Vapor Barrier Properties of Cold Plasma Treated Corn Starch Films
Abstract
:1. Introduction
2. Material and Methods
2.1. Films Extrusion
2.2. Plasma Modification
2.3. Contact Angle Measurements
2.4. Water Vapor Permeation Analysis
2.5. Water Absorption
2.6. SEM Imaging
2.7. AFM Imaging and Data Processing
2.7.1. AFM Measurements
2.7.2. Height Distribution Analysis
2.7.3. Power Spectrum Density and Fractal Parameters
2.7.4. Surface Uniformity Analysis
3. Results and Discussion
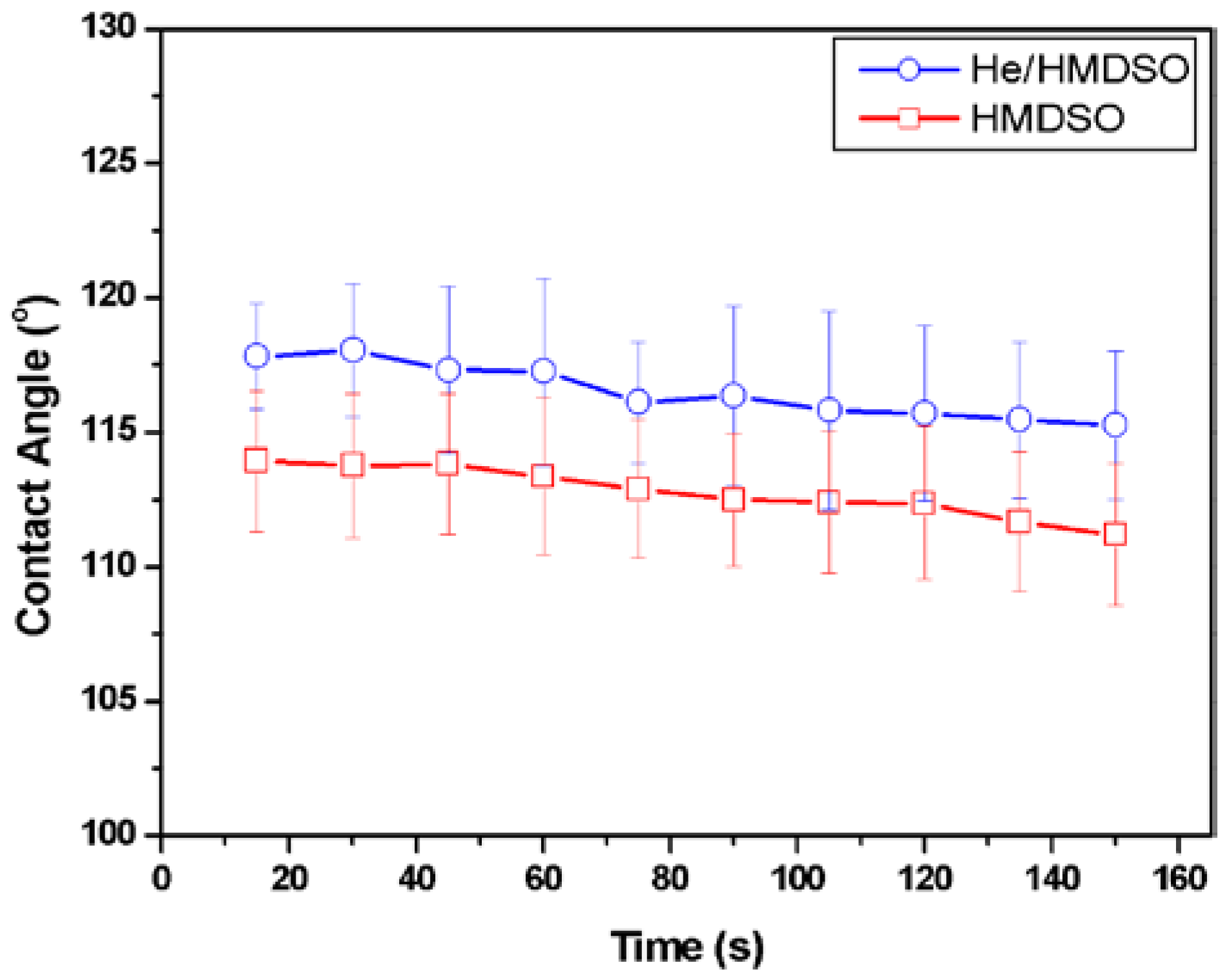
3.1. Contact Angle Measurements
3.2. Water Vapor Permeation Analysis
3.3. Water Absorption
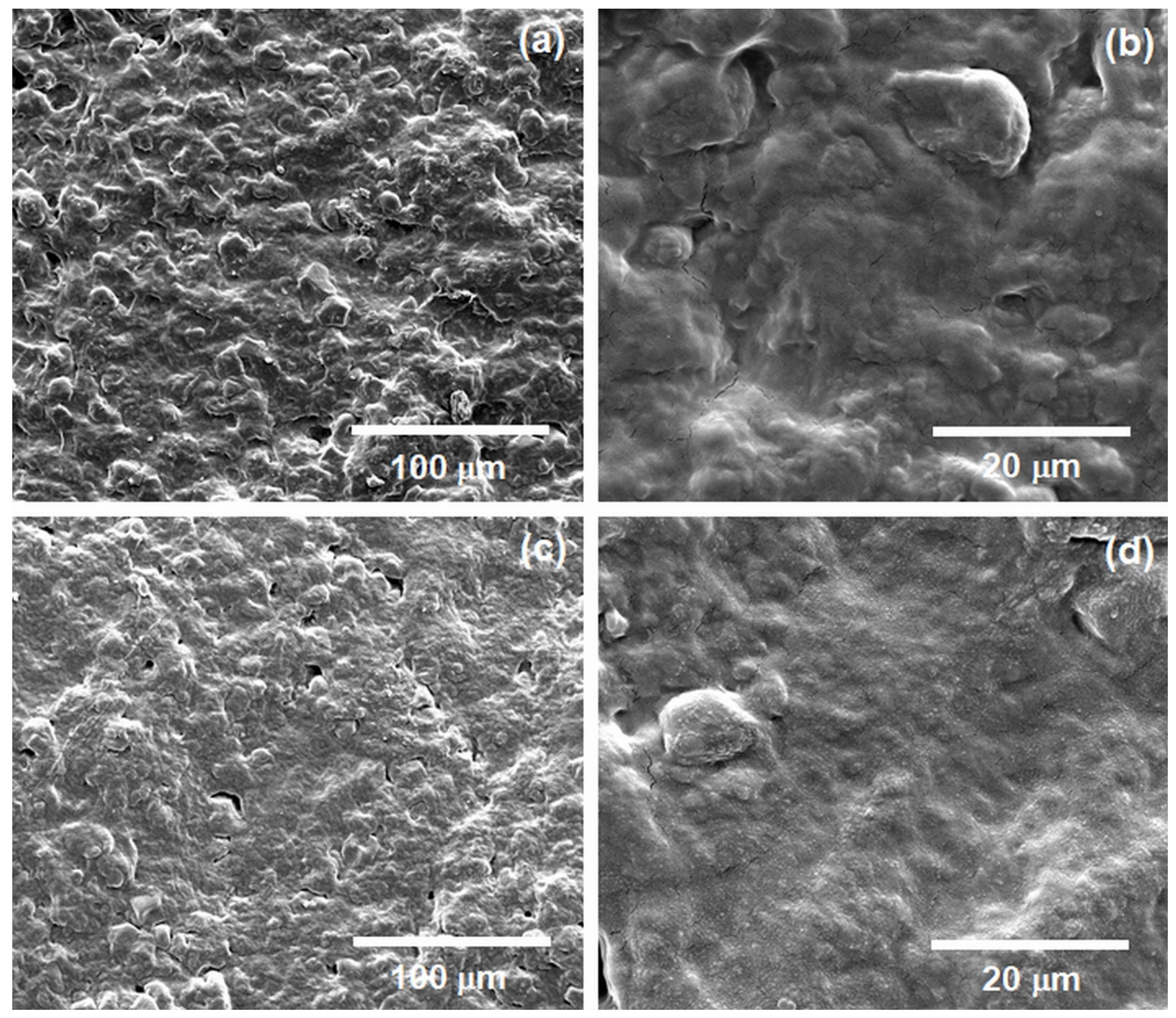
3.4. SEM Analysis
3.5. AFM Measurements
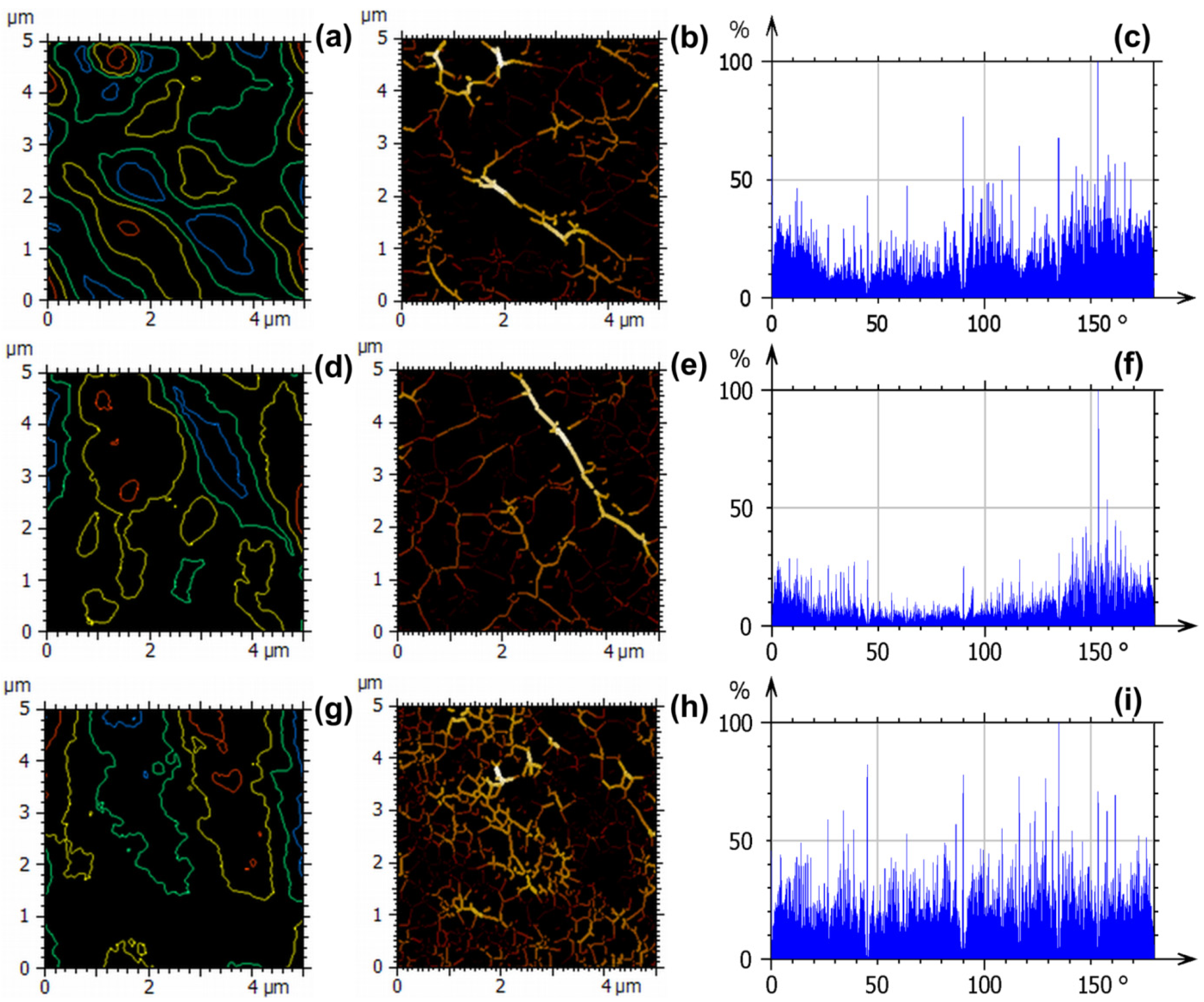
3.5.1. Evaluation of Morphology
3.5.2. Evaluation of Nanotexture
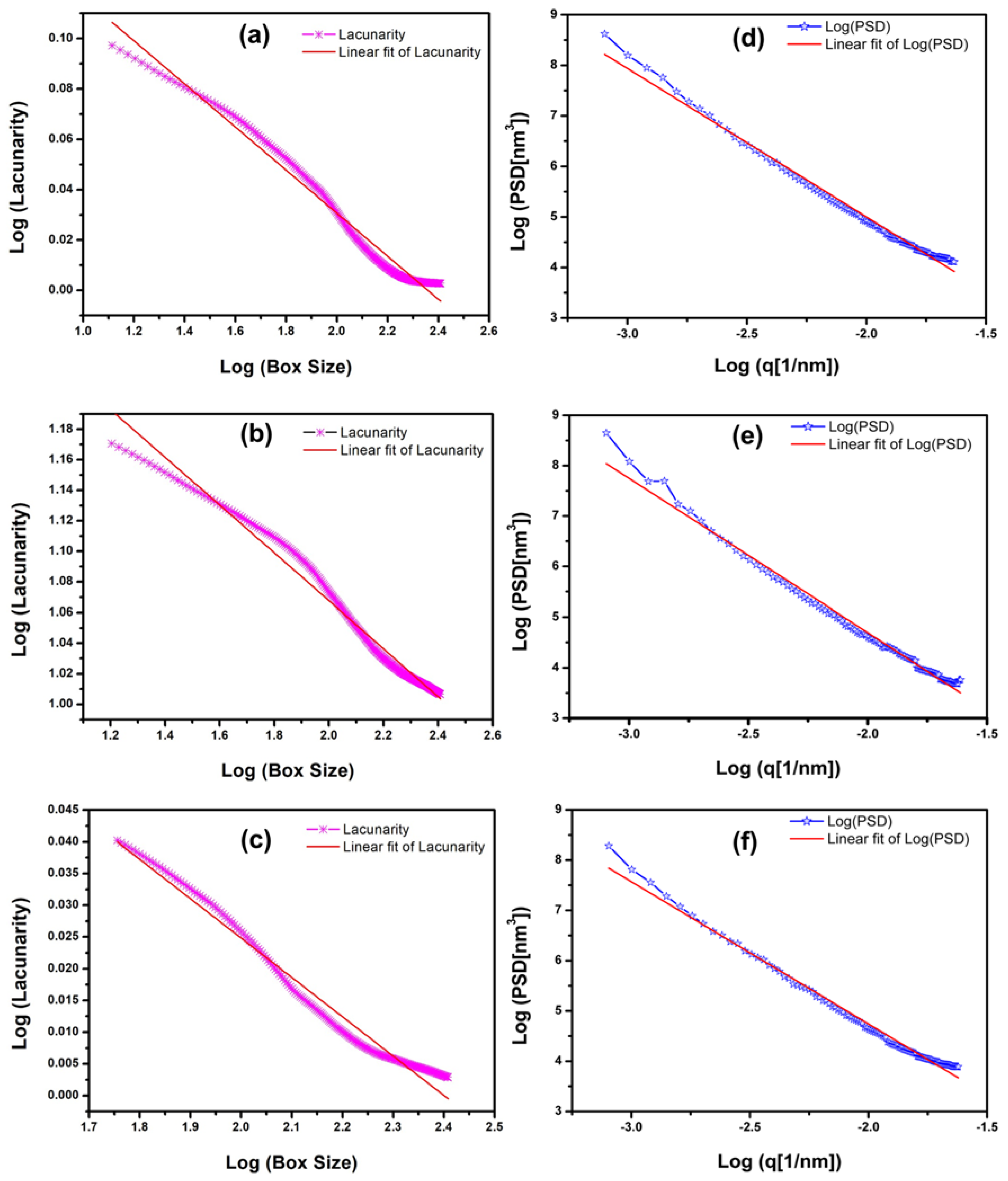
3.5.3. Advanced Fractal and Power Spectral Density Analysis of the Nanotexture
3.5.4. Uniformity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mali, S.; Grossmann, M.V.E.; Yamashita, F. Filmes de Amido: Produção, Propriedades e Potencial de Utilização. Semin. Ciênc. Agrárias 2010, 31, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.; Barros, M.M.; da Costa Pereira, P.S.; Bastos, D.C. Use of Manufacture Residue of Fluidized-Bed Catalyst-Cracking Catalyzers as Flame Retardant in Recycled High Density Polyethylene. J. Mater. Res. Technol. 2019, 8, 2386–2394. [Google Scholar] [CrossRef]
- Gerardo, C.F.; França, S.C.A.; Santos, S.F.; Bastos, D.C.A. A Study of Recycled High-Density Polyethylene with Mica Addition: Influence of Mica Particle Size on Wetting Behavior, Morphological, Physical, and Chemical Properties. Int. J. Dev. Res. 2020, 10, 37223–37228. [Google Scholar] [CrossRef]
- Marengo, V.A.; Vercelheze, A.E.S.; Mali, S. Compósitos Biodegradáveis de Amido de Mandioca e Resíduos Da Agroindústria. Quim. Nova 2013, 36, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.C.R.; Andrade, C.T. Investigação Dos Processos de Gelatinização e Extrusão de Amido de Milho. Polímeros 2000, 10, 24–30. [Google Scholar] [CrossRef]
- Bastos, D.C.; Santos, A.E.F.; da Fonseca, M.D.; Simão, R.A. Inducing Surface Hydrophobization on Cornstarch Film by SF6 and HMDSO Plasma Treatment. Carbohydr. Polym. 2013, 91, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.E.F.; Bastos, D.C.; da Silva, M.L.V.J.; Thiré, R.M.S.M.; Simão, R.A. Chemical Analysis of a Cornstarch Film Surface Modified by SF6 Plasma Treatment. Carbohydr. Polym. 2012, 87, 2217–2222. [Google Scholar] [CrossRef] [Green Version]
- Thiré, R.M.S.M.; Simão, R.A.; Araújo, P.J.G.; Achete, C.A.; Andrade, C.T. Redução Da Hidrofilicidade de Filmes Biodegradáveis à Base de Amido Por Meio de Polimerização Por Plasma. Polímeros 2004, 14, 57–62. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, H.; Guo, P.; Dong, H. Effects of Extrusion and Glycerol Content on Properties of Oxidized and Acetylated Corn Starch-Based Films. Carbohydr. Polym. 2012, 87, 707–712. [Google Scholar] [CrossRef]
- Hulleman, S.H.; Kalisvaart, M.; Janssen, F.H.; Feil, H.; Vliegenthart, J.F. Origins of B-Type Crystallinity in Glycerol-Plasticised, Compression-Moulded Potato Starches. Carbohydr. Polym. 1999, 39, 351–360. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Qiu, Y. Aging of Surface Properties of Ultra High Modulus Polyethylene Fibers Treated with He/O2 Atmospheric Pressure Plasma Jet. Surf. Coatings Technol. 2008, 202, 2670–2676. [Google Scholar] [CrossRef]
- Zanini, S.; Massini, P.; Mietta, M.; Grimoldi, E.; Riccardi, C. Plasma Treatments of PET Meshes for Fuel–Water Separation Applications. J. Colloid Interface Sci. 2008, 322, 566–571. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; van der Mullen, J. Gas Discharge Plasmas and Their Applications. Spectrochim. Acta Part B Spectrosc. 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Chan, C.-M.; Ko, T.-M.; Hiraoka, H. Polymer Surface Modification by Plasmas and Photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Choudhury, A.J.; Barve, S.A.; Chutia, J.; Pal, A.R.; Kishore, R.; Jagannath; Pande, M.; Patil, D.S. RF-PACVD of Water Repellent and Protective HMDSO Coatings on Bell Metal Surfaces: Correlation between Discharge Parameters and Film Properties. Appl. Surf. Sci. 2011, 257, 8469–8477. [Google Scholar] [CrossRef]
- García-Luis, A.; Corengia, P.; González-Santamaría, D.; Brizuela, M.; Braceras, I.; Briz, N.; Azpiroz, P.; Bellido-González, V.; Powell, S. Synthesis and Characterization of Plasma-Polymerized HMDSO Films Using an Ion Gun Inverse Magnetron Source. Plasma Process. Polym. 2007, 4, S766–S770. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Leys, C.; Schacht, E. Organic–Inorganic Behaviour of HMDSO Films Plasma-Polymerized at Atmospheric Pressure. Surf. Coatings Technol. 2009, 203, 1366–1372. [Google Scholar] [CrossRef]
- de Albuquerque, M.D.F.; Bastos, D.C.; Simão, R.A. Surface Modification of Starch Films by Plasma. Macromol. Symp. 2014, 343, 96–101. [Google Scholar] [CrossRef]
- Fattahi, M.; Nezafat, N.B.; Ţălu, Ş.; Solaymani, S.; Ghoranneviss, M.; Elahi, S.M.; Shafiekhani, A.; Rezaee, S. Topographic Characterization of Zirconia-Based Ceramics by Atomic Force Microscopy: A Case Study on Different Laser Irradiations. J. Alloys Compd. 2020, 831, 154763. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Achour, A.; Solaymani, S.; Nikpasand, K.; Dalouji, V.; Sari, A.; Rezaee, S.; Nezafat, N.B. Micromorphology Analysis of TiO2 Thin Films by Atomic Force Microscopy Images: The Influence of Postannealing. Microsc. Res. Technol. 2020, 83, 457–463. [Google Scholar] [CrossRef]
- Matos, R.S.; Ramos, G.Q.; da Fonseca Filho, H.D.; Ţălu, Ş. Advanced Micromorphology Study of Microbial Films Grown on Kefir Loaded with Açaí extract. Micron 2020, 137, 102912. [Google Scholar] [CrossRef] [PubMed]
- Rashid, D.; Stach, S.; Ţălu, Ş.; Sobola, D.; Méndez-Albores, A.; Córdova, G.T.; Grmela, L. Stereometric Analysis of Effects of Heat Stressing on Micromorphology of Si Single Crystals. Silicon 2019, 11, 2945–2959. [Google Scholar] [CrossRef]
- Barcelay, Y.R.; Moreira, J.A.G.; Almeida, A.J.M.; Brito, W.R.; Matos, R.S.; da Fonseca Filho, H.D. Nanoscale Stereometric Evaluation of BiZn0.5Ti0.5O3 Thin Films Grown by RF Magnetron Sputtering. Mater. Lett. 2020, 279, 128477. [Google Scholar] [CrossRef]
- Matos, R.S.; Pinto, E.P.; Ramos, G.Q.; de Albuquerque, F.M.D.; Filho, F.H.D. Stereometric Characterization of Kefir Microbial Films Associated with Maytenus Rigida Extract. Microsc. Res. Technol. 2020, 83, 1401–1410. [Google Scholar] [CrossRef]
- Arman, A.; Ţălu, Ş.; Luna, C.; Ahmadpourian, A.; Naseri, M.; Molamohammadi, M. Micromorphology Characterization of Copper Thin Films by AFM and Fractal Analysis. J. Mater. Sci. Mater. Electron. 2015, 26, 9630–9639. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Sahoo, N.K.; Thakur, S.; Tokas, R.B. Characterization of Microroughness Parameters in Gadolinium Oxide Thin Films: A Study Based on Extended Power Spectral Density Analyses. Appl. Surf. Sci. 2005, 252, 1608–1619. [Google Scholar] [CrossRef]
- Ţǎlu, Ş.; Stach, S.; Valedbagi, S.; Elahi, S.M.; Bavadi, R. Surface Morphology of Titanium Nitride Thin Films Synthesized by DC Reactive Magnetron Sputtering. Mater. Sci. 2015, 33, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Ţălu, Ş.; Bramowicz, M.; Kulesza, S.; Shafiekhani, A.; Ghaderi, A.; Mashayekhi, F.; Solaymani, S. Microstructure and Tribological Properties of FeNPs@a-C:H Films by Micromorphology Analysis and Fractal Geometry. Ind. Eng. Chem. Res. 2015, 54, 8212–8218. [Google Scholar] [CrossRef]
- Silva, M.R.P.; Matos, R.S.; Estevam, C.S.; Santos, S.B.; da Silva, F.M.A.; Sousa, I.G.P.P.; Filho, F.H.D.; Almeida, L.E. Structural Evaluation of Polymeric Microbial Films Grown on Kefir Loaded with Maytenus Rigida Extract. Microsc. Res. Technol. 2021, 84, 627–638. [Google Scholar] [CrossRef]
- Shakoury, R.; Rezaee, S.; Mwema, F.; Luna, C.; Ghosh, K.; Jurečka, S.; Ţălu, Ş.; Arman, A.; Grayeli Korpi, A. Multifractal and Optical Bandgap Characterization of Ta2O5 Thin Films Deposited by Electron Gun Method. Opt. Quantum Electron. 2020, 52, 95. [Google Scholar] [CrossRef]
- Yadav, R.P.; Kumar, M.; Mittal, A.K.; Pandey, A.C. Fractal and Multifractal Characteristics of Swift Heavy Ion Induced Self-Affine Nanostructured BaF2 Thin Film Surfaces. Chaos Interdiscip. J. Nonlinear Sci. 2015, 25, 083115. [Google Scholar] [CrossRef] [PubMed]
- Sapota, W.; Szczepanik, P.; Stach, S.; Wróbel, Z. Fractal and Multifractal Analyses of the Porosity Degree of Ceramics Used in Biomedicine. Adv. Sci. Eng. Med. 2020, 12, 450–456. [Google Scholar] [CrossRef]
- Almeida, P.A.; Pinto, E.P.; Filho, H.D.F.; Matos, R.S. Distribution of Microorganisms on Surface of Kefir Biofilms Associated with Açaí Extract Distribution of Microorganisms on Surface of Kefir Biofilms Associated with Açaí Extract. Sci. Amaz. 2019, 8, C10–C18. [Google Scholar]
- Ferraro, M.A.N.; Pinto, E.P.; Matos, R.S. Study of the Superficial Distribution of Microorganisms in Kefir Biofilms Prepared with Cupuaçu Juice. J. Bioenergy Food Sci. 2020, 7, 2732019. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Matos, R.S.; Campelo, P.H.; Sanches, E.A.; Filho, F.H.D. Evaluation of the Nanoscale Surface Applied to Biodegradable Nanoparticles Containing Allium Sativum Essential Oil. Mater. Lett. 2020, 275, 128111. [Google Scholar] [CrossRef]
- Pinto, E.; Tavares, W.; Matos, R.; Ferreira, A.; Menezes, R.; Costa, M.; Souza, T.; Ferreira, I.; Sousa, F.; Zamora, R. Influence of Low and High Glycerol Concentrations on Wettability and Flexibility of Chitosan Biofilms. Quim. Nova 2018, 41, 1109–1116. [Google Scholar] [CrossRef]
- Corradini, E.; Lotti, C.; de Medeiros, E.S.; Carvalho, A.J.F.; Curvelo, A.A.S.; Mattoso, L.H.C. Estudo Comparativo de Amidos Termoplásticos Derivados Do Milho Com Diferentes Teores de Amilose. Polímeros 2005, 15, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Blateyron, F. Characterisation of Areal Surface Texture; Leach, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642364, ISBN 978-3-642-36457-0. [Google Scholar]
- Franco, L.A.; Sinatora, A. 3D Surface Parameters (ISO 25178-2): Actual Meaning of Spk and Its Relationship to Vmp. Precis. Eng. 2015, 40, 106–111. [Google Scholar] [CrossRef]
- Jacobs, T.D.B.; Junge, T.; Pastewka, L. Quantitative Characterization of Surface Topography Using Spectral Analysis. Surf. Topogr. Metrol. Prop. 2017, 5, 013001. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Salcedo, M.O.C.; Zamora, R.R.M.; Carvalho, J.C.T. Study Fractal Leaf Surface of the Plant Species Copaifera Sp. Using the Microscope Atomic-Force-AFM. Revista ECIPerú 2016, 13, 10–16. [Google Scholar] [CrossRef]
- Heitor, R.; De Melo, C.; De Melo, R.H.C.; Conci, A. Succolarity: Defining a Method to Calculate This Fractal Measure. In Proceedings of the 15th International Conference on Systems, Signals and Image Processing, Bratislava, Slovakia, 25–28 June 2008. [Google Scholar] [CrossRef]
- Nosonovsky, M. Entropy in Tribology: In the Search for Applications. Entropy 2010, 12, 1345–1390. [Google Scholar] [CrossRef]
- Woodcock, C.E.; Strahler, A.H. The Factor of Scale in Remote Sensing. Remote Sens. Environ. 1987, 21, 311–332. [Google Scholar] [CrossRef]
- Aja-Fernandez, S.; Estepar, R.S.J.; Alberola-Lopez, C.; Westin, C.-F. Image Quality Assessment Based on Local Variance. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 15 December 2016; pp. 4815–4818. [Google Scholar]
- Drăguţ, L.; Eisank, C.; Strasser, T. Local Variance for Multi-Scale Analysis in Geomorphometry. Geomorphology 2011, 130, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, Mechanical and Optical Properties of Plasticized Yam Starch Films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, W.; Martínez-Bustos, F.; Jiménez-Arévalo, O.; González-Núñez, R.; Galicia-García, T. Functional Properties of Extruded and Tubular Films of Sorghum Starch-Based Glycerol and Yucca Schidigera Extract. Ind. Crops Prod. 2013, 44, 405–412. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch–Gelatin Edible Films: Water Vapor Permeability and Mechanical Properties as Affected by Plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Lipid Addition to Improve Barrier Properties of Edible Starch-Based Films and Coatings. J. Food Sci. 2000, 65, 941–944. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Grossmann, M.V.E.; Mali, S.; Bello-Perez, L.A.; Garcia, M.A.; Zamudio-Flores, P.B. Effects of Production Process and Plasticizers on Stability of Films and Sheets of Oat Starch. Mater. Sci. Eng. C 2009, 29, 492–498. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water Vapor Permeability of Edible Starch Based Films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect of Glycerol and Amylose Enrichment on Cassava Starch Film Properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Yew, G.H.; Yusof, M.A.M.; Ishak, M.Z.A.; Ishiaku, U.S. Water Absorption and Enzymatic Degradation of Poly(Lactic Acid)/Rice Starch Composites. Polym. Degrad. Stab. 2005, 90, 488–500. [Google Scholar] [CrossRef]
- Preechawong, D.; Peesan, M.; Supaphol, P.; Rujiravanit, R. Characterization of Starch/Poly(ε-Caprolactone) Hybrid Foams. Polym. Test. 2004, 23, 651–657. [Google Scholar] [CrossRef]
- Matsuda, D.K.M.; Verceheze, A.E.S.; Carvalho, G.M.; Yamashita, F.; Mali, S. Baked Foams of Cassava Starch and Organically Modified Nanoclays. Ind. Crops Prod. 2013, 44, 705–711. [Google Scholar] [CrossRef]
- Shimazu, A.A.; Mali, S.; Grossmann, M.V.E. Efeitos Plastificante e Antiplastificante Do Glicerol e Do Sorbitol Em Filmes Biodegradáveis de Amido de Mandioca. Semin. Ciênc. Agrárias 2007, 28, 79. [Google Scholar] [CrossRef] [Green Version]
- Mendes, J.F.; Paschoalin, R.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable Polymer Blends Based on Corn Starch and Thermoplastic Chitosan Processed by Extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Kuutti, L.; Peltonen, J.; Myllärinen, P.; Teleman, O.; Forssell, P. AFM in Studies of Thermoplastic Starches during Ageing. Carbohydr. Polym. 1998, 37, 7–12. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Marx, D.B.; Hanna, M.A. Effects of Extrusion Temperature and Plasticizers on the Physical and Functional Properties of Starch Films. Starch—Stärke 2008, 60, 527–538. [Google Scholar] [CrossRef]
- Thiré, R.M.S.; Simão, R.A.; Andrade, C.T. High Resolution Imaging of the Microstructure of Maize Starch Films. Carbohydr. Polym. 2003, 54, 149–158. [Google Scholar] [CrossRef]
- Rindlav-Westling, Å.; Gatenholm, P. Surface Composition and Morphology of Starch, Amylose, and Amylopectin Films. Biomacromolecules 2003, 4, 166–172. [Google Scholar] [CrossRef]
- Ito, R.M.; de Souza, C.C.; Gandarilla, A.M.D.; de Oliveira, L.M.; Brito, W.R.; Sanches, E.A.; Matos, R.S.; da Fonseca Filho, H.D. Micromorphology and Microtexture Evaluation of Poly(o-Ethoxyaniline) Films Using Atomic Force Microscopy and Fractal Analysis. J. Polym. Res. 2020, 27, 299. [Google Scholar] [CrossRef]
- Matos, R.S.; Lopes, G.A.C.; Ferreira, N.S.; Pinto, E.P.; Carvalho, J.C.T.; Figueiredo, S.S.; Oliveira, A.F.; Zamora, R.R.M. Superficial Characterization of Kefir Biofilms Associated with Açaí and Cupuaçu Extracts. Arab. J. Sci. Eng. 2018, 43, 3371–3379. [Google Scholar] [CrossRef]

| Sample | Thickness (mm) | WVTR (×10−3 g·s−1·m−2) | WVP (×10−10 g·Pa−1·s−1·m−1) |
|---|---|---|---|
| untreated | 1.30 ± 0.08 | 2.11 ± 0.38 | 8.64 ± 1.47 |
| HMDSO | 1.10 ± 0.09 | 2.45 ± 0.16 | 8.49 ± 0.12 |
| He/HMDSO | 1.41 ± 0.26 | 1.23 ± 0.67 | 9.74 ± 1.16 |
| Parameter | Unit | Untreated | HDMSO | He/HDMSO |
|---|---|---|---|---|
| Sq | [nm] | 97 ± 15 | 60 ± 13 | 37 ± 8 |
| Ssk | [-] | 0.0 ± 0.1 | −0.4 ± 0.1 | 0.1 ± 0.3 |
| Sku | [-] | 3.5 ± 0.7 | 3.7 ± 0.5 | 3.2 ± 0.3 |
| Sp | [nm] | 389 ± 15 | 238 ± 36 | 131 ± 23 |
| Sv | [nm] | 316 ± 15 | 233 ± 43 | 123 ± 33 |
| Sz | [nm] | 706 ± 0 | 471 ± 77 | 254 ± 45 |
| Sa | [nm] | 76 ± 15 | 46 ± 10 | 29 ± 6 |
| Parameter | Unit | Untreated | HDMSO | He/HDMSO |
|---|---|---|---|---|
| MFD | [nm] | 193 ± 54 | 140 ± 14 | 53 ± 6 |
| AFD | [nm] | 71 ± 18 | 47 ± 7 | 21 ± 3 |
| AFDsty | [cm/cm2] | 25,040 ± 992 | 26,883 ± 537 | 38,408 ± 1383 |
| TI * | [%] | 35 ± 12 | 49 ± 21 | 48 ± 15 |
| Sal * | [nm] | 0.47 ± 0.03 | 0.47 ± 0.04 | 0.48 ± 0.05 |
| Str | [-] | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.28 ± 0.1 |
| Std * | [°] | 165 ± 9 | 134 ± 39 | 117 ± 14 |
| 1st direction * | [°] | 144 ± 9 | 117 ± 30 | 101 ± 19 |
| 2nd direction | [°] | 159 ± 3 | 74 ± 38 | 113 ± 39 |
| 3rd direction * | [°] | 121 ± 64 | 83 ± 47 | 119 ± 23 |
| Parameter | Untreated | HDMSO | He/HDMSO |
|---|---|---|---|
| FD * | 2.11 ± 0.03 | 2.13 ± 0.01 | 2.12 ± 0.01 |
| FS * | 0.49 ± 0.02 | 0.46 ± 0.01 | 0. 50 ± 0.02 |
| |β| * | 0.05 ± 0.03 | 0.06 ± 0.05 | 0.09 ± 0.05 |
| H * | 0.990 ± 0.111 | 0.996 ± 0.004 | 0.985 ± 0.090 |
| Parameter | Untreated | HMDSO | He/HMDSO |
|---|---|---|---|
| E * | 0.97 ± 0.02 | 0.96 ± 0.02 | 0.98 ± 0.00 |
| * | 0.017 ± 0.017 | 0.025 ± 0.017 | 0.010 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca de Albuquerque, M.D.; Bastos, D.C.; Ţălu, Ş.; Matos, R.S.; Pires, M.A.; Salerno, M.; da Fonseca Filho, H.D.; Simão, R.A. Vapor Barrier Properties of Cold Plasma Treated Corn Starch Films. Coatings 2022, 12, 1006. https://doi.org/10.3390/coatings12071006
da Fonseca de Albuquerque MD, Bastos DC, Ţălu Ş, Matos RS, Pires MA, Salerno M, da Fonseca Filho HD, Simão RA. Vapor Barrier Properties of Cold Plasma Treated Corn Starch Films. Coatings. 2022; 12(7):1006. https://doi.org/10.3390/coatings12071006
Chicago/Turabian Styleda Fonseca de Albuquerque, Marta D., Daniele C. Bastos, Ştefan Ţălu, Robert S. Matos, Marcelo A. Pires, Marco Salerno, Henrique D. da Fonseca Filho, and Renata A. Simão. 2022. "Vapor Barrier Properties of Cold Plasma Treated Corn Starch Films" Coatings 12, no. 7: 1006. https://doi.org/10.3390/coatings12071006
APA Styleda Fonseca de Albuquerque, M. D., Bastos, D. C., Ţălu, Ş., Matos, R. S., Pires, M. A., Salerno, M., da Fonseca Filho, H. D., & Simão, R. A. (2022). Vapor Barrier Properties of Cold Plasma Treated Corn Starch Films. Coatings, 12(7), 1006. https://doi.org/10.3390/coatings12071006











