Preparation of Core–Shell Structure W/Gd2O3 and Study on the Properties of Radiation Protection Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
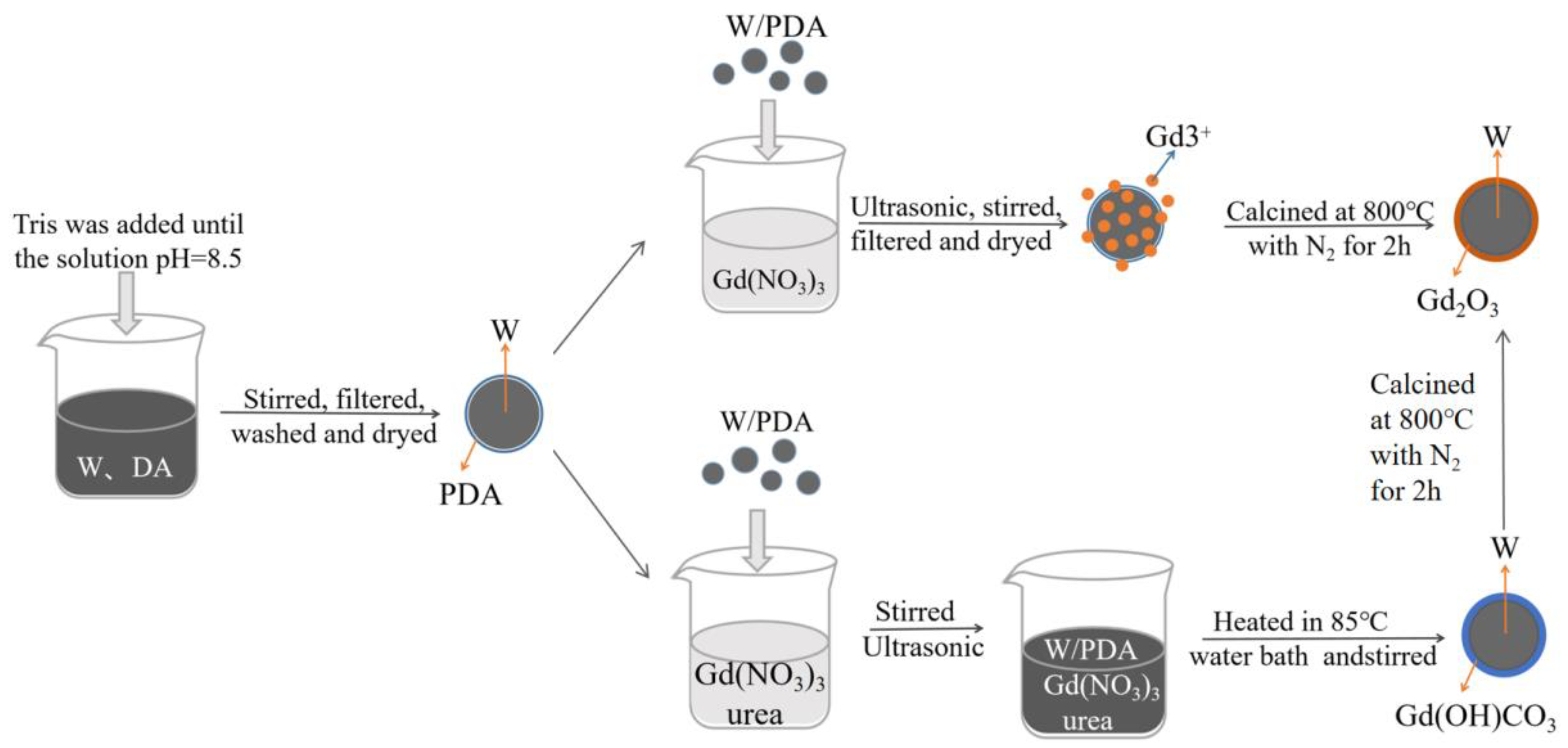
2.2. Preparation and Characterization of W/PDA
2.3. Preparation and Characterization of W/Gd2O3 by Self-Assembly Method
2.4. Preparation and Characterization of W/Gd2O3 by Homogeneous Precipitation
2.5. Preparation and Characterization of Radiation Protection Fabric
2.6. Radiation Protection Performance Test
2.7. Mechanical Performance Test
3. Results
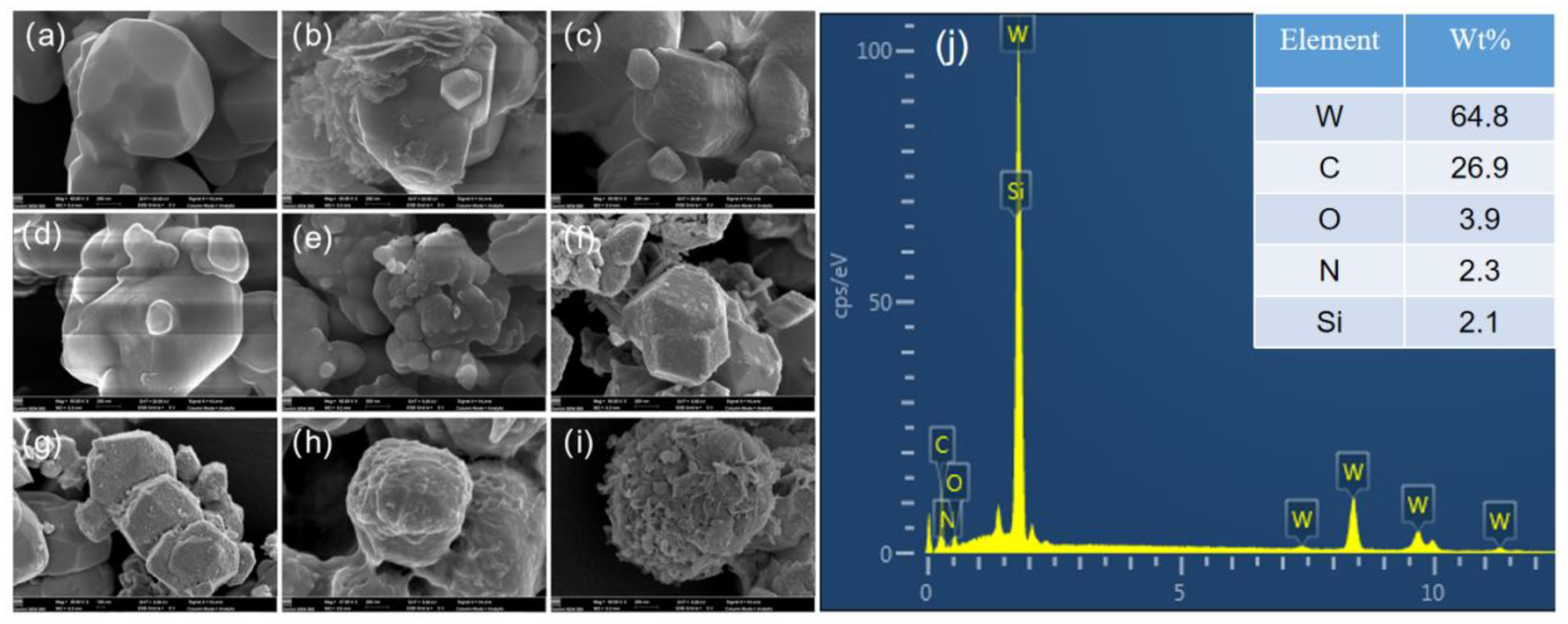
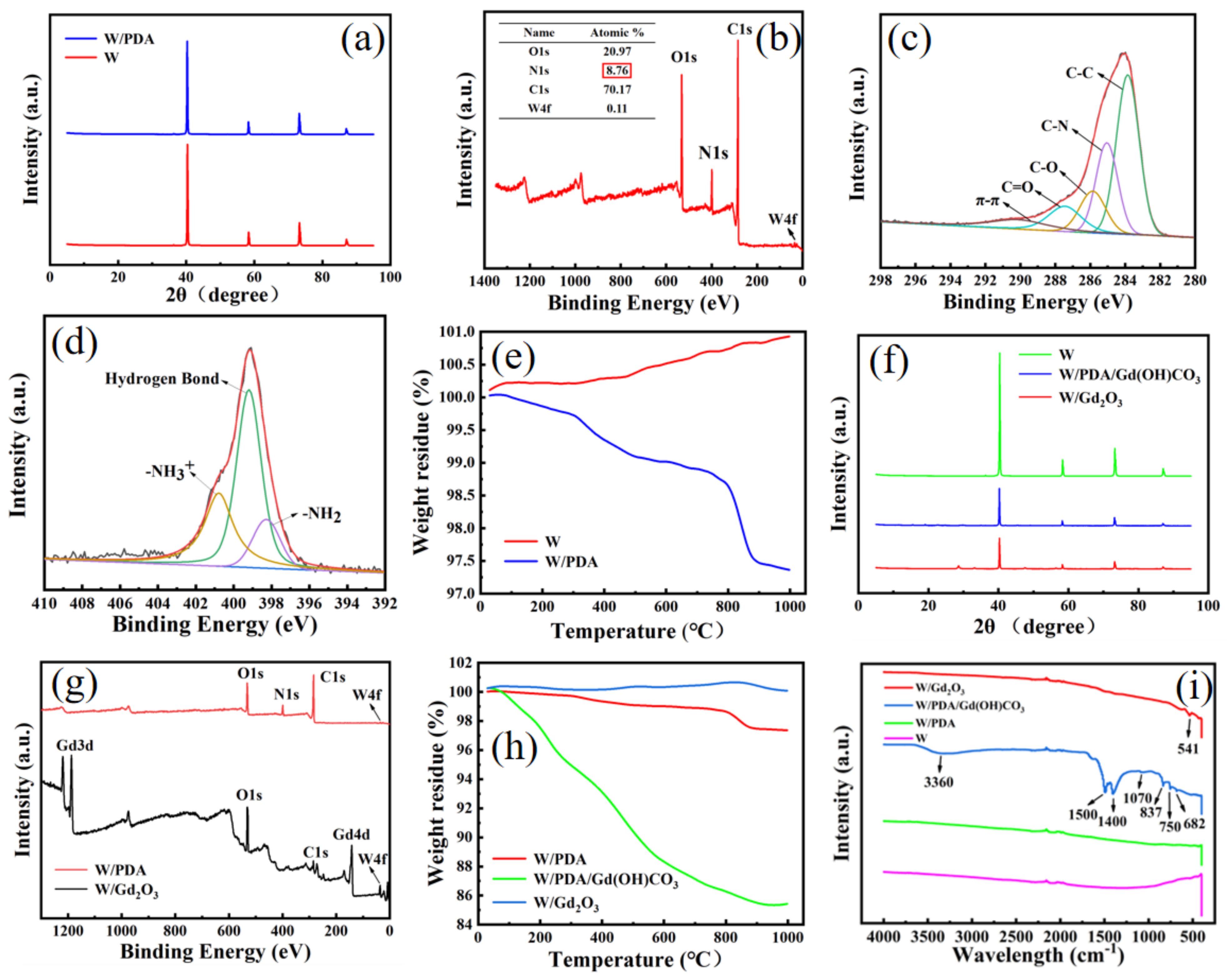
3.1. Preparation and Characterization of W/PDA
3.2. Preparation and Characterization of W/Gd2O3 by Self-Assembly Method
3.3. Preparation and Characterization of W/Gd2O3 by Homogeneous Precipitation
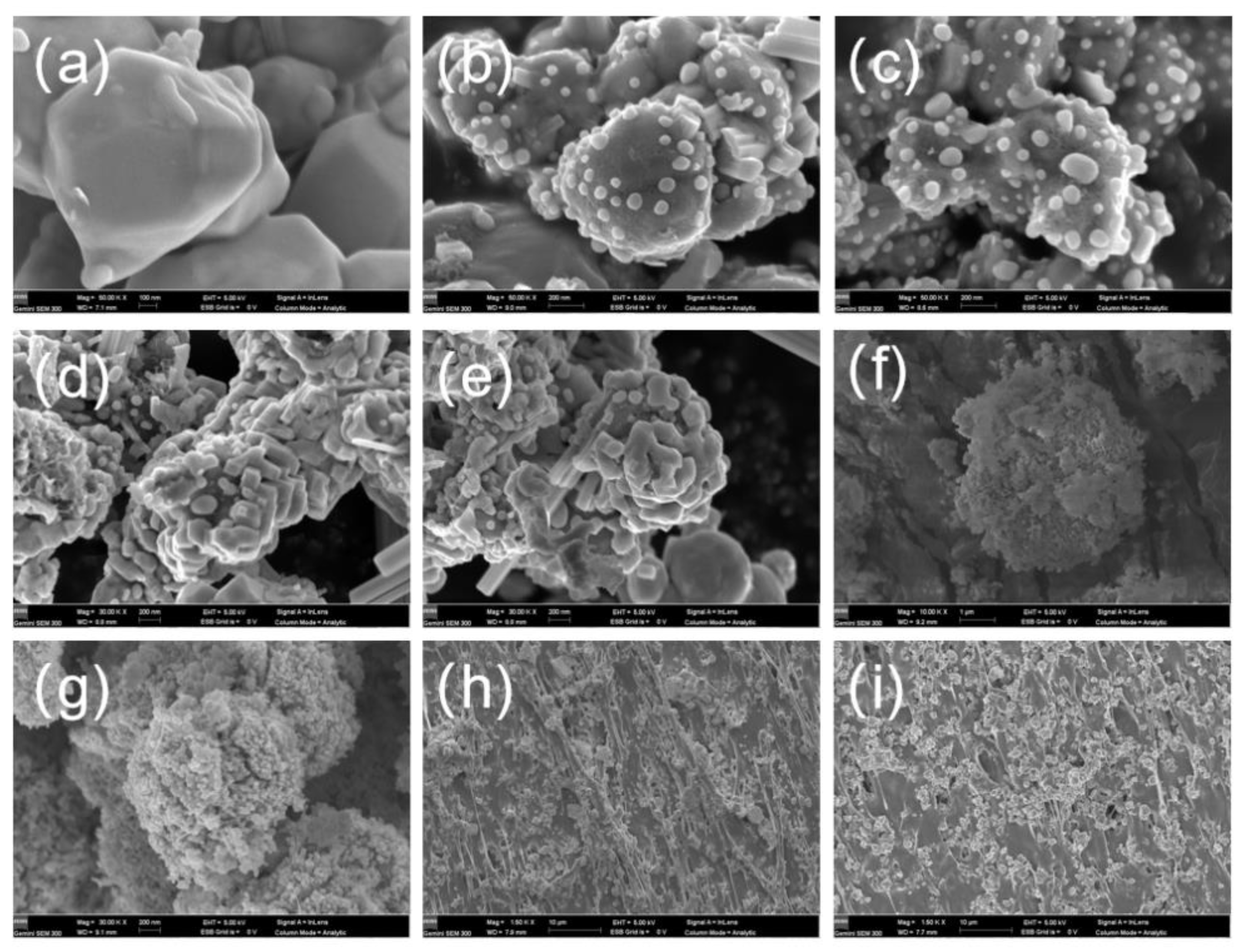
3.4. Preparation and Characterization of Radiation Protection Fabric
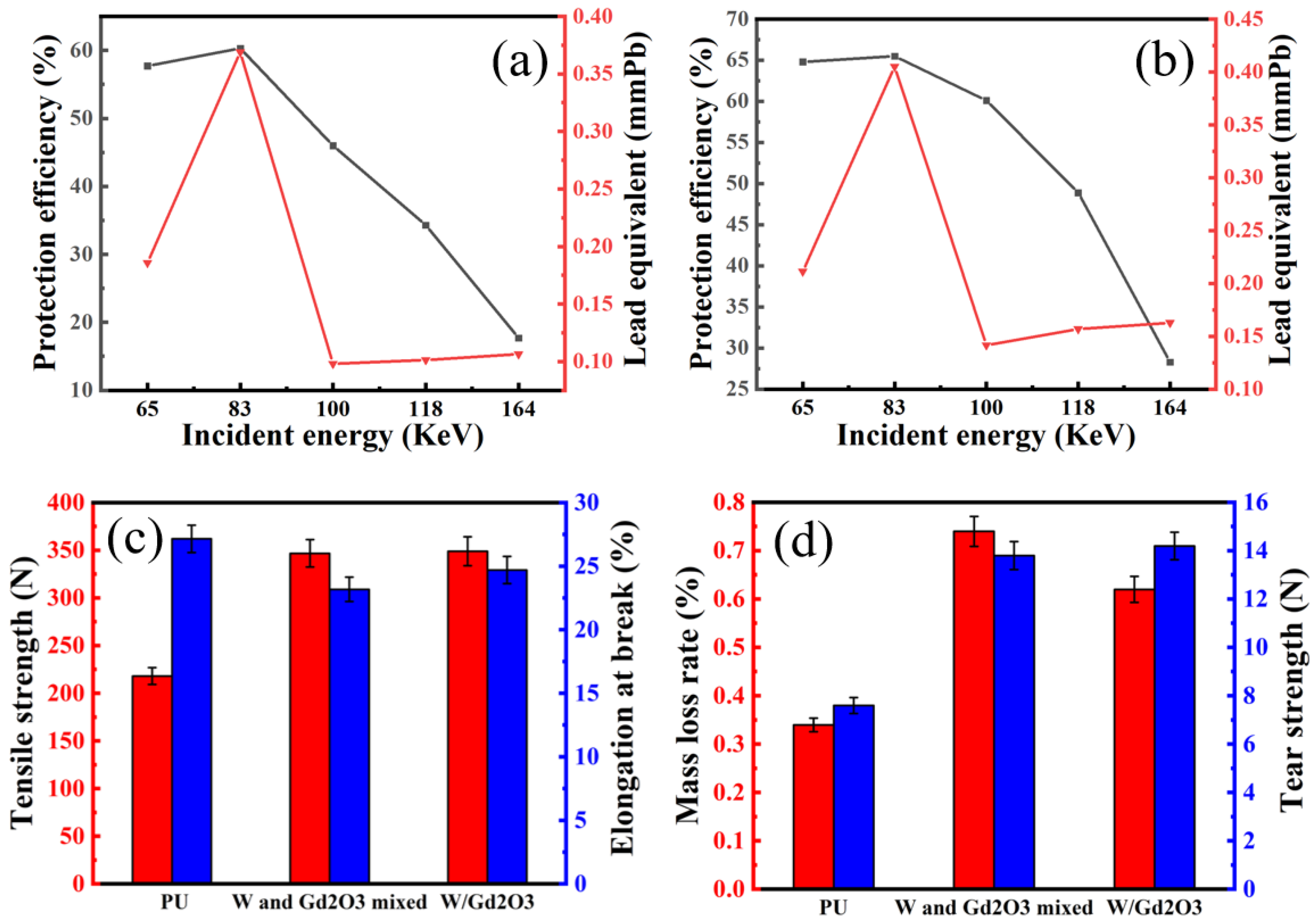
3.5. Radiation Protection Performance Test
3.6. Mechanical Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daniele, G. Individual Radiation Protection: Idea and Research Needs. J. Radiol. Prot. 2019, 39, 641–646. [Google Scholar] [CrossRef]
- Reading, R. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Child Care Health Dev. 2012, 38, 910. [Google Scholar] [CrossRef]
- Boaira, M.S.; Chaffey, C.E. Effects of coupling agents on the mechanical and rheological properties and mica-reinforced polypropylene. Polym. Eng. Sci. 1977, 17, 715–718. [Google Scholar] [CrossRef]
- Bibbo, G. Shielding of medical imaging X-ray facilities: A simple and practical method. Australas. Phys. Eng. Sci. Med. 2017, 40, 925–930. [Google Scholar] [CrossRef]
- Fan, G.; Geng, L.; Wang, G.; Zheng, Z. A novel radiation protection material: BaPbO3/Al composite. Mater. Des. 2009, 30, 862–866. [Google Scholar] [CrossRef]
- Spillantini, P.; Casolino, M.; Durante, M. Shielding from cosmic radiation for interplanetary missions: Active and passive methods. Radiat. Meas. 2009, 42, 14–23. [Google Scholar] [CrossRef]
- Nuñez-Briones, A.G.; Benavides, R.; Mendoza-Mendoza, E.; Martínez-Pardo, M.E.; Carrasco-Abrego, H.; Kotzian, C.; Saucedo-Zendejo, F.R.; García-Cerda, L.A. Preparation of PVC/Bi2O3 composites and their evaluation as low energy X-ray radiation shielding. Radiat. Phys. Chem. 2021, 179, 109198. [Google Scholar] [CrossRef]
- Cherkashina, N.I.; Pavlenko, V.I.; Noskov, A.V. Radiation shielding properties of polyimide composite materials. Radiat. Phys. Chem. 2019, 159, 111–117. [Google Scholar] [CrossRef]
- Kijima, K.; Krisanachinda, A.; Pasawang, P.; Hanaoka, K.; Nishimura, Y. Shielding Efficiency of Novel Tungsten Rubber against Radionuclides of 99mTc, 131I, 18F and 68Ga. Radiat. Phys. Chem. 2020, 172, 108755. [Google Scholar] [CrossRef]
- Bilous, V.A.; Borysenko, V.M.; Voevodin, V.M.; Didenko, S.Y.; Chenko, M.I.; Neklyudov, I.M.; Rybka, O.V. Dependence of the Radiation-Protective Efficiency of AL-PB Multilayer Composites on Their Structure. Mater. Sci. 2015, 50, 600–603. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.; Kim, J.; Lee, J.; Seo, Y. Multilayer Structuring of Nonleaded Metal (BiSn)/Polymer/Tungsten Composites for Enhanced γ-Ray Shielding. Adv. Eng. Mater. 2020, 22, 1901448. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Wang, Y.; Chen, P.; Xin, S.; Jiu, H.; Liu, J. Hollow and Hollow Core/shell CeO2/SiO2@CeO2 Spheres: Synthesis, Structure Evolution and Catalytic Properties. J. Alloys Compd. 2014, 586, 441–447. [Google Scholar] [CrossRef]
- Zhou, J.; Ao, J.; Xia, Y.; Xiong, H. Stable photoluminescent ZnO@Cd(OH)2 core-shell nanoparticles synthesized via ultrasonication-assisted sol-gel method. J. Colloid Interface Sci. 2013, 393, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Soylu, H.M.; Lambrecht, F.Y.; Ersöz, O.A. Gamma radiation shielding efficiency of a new lead-free composite material. J. Radioanal. Nucl. Chem. 2015, 305, 529–534. [Google Scholar] [CrossRef]
- Shang, W.; Meng, H.; Wen, Y. Influence of preparation temperature on the electrochemical characteristics of the Dodecafluoroheptyl-propyl-trimethoxysilane SAMs on aluminum alloy 6061. Surf. Coat. Technol. 2014, 258, 574–579. [Google Scholar] [CrossRef]
- Ciliberto, E.; Condorelli, G.G.; Delfa, S.L.; Viscuso, E. Nanoparticles of Sr(OH)2: Synthesis in homogeneous phase at low temperature and application for cultural heritage artefacts. Appl. Phys. A 2008, 92, 137–141. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Liang, L.; Xu, J.; Chen, Z. A “Sandwich” type of neutron shielding composite filled with boron carbide reinforced by carbon fiber. Chem. Eng. J. 2013, 220, 143–150. [Google Scholar] [CrossRef]
- Yin, J.; Chen, D.; Zhang, Y.; Li, C.; Liu, L.; Shao, Y. MRI relaxivity enhancement of gadolinium oxide nanoshells with a controllable shell thickness. Phys. Chem. Chem. Phys. PCCP 2018, 20, 10038–10047. [Google Scholar] [CrossRef]
- Li, W.; Zheng, X.; Li, F.; Wang, J. Facile Synthetic Route to Hollow Gadolinium Oxide Spheres with TunableThickness. Micro Nano Lett. 2012, 7, 1267–1269. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; You, H.P.; Liu, K.; Zheng, Y.H.; Guo, N.; Zhang, H.J. Highly uniform Gd2O3 hollow microspheres: Template-directed synthesis and luminescence properties. Langmuir ACS J. Surf. Colloids 2010, 26, 5122–5128. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, J.; Li, H.; Li, L.; Jin, Q.; Ren, K.; Ji, J. Mussel-inspired polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tian, M.; Liu, L. Fabrication and Properties of Silverized Glass Fiber by Dopamine Functionalization and Electroless Plating. J. Electrochem. Soc. 2012, 159, 1945–7111. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Y.; Li, Y.; Huang, Y.; Parent, L.R.; Ditri, T.; Zang, N.; Rinehart, J.D.; Gianneschi, N.C. Tunable, metal-loaded polydopamine nanoparticles analyzed by magnetometry. Chem. Mater. 2017, 29, 8195–8201. [Google Scholar] [CrossRef]
- Kong, J.; Yee, W.A.; Yang, L.; Wei, Y.; Phua, S.L.; Ong, H.G.; Ang, J.M.; Li, X.; Lu, X. Highly electrically conductive layered carbon derived from polydopamine and its functions in SnO2-based lithium ion battery anodes. Chem. Commun. 2012, 48, 10316. [Google Scholar] [CrossRef]
- Khakzadian, J.; Hosseini, H.R.M.; Bagherzadeh, E. A three-layered multifunctional photoactive core-shell gadolinium based nanocomposite. Ceram. Int. 2019, 45, 21228–21234. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, K.J.; Lee, J.K. Synthesis and characterization of hollow nanoparticles of crystalline Gd2O3. J. Nanopart. Res. 2011, 13, 2311–2318. [Google Scholar] [CrossRef]
- Liu, G.; Hong, G.; Wang, J.; Dong, X. Hydrothermal synthesis of spherical and hollow Gd2O3: Eu3+ phosphors. J. Alloys Compd. 2006, 432, 200–204. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Yang, T.; Pan, G.; Xu, S.; Yao, L. Preparation of Core–Shell Structure W/Gd2O3 and Study on the Properties of Radiation Protection Materials. Coatings 2022, 12, 851. https://doi.org/10.3390/coatings12060851
Xia Y, Yang T, Pan G, Xu S, Yao L. Preparation of Core–Shell Structure W/Gd2O3 and Study on the Properties of Radiation Protection Materials. Coatings. 2022; 12(6):851. https://doi.org/10.3390/coatings12060851
Chicago/Turabian StyleXia, Yong, Tao Yang, Gangwei Pan, Sijun Xu, and Lirong Yao. 2022. "Preparation of Core–Shell Structure W/Gd2O3 and Study on the Properties of Radiation Protection Materials" Coatings 12, no. 6: 851. https://doi.org/10.3390/coatings12060851
APA StyleXia, Y., Yang, T., Pan, G., Xu, S., & Yao, L. (2022). Preparation of Core–Shell Structure W/Gd2O3 and Study on the Properties of Radiation Protection Materials. Coatings, 12(6), 851. https://doi.org/10.3390/coatings12060851




