Argon and Other Defects in Amorphous SiO2 Coatings for Gravitational-Wave Detectors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effusion of Argon by Mass Spectrometry
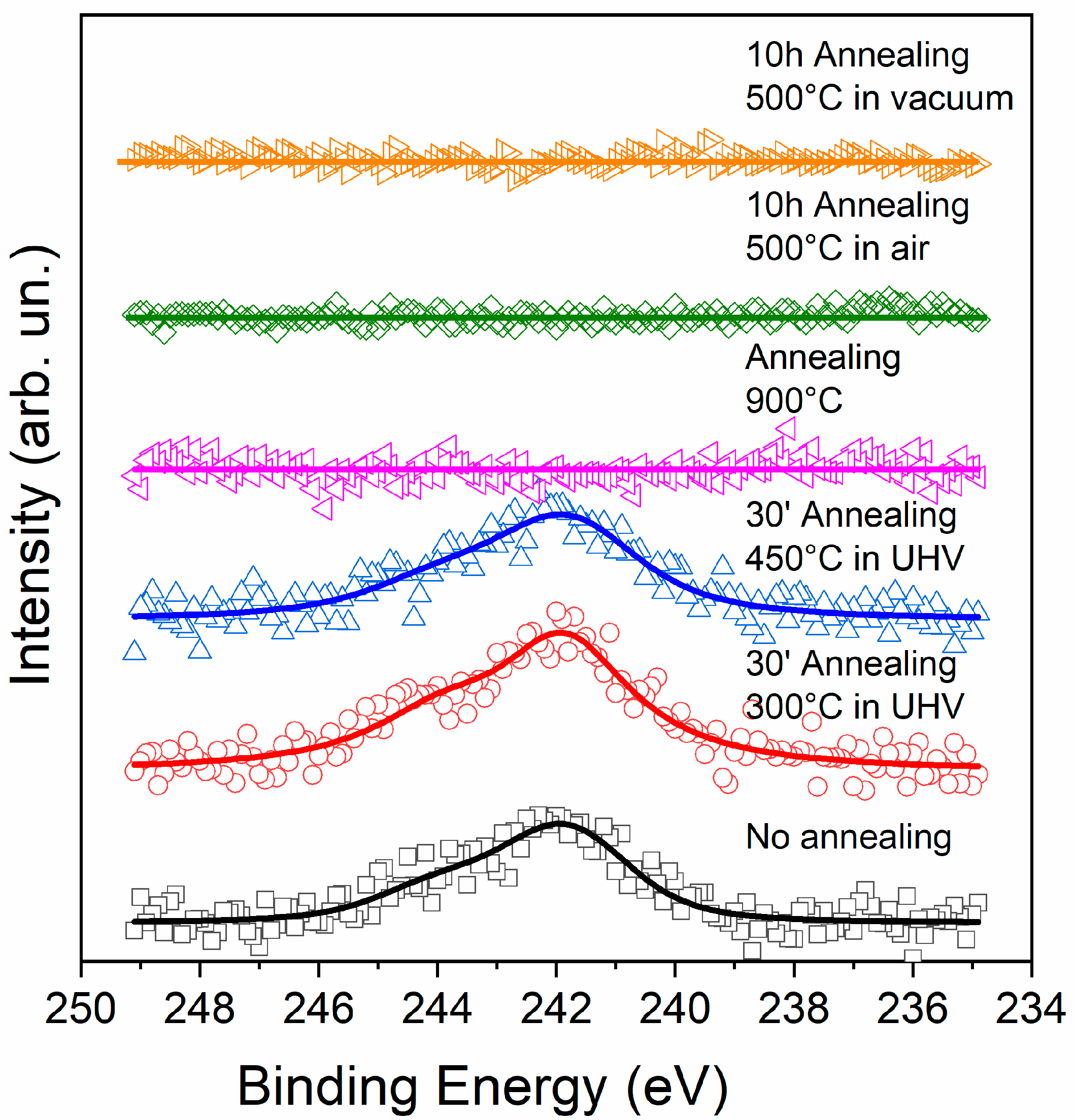
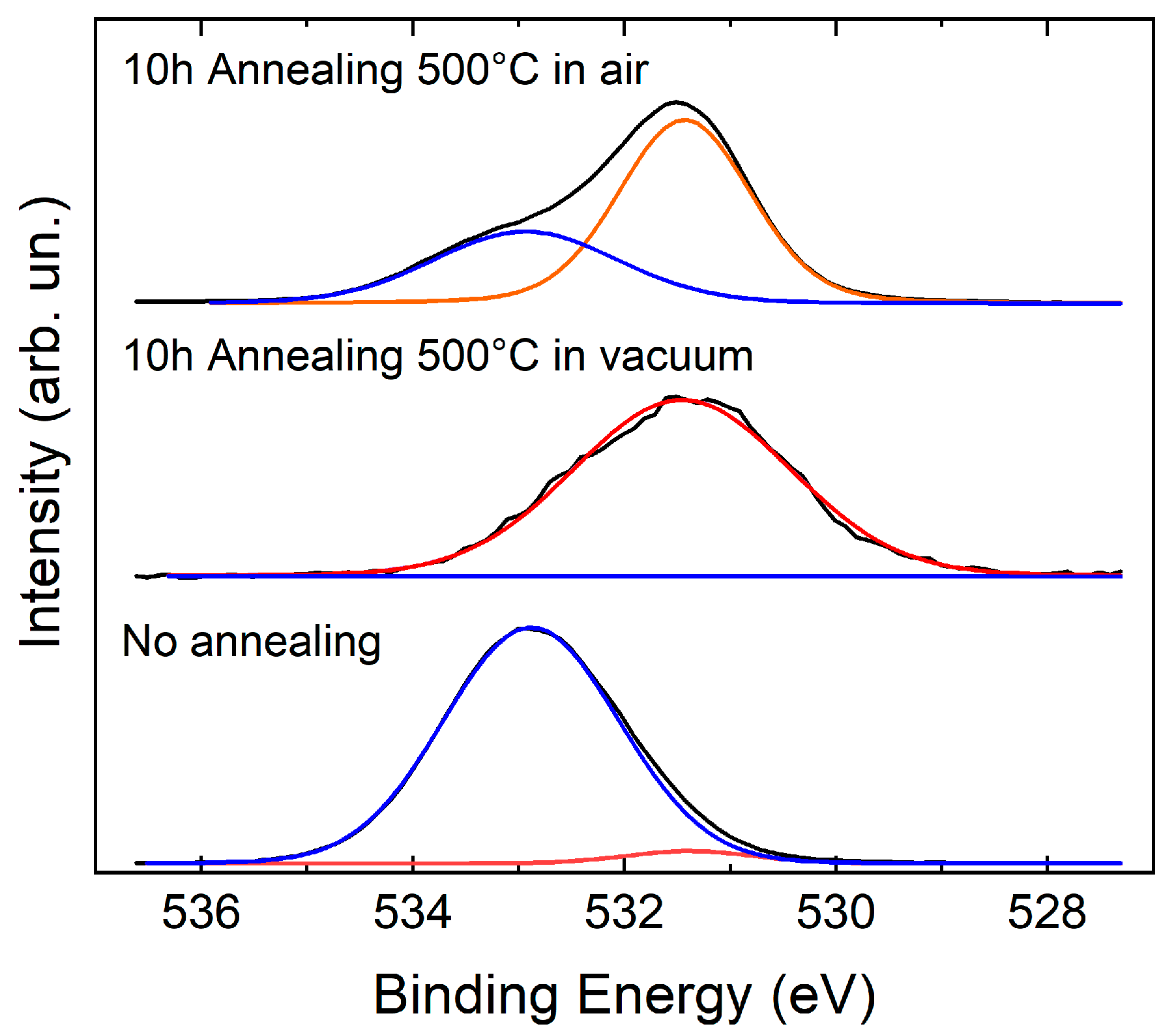
3.2. Effusion of Argon by XPS
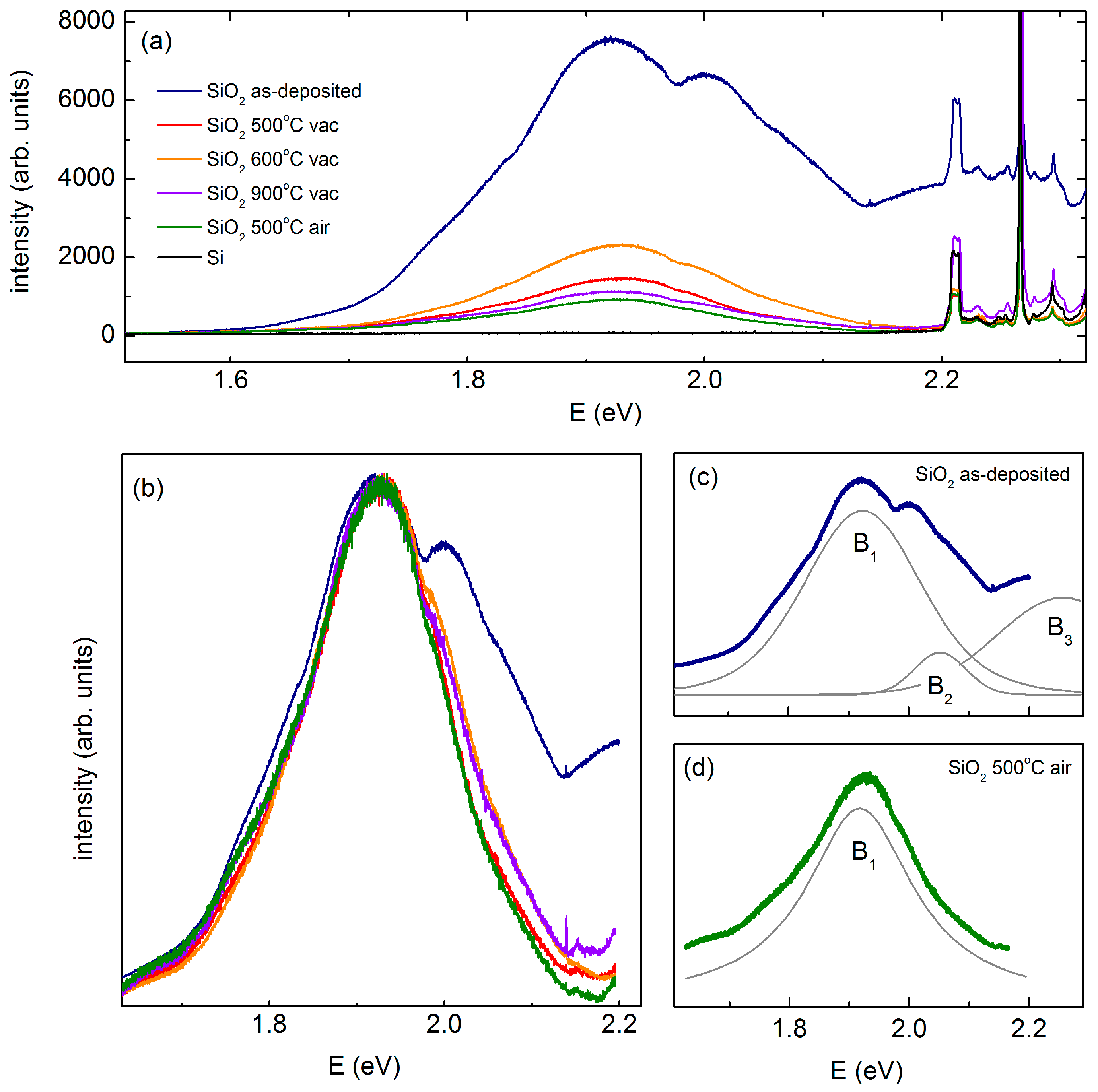
3.3. Defects in the Coating Layer by Photoluminescence
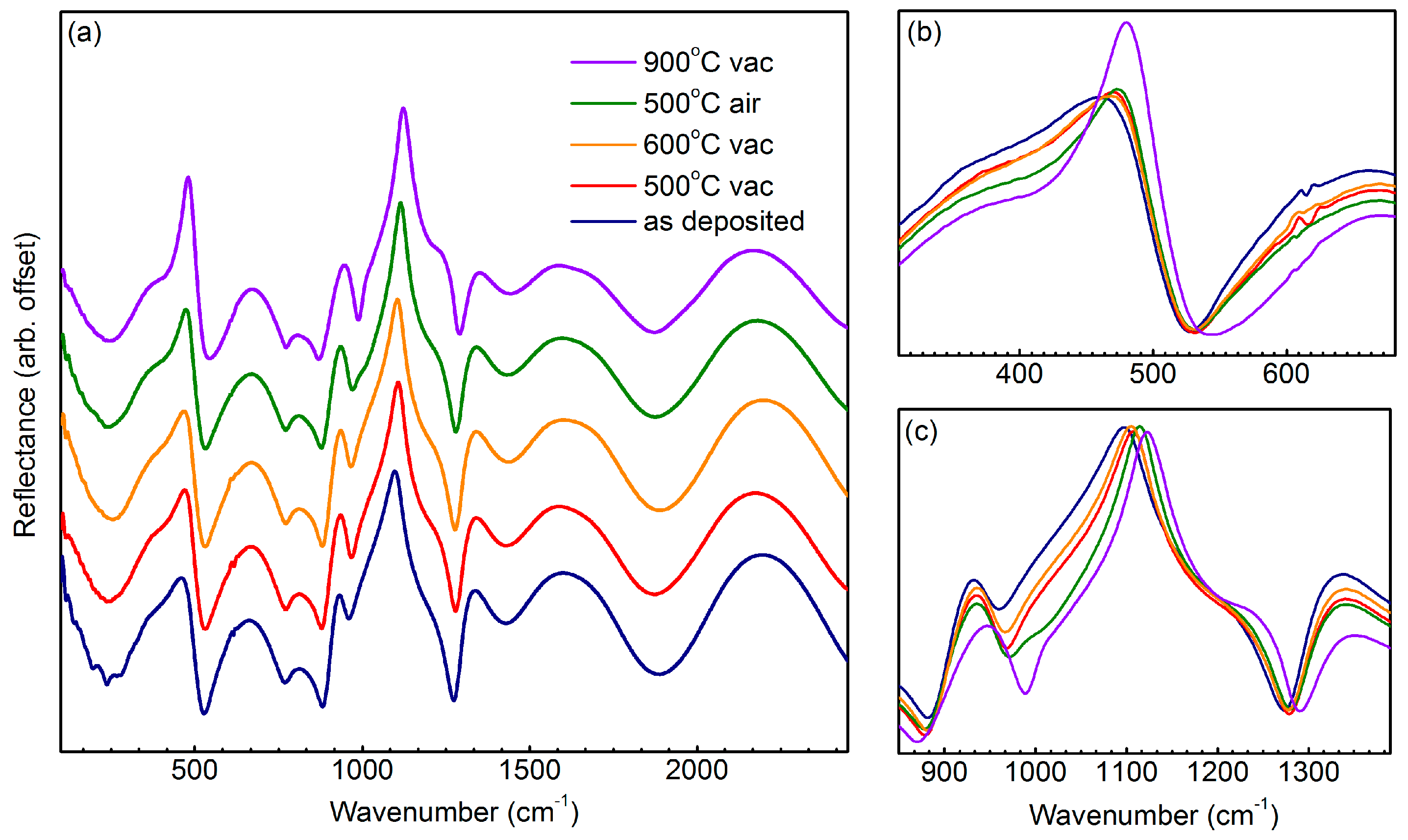
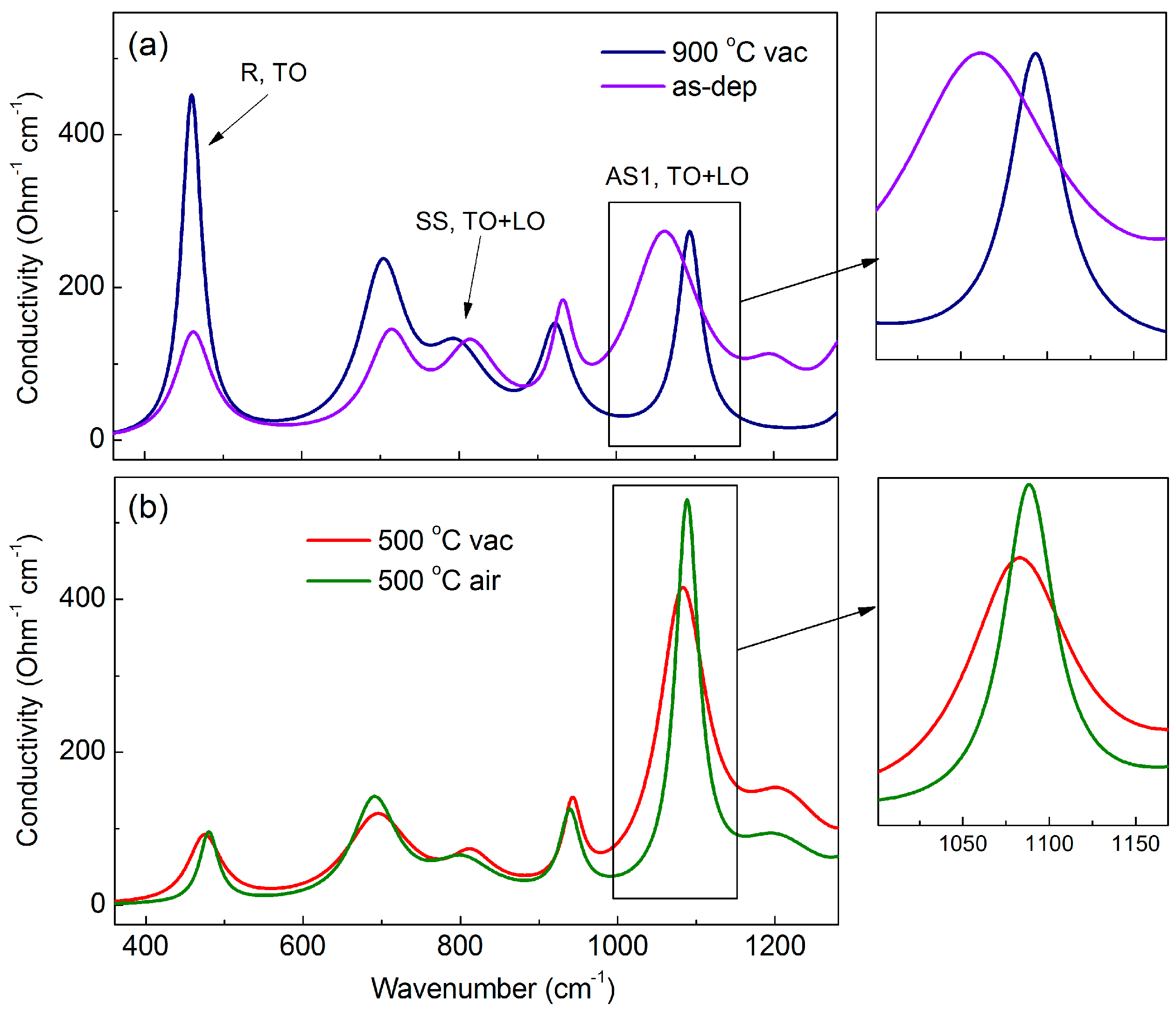
3.4. Infrared Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikari, R.X. Gravitational radiation detection with laser interferometry. Rev. Mod. Phys. 2014, 86, 121–151. [Google Scholar] [CrossRef]
- Aspelmeyer, M.; Kippenberg, T.J.; Marquardt, F. Cavity optomechanics. Rev. Mod. Phys. 2014, 86, 1391–1452. [Google Scholar] [CrossRef]
- Matei, D.G.; Legero, T.; Häfner, S.H.; Grebing, C.; Weyrich, R.; Zhang, W.; Sonderhouse, L.; Robinson, J.M.; Ye, J.; Riehle, F.; et al. 1.5 μm Lasers with Sub-10 mHz Linewidth. Phys. Rev. Lett. 2017, 118, 263202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulson, P.R. Thermal noise in mechanical experiments. Phys. Rev. D 1990, 42, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y. Internal thermal noise in the LIGO test masses: A direct approach. Phys. Rev. D 1998, 57, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.M.; Gretarsson, A.M.; Saulson, P.R.; Kittelberger, S.E.; Penn, S.D.; Startin, W.J.; Rowan, S.; Fejer, M.M.; Crooks, D.R.M.; Cagnoli, G.; et al. Thermal noise in interferometric gravitational wave detectors due to dielectric optical coatings. Class. Quantum Grav. 2002, 19, 897–917. [Google Scholar] [CrossRef]
- Aasi, J.; Abbott, B.P.; Abbott, R.; Abbott, T.; Abernathy, R.M.; Ackley, K.; Adams, C.; Adams, T.; Addesso, P.; Adhikari, R.X.; et al. Advanced LIGO. Class. Quantum Grav. 2015, 32, 074001. [Google Scholar]
- Acernese, R.; Agathos, M.; Agatsuma, K.; Aisa, D.; Allemandou, L.; Allocca, A.; Amarni, J.; Astone, P.; Balestri, G.; Ballardin, G.; et al. Advanced Virgo: A second-generation interferometric gravitational wave detector. Class. Quantum Grav. 2015, 32, 024001. [Google Scholar] [CrossRef] [Green Version]
- Aso, Y.; Michimura, Y.; Somiya, K.; Ando, M.; Miyakawa, O.; Sekiguchi, T.; Tatsumi, D.; Yamamoto, H.; The KAGRA Collaboration. Interferometer design of the KAGRA gravitational wave detector. Phys. Rev. D 2013, 88, 043007. [Google Scholar] [CrossRef] [Green Version]
- Villar, A.E.; Black, E.D.; DeSalvo, R.; Libbrecht, K.G.; Michel, C.; Morgado, N.; Pinard, L.; Pinto, I.M.; Pierro, V.; Galdi, V.; et al. Measurement of thermal noise in multilayer coatings with optimized layer thickness. Phys. Rev. D 2010, 81, 122001. [Google Scholar] [CrossRef] [Green Version]
- Pinard, L.; Michel, C.; Sassolas, B.; Balzarini, L.; Degallaix, J.; Dolique, V.; Flaminio, R.; Forest, D.; Granata, M.; Lagrange, B.; et al. Mirrors used in the LIGO interferometers for first detection of gravitational waves. Appl. Opt. 2017, 56, C11–C15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degallaix, J.; Michel, C.; Sassolas, B.; Allocca, A.; Cagnoli, G.; Balzarini, L.; Dolique, V.; Flaminio, R.; Forest, D.; Granata, M.; et al. Large and extremely low loss: The unique challenges of gravitational wave mirrors. J. Opt. Soc. Am. A 2019, 36, C85–C94. [Google Scholar] [CrossRef] [PubMed]
- Granata, M.; Amato, A.; Balzarini, L.; Canepa, M.; Degallaix, J.; Forest, D.; Dolique, V.; Mereni, L.; Michel, C.; Pinard, L.; et al. Amorphous optical coatings of presentgravitational-waveinterferometers. Class. Quantum Grav. 2020, 37, 095004. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.M.; Abernathy, M.R.; Becerra-Toledo, A.E.; Armandula, H.; Black, E.; Dooley, K.; Eichenfield, M.; Nwabugwu, C.; Villar, A.; Crooks, D.R.M.; et al. Titania-doped tantala/silica coatings for gravitational-wave detection. Class. Quantum Grav. 2007, 24, 405–415. [Google Scholar] [CrossRef]
- Amato, A.; Terreni, S.; Dolique, V.; Forest, D.; Gemme, G.; Granata, M.; Mereni, L.; Michel, C.; Pinard, L.; Sassolas, B.; et al. Optical properties of high-quality oxide coating materials used in gravitational-wave advanced detectors. J. Phys. Mater. 2019, 2, 035004. [Google Scholar] [CrossRef]
- Granata, M.; Amato, A.; Cagnoli, G.; Coulon, M.; Degallaix, J.; Forest, D.; Mereni, L.; Michel, C.; Pinard, L.; Sassolas, B.; et al. Progress in the measurement and reduction of thermal noise in optical coatings for gravitational-wave detectors. Appl. Opt. 2020, 59, A229–A235. [Google Scholar] [CrossRef]
- Hild, S.; Abernathy, M.; Acernese, F.; Amaro-Seoane, P.; Andersson, N.; Arun, K.; Barone, F.; Barr, B.; Barsuglia, M.; Beker, M.; et al. Sensitivity studies for third-generation gravitational wave observatories. Class. Quantum Grav. 2011, 28, 094013. [Google Scholar] [CrossRef]
- Abernathy, M.; Acernese, F.; Ajith, P.; Allen, B.; Amaro-Seoane, P.; Andersson, N.; Aoudia, S.; Astone, P.; Krishnan, B.; Barack, L.; et al. Einstein Telescopec onceptual Design Study, ET Technical Note ET-0106C-10. 2011. Available online: https://tds.ego-gw.it/ql/?c=7954 (accessed on 8 July 2022).
- Abbott, B.P.; Abbott, R.; Abbott, T.D.; Abernathy, M.R.; Ackley, K.; Adams, C.; Addesso, P.; Adhikari, R.X.; Adya, V.B.; Affeldt, C.; et al. The LIGO Scientific Collaboration. Exploring the Sensitivity of Next Generation Gravitational Wave Detectors. Class. Quantum Grav. 2017, 34, 044001. [Google Scholar] [CrossRef] [Green Version]
- Amato, A.; Cagnoli, G.; Canepa, M.; Coillet, E.; Degallaix, J.; Dolique, V.; Forest, D.; Granata, M.; Martinez, V.; Michel, C.; et al. High-reflection coatings for gravitational-wave detectors: State of the art and future developments. J. Phys. Conf. Ser. 2018, 957, 012006. [Google Scholar] [CrossRef] [Green Version]
- Bassiri, R.; Liou, F.; Abernathy, M.R.; Lin, A.C.; Kim, N.; Mehta, A.; Shyam, B.; Byer, R.L.; Gustafson, E.K.; Hart, M.; et al. Order within disorder: The atomic structure of ion-beam sputtered amorphous tantala (a-Ta2O5). APL Mater. 2015, 3, 036103. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.J.; Bassiri, R.; Borisenko, K.B.; Véron, M.; Rauch, E.F.; Martin, I.W.; Rowan, S.; Fejer, M.M.; MacLaren, I. Medium range structural order in amorphous tantala spatially resolved with changes to atomic structure by thermal annealing. J. Non-Cryst. Solids 2016, 438, 10–17. [Google Scholar] [CrossRef]
- Prasai, K.; Jiang, J.; Mishkin, A.; Shyam, B.; Angelova, S.; Birney, R.; Drabold, D.A.; Fazio, M.; Gustafson, E.K.; Harry, G.; et al. High Precision Detection of Change in Intermediate Range Order of AmorphousZirconia-Doped Tantala Thin Films Due to Annealing. Phys. Rev. Lett. 2019, 123, 045501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolone, A.; Placidi, E.; Stellino, E.; Betti, M.G.; Majorana, E.; Mariani, C.; Nucara, A.; Palumbo, O.; Postorino, P.; Rago, I.; et al. Effects of the annealing of amorphous Ta2O5 coatings produced by ion beam sputtering concerning the effusion of argon and the chemical composition. J Non-Cryst. Solids 2021, 557, 120651. [Google Scholar] [CrossRef]
- Fazio, M.A.; Vajente, G.; Ananyeva, A.; Markosyan, A.; Bassiri, R.; Fejer, M.M.; Menoni, C.S. Structure and morphology of low mechanical loss TiO2-doped Ta2O5. Opt. Mater. Express 2020, 10, 1687–1703. [Google Scholar] [CrossRef]
- Cummings, R.B.; Bassiri, R.; Martin, I.W.; MacLaren, I. Argon bubble formation in tantalum oxide-based films for gravitational wave interferometer mirrors. Opt. Mater. Express 2021, 11, 707–718. [Google Scholar] [CrossRef]
- Harthcock, C.; Qiu, S.R.; Negres, R.A.; Hammons, J.A.; Voisin, T.; Guss, G.; Martin, A.A.; Stolz, C.J.; Menor, M.G.; Bhowmik, G.; et al. The impact of nano-bubbles on the laser performance of hafnia films deposited by oxygen assisted ion beam sputtering method. Appl. Phys. Lett. 2019, 115, 251902. [Google Scholar] [CrossRef]
- Amato, A.; Cagnoli, G.; Granata, M.; Sassolas, B.; Degallaix, J.; Forest, D.; Michel, C.; Pinard, L.; Demos, N.; Gras, S.; et al. Optical and mechanical properties of ion-beam-sputtered Nb2O5 and TiO2−Nb2O5 thin films for gravitational-wave interferometers and an improved measurement of coating thermal noise in Advanced LIGO. Phys. Rev D 2021, 103, 072001. [Google Scholar] [CrossRef]
- Cisneros, J.I. Optical characterization of dielectric and semiconductor thin films by use of transmission data. Appl. Opt. 1998, 37, 5262–5270. [Google Scholar] [CrossRef]
- Perkins, W.G.; Begeal, D.R. Diffusion and Permeation of He, Ne, Ar, Kr, and D2 through Silicon Oxide Thin Films. J. Chem. Phys. 1971, 54, 1683–1694. [Google Scholar] [CrossRef]
- Carroll, M.R.; Stolper, E.M. Argon solubility and diffusion in silica glass: Implications for the solution behavior of molecular gases. Geochim. Cosmochim. Acta 1991, 55, 211–225. [Google Scholar] [CrossRef]
- Hara, S.; Caravella, A.; Ishitsuka, M.; Suda, H.; Mukaida, M.; Haraya, K.; Shimano, E.; Tsuji, T. Hydrogen diffusion coefficient and mobility in palladium as a function of equilibrium pressure evaluated by permeation measurement. J. Membr. Sci. 2012, 421–422, 355–360. [Google Scholar] [CrossRef]
- Mayo, S.; Evans, W.H. Development of Hydrogen and Hydroxyl Contamination in Thin Silicon Dioxide Thermal Films; Final Report National Bureau of Standards; National Engineering Lab.: Washington, DC, USA, 1979; Available online: https://ui.adsabs.harvard.edu/abs/1979nbs..reptQ....M (accessed on 8 July 2022).
- Sakurai, Y. Photoluminescence band near 2.2 eV in γ-irradiated oxygen-deficient silica glass. J. Non-Cryst. Solids. 2004, 342, 54–58. [Google Scholar] [CrossRef]
- Sakurai, Y. Oxygen-related red photoluminescence bands in silica glasses. J. Non-Cryst. Solids. 2003, 316, 389–392. [Google Scholar] [CrossRef]
- Skuja, L. The origin of the intrinsic 1.9 eV luminescence band in glassy SiO2. J. Non-Cryst. Solids 1994, 179, 51–69. [Google Scholar] [CrossRef]
- Palik, E.D. (Ed.) Handbook of Optical Constants of Solids; Academic Press: Cambridge, MA, USA, 1998; Volume 3. [Google Scholar]
- Gunde, M.K. Vibrational modes in amorphous silicon dioxide. Phys. B Cond. Mat. 2000, 292, 286–295. [Google Scholar] [CrossRef]
- Hirose, T.; Saito, K.; Ikushima, A.J. Structural relaxation in sputter-deposited silica glass. J. Non-Cryst. Solids 2006, 352, 2198–2203. [Google Scholar] [CrossRef]
- Granata, M.; Coillet, E.; Martinez, V.; Dolique, V.; Amato, A.; Canepa, M.; Margueritat, J.; Martinet, C.; Mermet, A.; Michel, C.; et al. Correlated evolution of structure and mechanical loss of a sputtered silica film. Phys. Rev. Mater. 2018, 2, 053607. [Google Scholar] [CrossRef] [Green Version]
- Tomozeiu, N. Silicon Oxide (SiOx, 0 < x < 2):a Challenging Material for Optoelectronics. In Optoelectronics: Materials and Techniques; Predeep, P., Ed.; IntechOpen: London, UK, 2011; pp. 1–46. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolone, A.; Placidi, E.; Stellino, E.; Betti, M.G.; Majorana, E.; Mariani, C.; Nucara, A.; Palumbo, O.; Postorino, P.; Sbroscia, M.; et al. Argon and Other Defects in Amorphous SiO2 Coatings for Gravitational-Wave Detectors. Coatings 2022, 12, 1001. https://doi.org/10.3390/coatings12071001
Paolone A, Placidi E, Stellino E, Betti MG, Majorana E, Mariani C, Nucara A, Palumbo O, Postorino P, Sbroscia M, et al. Argon and Other Defects in Amorphous SiO2 Coatings for Gravitational-Wave Detectors. Coatings. 2022; 12(7):1001. https://doi.org/10.3390/coatings12071001
Chicago/Turabian StylePaolone, Annalisa, Ernesto Placidi, Elena Stellino, Maria Grazia Betti, Ettore Majorana, Carlo Mariani, Alessandro Nucara, Oriele Palumbo, Paolo Postorino, Marco Sbroscia, and et al. 2022. "Argon and Other Defects in Amorphous SiO2 Coatings for Gravitational-Wave Detectors" Coatings 12, no. 7: 1001. https://doi.org/10.3390/coatings12071001
APA StylePaolone, A., Placidi, E., Stellino, E., Betti, M. G., Majorana, E., Mariani, C., Nucara, A., Palumbo, O., Postorino, P., Sbroscia, M., Trequattrini, F., Granata, M., Hofman, D., Michel, C., Pinard, L., Lemaitre, A., Shcheblanov, N., Cagnoli, G., & Ricci, F. (2022). Argon and Other Defects in Amorphous SiO2 Coatings for Gravitational-Wave Detectors. Coatings, 12(7), 1001. https://doi.org/10.3390/coatings12071001









