Preparation and Performance Study of Carboxy-Functionalized Graphene Oxide Composite Polyaniline Modified Water-Based Epoxy Zinc-Rich Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CGO
2.3. Preparation of CGO/PANI
2.4. Preparation of CGO/PANI/W-B EZRC
2.5. Characterization
2.5.1. Characterization of CGO/PANI
2.5.2. Characterization of CGO/PANI/W-B EZRC
3. Results and Discussion
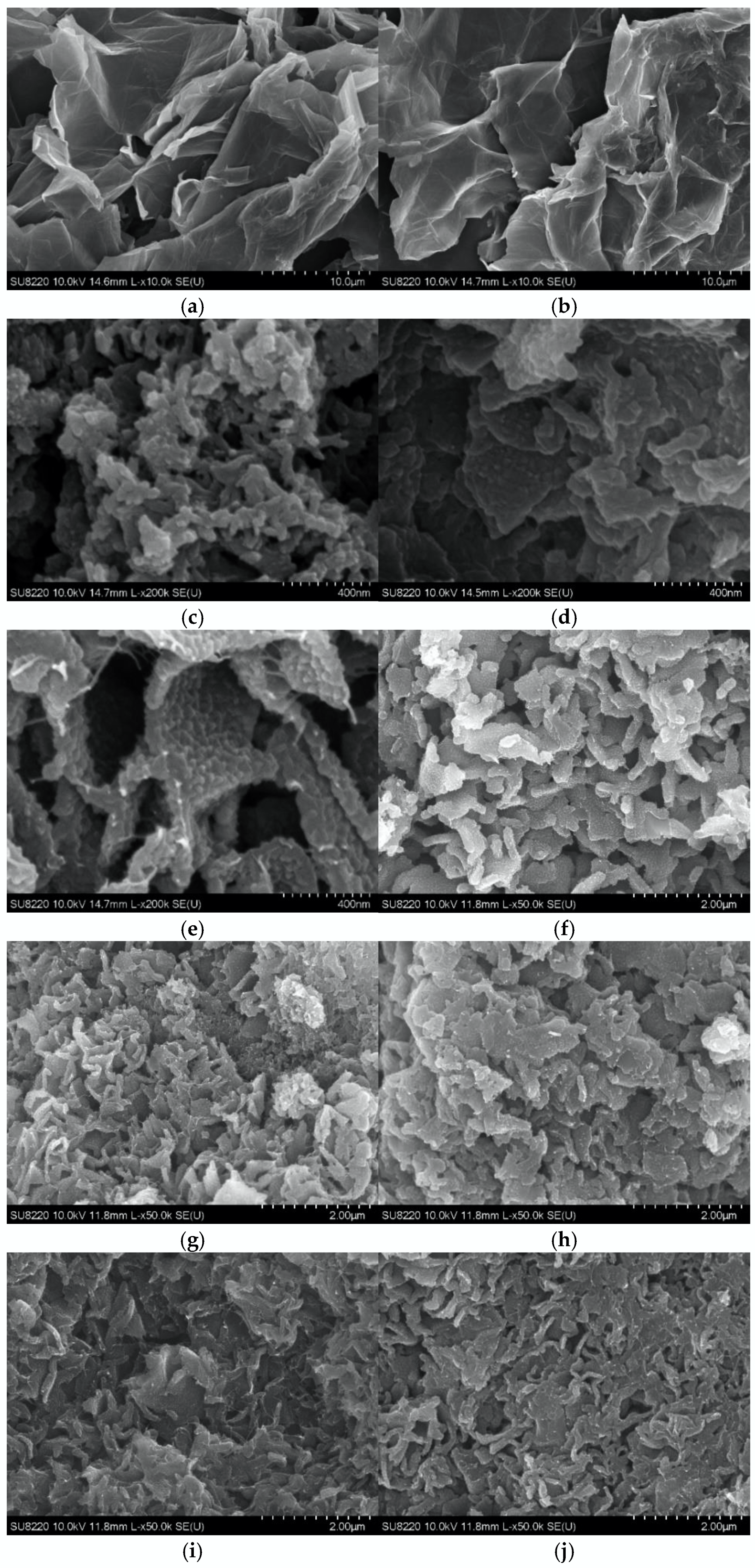
3.1. Morphological Study of CGO/PANI by SEM
3.2. FT-IR Spectroscopic Study
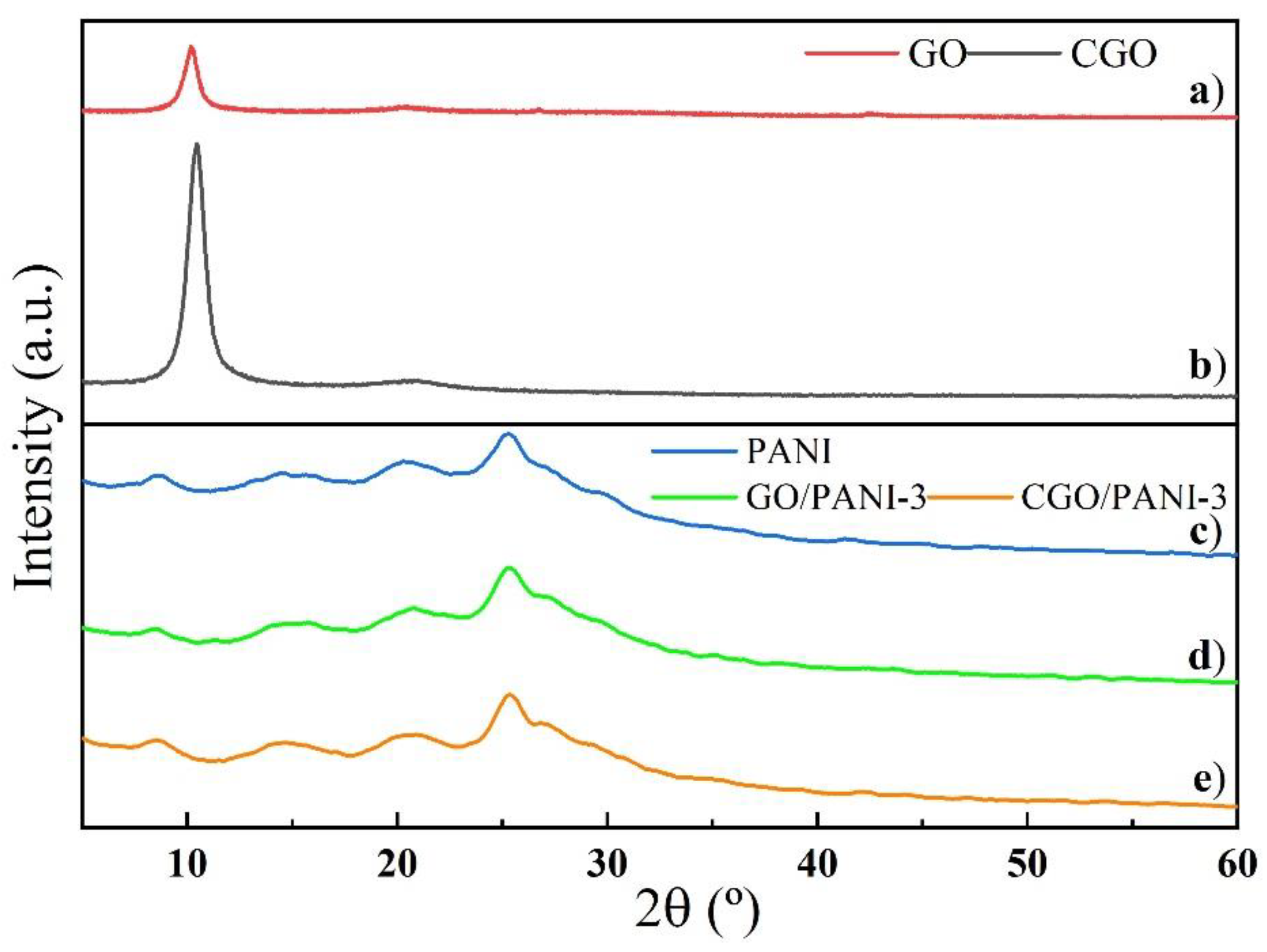
3.3. X-ray Diffraction (XRD) Study
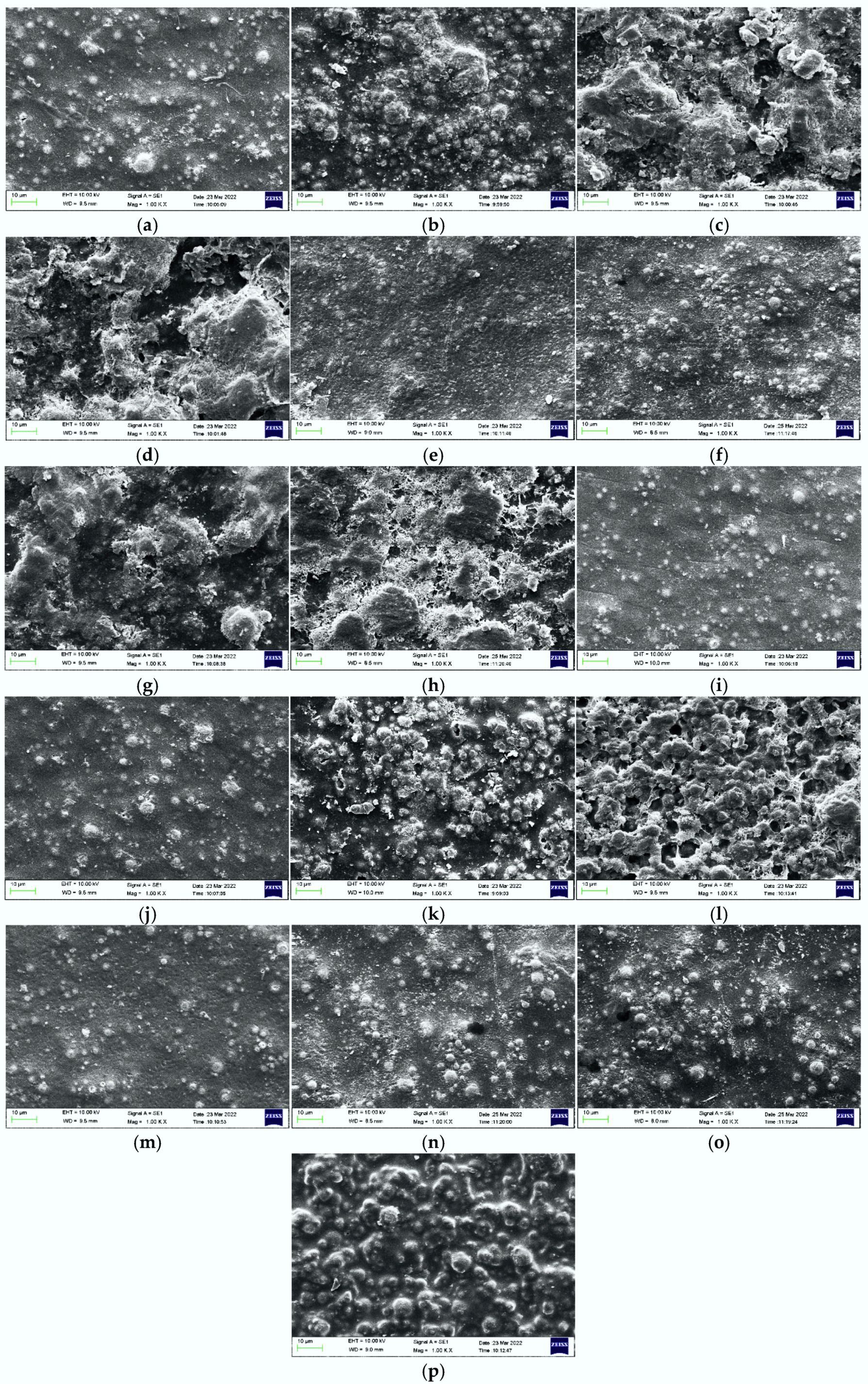
3.4. Morphological Study of CGO/PANI/W-B EZRC by SEM
3.5. Electrochemical Impedance Spectroscopy (EIS)
3.6. Comprehensive Anticorrosion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, F.; Qian, C.; Guo, M.; Wang, J.; Yan, X.; Li, H.; Yue, L. Anticorrosive durability of zinc-based waterborne coatings enhanced by highly dispersed and conductive polyaniline/graphene oxide composite. Prog. Org. Coat. 2018, 125, 79–88. [Google Scholar] [CrossRef]
- Jagtap, R.N.; Nambiar, R.; Hassan, S.Z.; Malshe, V.C. Predictive power for life and residual life of the zinc rich primer coatings with electrical measurement. Prog. Org. Coat. 2007, 58, 253–258. [Google Scholar] [CrossRef]
- Shreepathi, S.; Bajaj, P.; Mallik, B.P. Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: Role of Zn content on corrosion protection mechanism. Electrochim. Acta 2010, 55, 5129–5134. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mandavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Vilche, J.R.; Bucharsky, E.C.; Giudice, C.A. Application of EIS and SEM to evaluate the influence of pigment shape and content in ZRP formulations on the corrosion prevention of naval steel. Corros. Sci. 2002, 44, 1287–1309. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Park, K.C. Study on corrosive electrochemical behaviors of zinc-rich and graphite-filled epoxy coatings in 3.5 wt% NaCl solution. Mater. Corros. 2011, 62, 1008–1014. [Google Scholar] [CrossRef]
- Cubides, Y.; Su, S.S.; Castaneda, H. Influence of Zinc Content and Chloride Concentration on the Corrosion Protection Performance of Zinc-Rich Epoxy Coatings Containing Carbon Nanotubes on Carbon Steel in Simulated Concrete Pore Environments. Corrosion 2016, 72, 1397–1423. [Google Scholar] [CrossRef]
- Park, S.; Shon, M. Effects of multi-walled carbon nano tubes on corrosion protection of zinc rich epoxy resin coating. J. Ind. Eng. Chem. 2015, 21, 1258–1264. [Google Scholar] [CrossRef]
- Cubides, Y.; Castaneda, H. Corrosion protection mechanisms of carbon nanotube and zinc-rich epoxy primers on carbon steel in simulated concrete pore solutions in the presence of chloride ions. Corros. Sci. 2016, 109, 145–161. [Google Scholar] [CrossRef] [Green Version]
- Hayatdavoudi, H.; Rahsepar, M. A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloy. Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Sari, M.G.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an epoxy composite coating with enhanced corrosion resistance through impregnation of functionalized graphene oxide-co-montmorillonite Nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Zhao, W.; Yu, J.; Jiang, F.; Wu, Y.; Ma, L. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 2019, 169, 107694. [Google Scholar] [CrossRef]
- Kratochvilova, I.; Ashcheulov, P.; Skarohlid, J.; Skoda, R.; Kopecek, J.; Sajdl, P.; Macak, J.; Lajcinova, M.; Novakova, A.; Neethling, J.; et al. Zr alloy protection against high-temperature oxidation: Coating by a double-layered structure with active and passive functional properties. Corros. Sci. 2020, 163, 108270. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.X.; Zhang, R.Y.; Liu, J.G.; Yu, X.; Li, Z.L. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, W.J.; Wang, K.; Xu, J.G. GO-Ti3C2 two-dimensional heterojunction nanomaterial for anticorrosion enhancement of epoxy zinc-rich coatings. J. Hazard. Mater. 2021, 417, 126048. [Google Scholar] [CrossRef]
- Liao, G.F.; Li, Q.; Xu, Z.S. The chemical modification of polyaniline with enhanced properties: A review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Ebrahimi, M.; Sharif, F.; Ghanbarzadeh, A. Evaluating protection performance of zinc rich epoxy paints modified with polyaniline and polyaniline-clay nanocomposite. Prog. Org. Coat. 2014, 77, 1299–1308. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, Z.; Liu, J.; Li, D.; Tan, N.; Liu, T.; Zhang, Y.; Chang, X.; Gu, Y.; Yin, Y. Effect of Conducting Polyaniline/Graphene Nanosheet Content on the Corrosion Behavior of Zinc-Rich Epoxy Primers in 3.5% NaCl Solution. Polymers 2019, 11, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalendova, A.; Vesely, D.; Stejskal, J. Organic coatings containing polyaniline and inorganic pigments as corrosion inhibitors. Prog. Org. Coat. 2008, 62, 105–116. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.H.; Li, J.; Lu, J.L.; Wang, F.S. Long-term anticorrosion behaviour of polyaniline on mild steel. Corros. Sci. 2007, 49, 3052–3063. [Google Scholar] [CrossRef]
- Meroufel, A.; Deslouis, C.; Touzain, S. Electrochemical and anticorrosion performances of zinc-rich and polyaniline powder coatings. Electrochim. Acta 2008, 53, 2331–2338. [Google Scholar] [CrossRef]
- Kalendova, A.; Sapurina, I.; Stejskal, J.; Vesely, D. Anticorrosion properties of polyaniline-coated pigments in organic coatings. Corros. Sci. 2008, 50, 3549–3560. [Google Scholar] [CrossRef]
- Basavaraja, C.; Kim, W.J.; Kim, Y.D.; Huh, D.S. Synthesis of polyaniline-gold/graphene oxide composite and microwave absorption characteristics of the composite films. Mater. Lett. 2011, 65, 3120–3123. [Google Scholar] [CrossRef]
- Bharadiya, P.; Jain, R.; Chaudhari, V.; Mishra, S. Graphene Oxide-Wrapped Polyaniline Nanorods for Supercapacitor Applications. Polym. Compos. 2019, 40, E1716–E1724. [Google Scholar] [CrossRef]
- Wang, H.L.; Hao, Q.L.; Yang, X.J.; Lu, L.D.; Wang, X. Effect of Graphene Oxide on the Properties of Its Composite with Polyaniline. ACS Appl. Mater. Interfaces 2010, 2, 821–828. [Google Scholar] [CrossRef]
- Wang, H.L.; Hao, Q.L.; Yang, X.J.; Lu, L.D.; Wang, X. A nanostructured graphene/polyaniline hybrid material for supercapacitors. Nanoscale 2010, 2, 2164–2170. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J.S. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Zhang, W.L.; Park, B.J.; Choi, H.J. Colloidal graphene oxide/polyaniline nanocomposite and its electrorheology. Chem. Commun. 2010, 46, 5596–5598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, R.J.; Wang, Z.; Liu, H.T. Carboxyl-functionalized graphene oxide-polyaniline composite as a promising supercapacitor material. J. Mater. Chem. 2012, 22, 13619–13624. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Gao, X.L.; Wei, Y.; Wang, X.Y.; Wang, J.; Zhang, Y.S.; Gao, C.J. Enhanced desalination performance of carboxyl functionalized graphene oxide nanofiltration membranes. Desalination 2017, 405, 29–39. [Google Scholar] [CrossRef]
- Xu, J.J.; Wang, K.; Zu, S.Z.; Han, B.H.; Wei, Z.X. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, S.; Hong, R. Graphene Oxide/Polyaniline Nanocomposites Used in Anticorrosive Coatings for Environmental Protection. Coatings 2020, 10, 1215. [Google Scholar] [CrossRef]
- Kumar, N.A.; Choi, H.-J.; Shin, Y.R.; Chang, D.W.; Dai, L.; Baek, J.-B. Polyaniline-Grafted Reduced Graphene Oxide for Efficient Electrochemical Supercapacitors. ACS Nano 2012, 6, 1715–1723. [Google Scholar] [CrossRef]
- Sanches, E.A.; Soares, J.C.; Iost, R.M.; Marangoni, V.S.; Trovati, G.; Batista, T.; Mafud, A.C.; Zucolotto, V.; Mascarenhas, Y.P. Structural Characterization of Emeraldine-Salt Polyaniline/Gold Nanoparticles Complexes. J. Nanomater. 2011, 2011, 697071. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.P.; Lei, S.B.; Song, Y.H. Graphene-based polyaniline nanocomposites: Preparation, properties and applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Htut, K.Z.; Kim, M.; Lee, E.; Lee, G.; Baeck, S.H.; Shim, S.E. Biodegradable polymer-modified graphene/polyaniline electrodes for supercapacitors. Synth. Met. 2017, 227, 61–70. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Cai, Y.; Lu, Y.; Cao, Q.; Lv, P.; Zhang, Y.; Liu, W. Preparation and Performance Study of Carboxy-Functionalized Graphene Oxide Composite Polyaniline Modified Water-Based Epoxy Zinc-Rich Coatings. Coatings 2022, 12, 824. https://doi.org/10.3390/coatings12060824
Chen Z, Cai Y, Lu Y, Cao Q, Lv P, Zhang Y, Liu W. Preparation and Performance Study of Carboxy-Functionalized Graphene Oxide Composite Polyaniline Modified Water-Based Epoxy Zinc-Rich Coatings. Coatings. 2022; 12(6):824. https://doi.org/10.3390/coatings12060824
Chicago/Turabian StyleChen, Zhonghua, Yuande Cai, Yunyun Lu, Qi Cao, Peibin Lv, Yiru Zhang, and Wenjie Liu. 2022. "Preparation and Performance Study of Carboxy-Functionalized Graphene Oxide Composite Polyaniline Modified Water-Based Epoxy Zinc-Rich Coatings" Coatings 12, no. 6: 824. https://doi.org/10.3390/coatings12060824




