; He, Y.; Gao, W. Electrodeposition of sol-enhanced nanostructured Ni-TiO2 composite coatings. Surf. Coat. Technol. 2010, 204, 2487–2492. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W. Sol-enhanced electroplating of nanostructured Ni–TiO2 composite coatings—The effects of sol concentration on the mechanical and corrosion properties. Electrochim. Acta 2010, 55, 6865–6871. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent progresses in electrocatalysts for water electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef] [Green Version]
- Danilov, F.I.; Kityk, A.A.; Shaiderov, D.A.; Bogdanov, D.A.; Korniy, S.A.; Protsenko, V.S. Electrodeposition of Ni–TiO2 composite coatings using electrolyte based on a deep eutectic solvent. Surf. Eng. Appl. Electrochem. 2019, 55, 138–149. [Google Scholar] [CrossRef]
- Danilov, F.I.; Protsenko, V.S.; Kityk, A.A.; Shaiderov, D.A.; Vasil’eva, E.A.; Kumar, U.P.; Kennady, C.J. Electrodeposition of nanocrystalline nickel coatings from a deep eutectic solvent with water addition. Prot. Met. Phys. Chem. Surf. 2017, 53, 1131–1138. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Walsh, F.C.; Larson, C. Towards improved electroplating of metal-particle composite coatings. Trans. IMF 2020, 98, 288–299. [Google Scholar] [CrossRef]
- Shao, W.; Nabb, D.; Renevier, N.; Sherrington, I.; Fu, Y.; Luo, J. Mechanical and anti-corrosion properties of TiO2 nanoparticle reinforced Ni coating by electrodeposition. J. Electrochem. Soc. 2012, 159, D671–D676. [Google Scholar] [CrossRef]
- Baghery, P.; Farzam, M.; Mousavi, A.B.; Hosseini, M. Ni–TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf. Coat. Technol. 2010, 204, 3804–3810. [Google Scholar] [CrossRef]
- Mohajeri, S.; Dolati, A.; Ghorbani, M. The influence of pulse plating parameters on the electrocodeposition of Ni-TiO2 nanocomposite single layer and multilayer structures on copper substrates. Surf. Coat. Technol. 2015, 262, 173–183. [Google Scholar] [CrossRef]
- Szczygiel, B.; Kolodziej, M. Corrosion resistance of Ni/Al2O3 coatings in NaCl solution. Trans. IMF 2005, 83, 181–187. [Google Scholar] [CrossRef]
- Danilov, F.I.; Tsurkan, A.V.; Vasil’eva, E.A.; Protsenko, V.S. Electrocatalytic activity of composite Fe/TiO2 electrodeposits for hydrogen evolution reaction in alkaline solutions. Int. J. Hydrogen Energy 2016, 41, 7363–7372. [Google Scholar] [CrossRef]
- Macdonald, D.D. Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Z.; Xin, S.; Sun, X.; Wu, X. Electrochemical impedance spectroscopy of ceramic coatings on Ti–6Al–4V by micro-plasma oxidation. Electrochim. Acta 2005, 50, 3273–3279. [Google Scholar] [CrossRef]
- Rammelt, U.; Reinhard, G. On the applicability of a constant phase element (CPE) to the estimation of roughness of solid metal electrodes. Electrochim. Acta 1990, 35, 1045–1049. [Google Scholar] [CrossRef]
- He, D.; Li, G.; Shen, D.; Guo, C.; Ma, H.; Cai, J. Effect mechanism of ultrasound on growth of micro-arc oxidation coatings on A96061 aluminum alloy. Vacuum 2014, 107, 99–102. [Google Scholar] [CrossRef]
- Sayar, P.N.; Bahrololoom, M.E. Comparison of anodic dissolution, surface brightness and surface roughness of nanocrystalline nickel coatings with conventional decorative chromium coatings. J. Appl. Electrochem. 2009, 39, 2489–2496. [Google Scholar] [CrossRef]
- Kaseem, M.; Yang, H.W.; Ko, Y.G. Toward a nearly defect-free coating via high-energy plasma sparks. Sci. Rep. 2017, 7, 2378. [Google Scholar] [CrossRef]

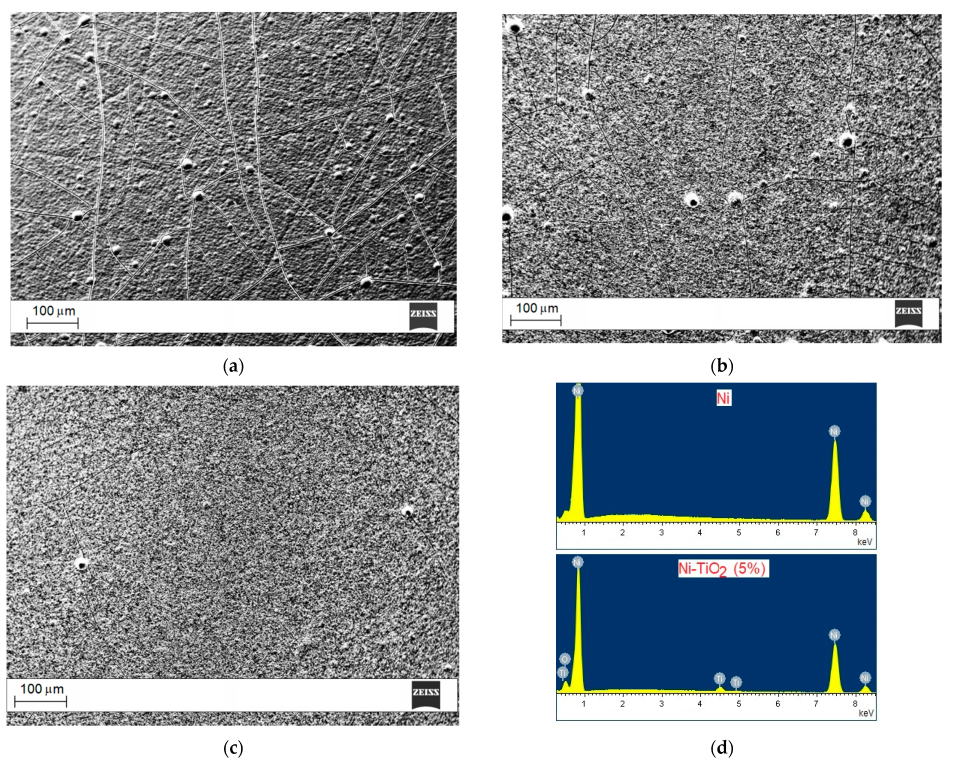
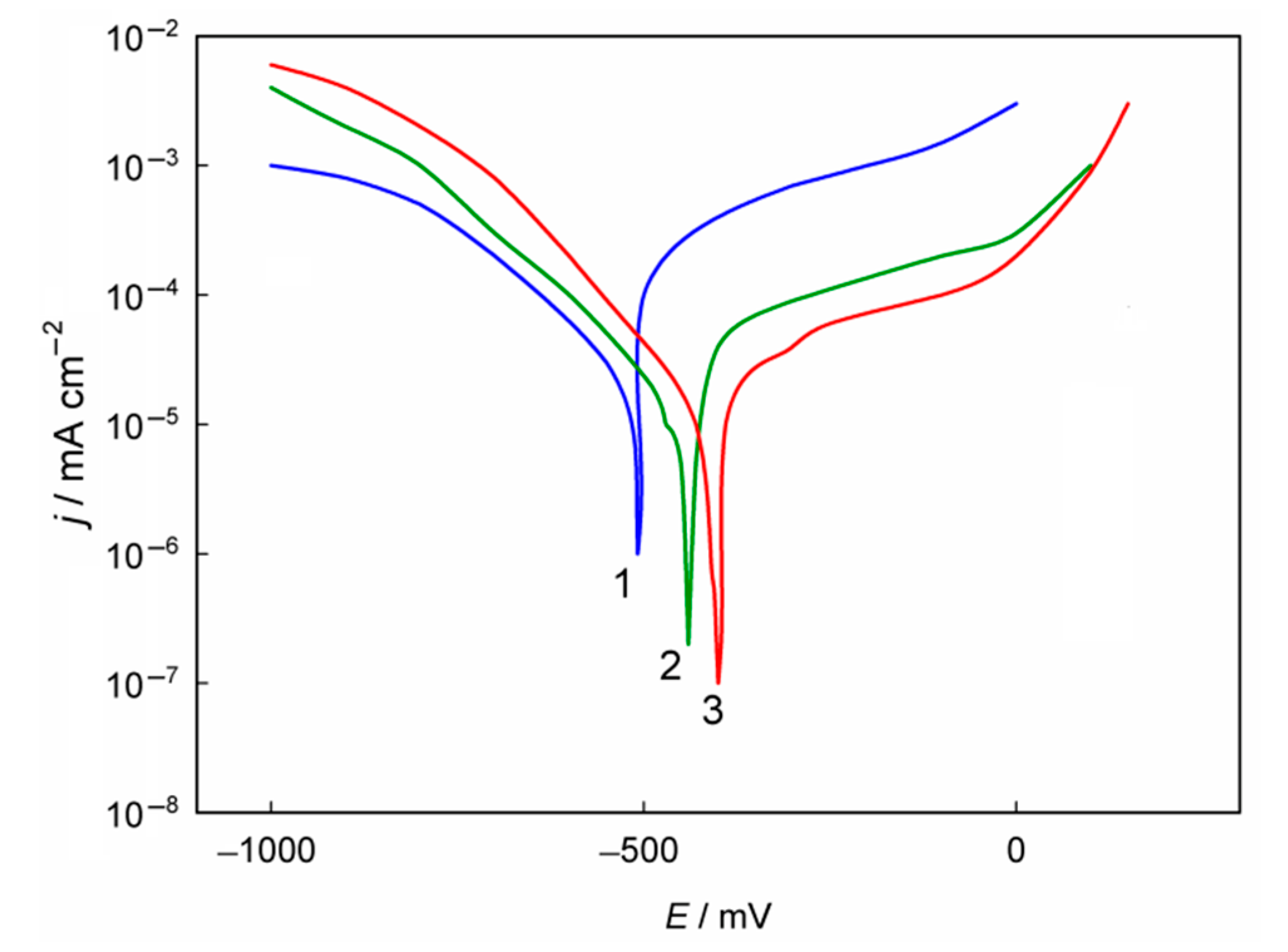
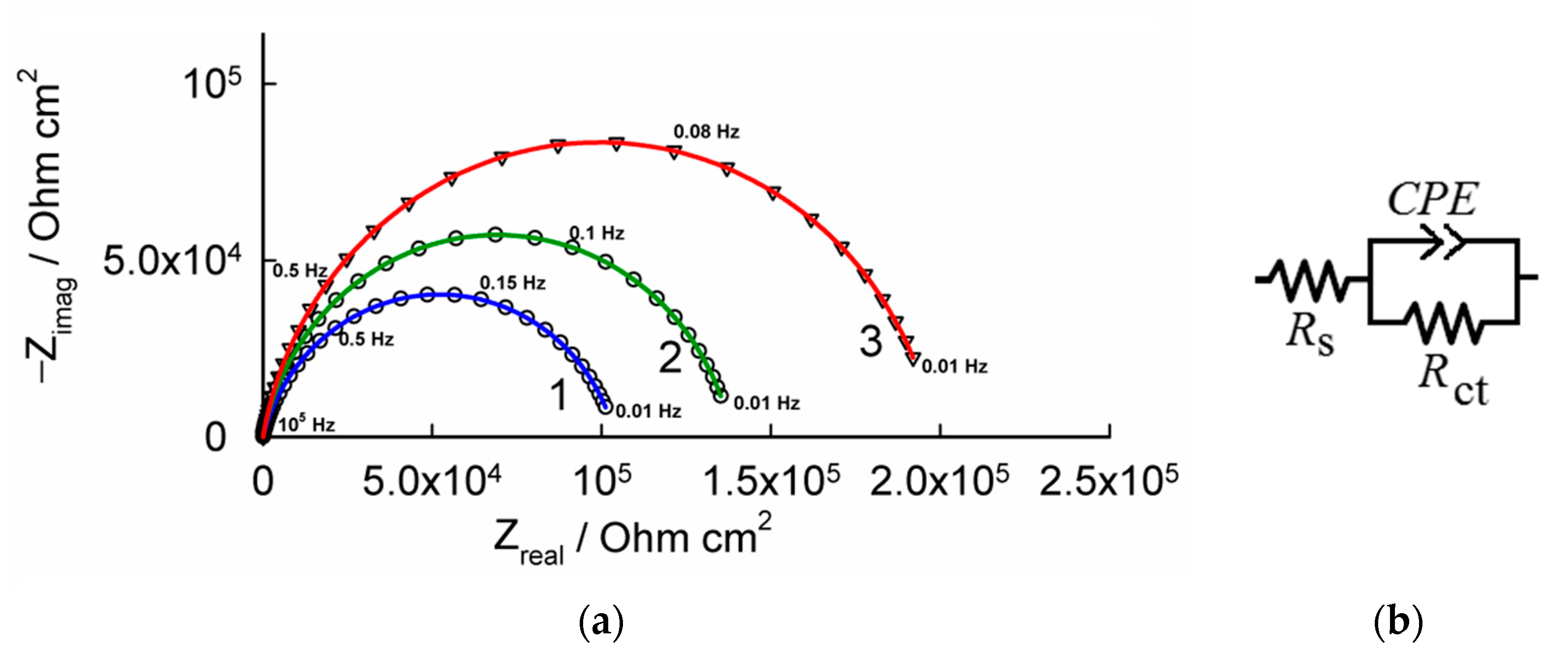
| Coating Type | Ecorr (mV) | jcorr (mA cm–2) | βa (mV dec–1) |
|---|---|---|---|
| Ni | –508 | 9.31 × 10–6 | 110 |
| Ni–TiO2(5%) | –451 | 3.45 × 10–6 | 113 |
| Ni–TiO2(10%) | –408 | 1.28 × 10–6 | 112 |
| Coating Type | Parameters of Equivalent Circuit | |||
|---|---|---|---|---|
| RS (Ω) | Rct (kΩ cm2) | Q × 106 (Ω–1 sn cm–2) | n | |
| Ni | 8.3 | 104.1 | 8.64 | 0.84 |
| Ni–TiO2(5%) | 8.2 | 138.4 | 7.32 | 0.88 |
| Ni–TiO2(10%) | 8.4 | 198.7 | 7.17 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protsenko, V.S.; Butyrina, T.E.; Bobrova, L.S.; Korniy, S.A.; Danilov, F.I. Electrochemical Corrosion Behavior of Ni–TiO2 Composite Coatings Electrodeposited from a Deep Eutectic Solvent-Based Electrolyte. Coatings 2022, 12, 800. https://doi.org/10.3390/coatings12060800
Protsenko VS, Butyrina TE, Bobrova LS, Korniy SA, Danilov FI. Electrochemical Corrosion Behavior of Ni–TiO2 Composite Coatings Electrodeposited from a Deep Eutectic Solvent-Based Electrolyte. Coatings. 2022; 12(6):800. https://doi.org/10.3390/coatings12060800
Chicago/Turabian StyleProtsenko, Vyacheslav S., Tetyana E. Butyrina, Lina S. Bobrova, Sergiy A. Korniy, and Felix I. Danilov. 2022. "Electrochemical Corrosion Behavior of Ni–TiO2 Composite Coatings Electrodeposited from a Deep Eutectic Solvent-Based Electrolyte" Coatings 12, no. 6: 800. https://doi.org/10.3390/coatings12060800







