Synthesis and Characterization of Novel NiW–CeO2 Composite Coating with Enhanced Corrosion and Wear Resistance
Abstract
:1. Introduction
2. Methodology
2.1. Electrolyte Components
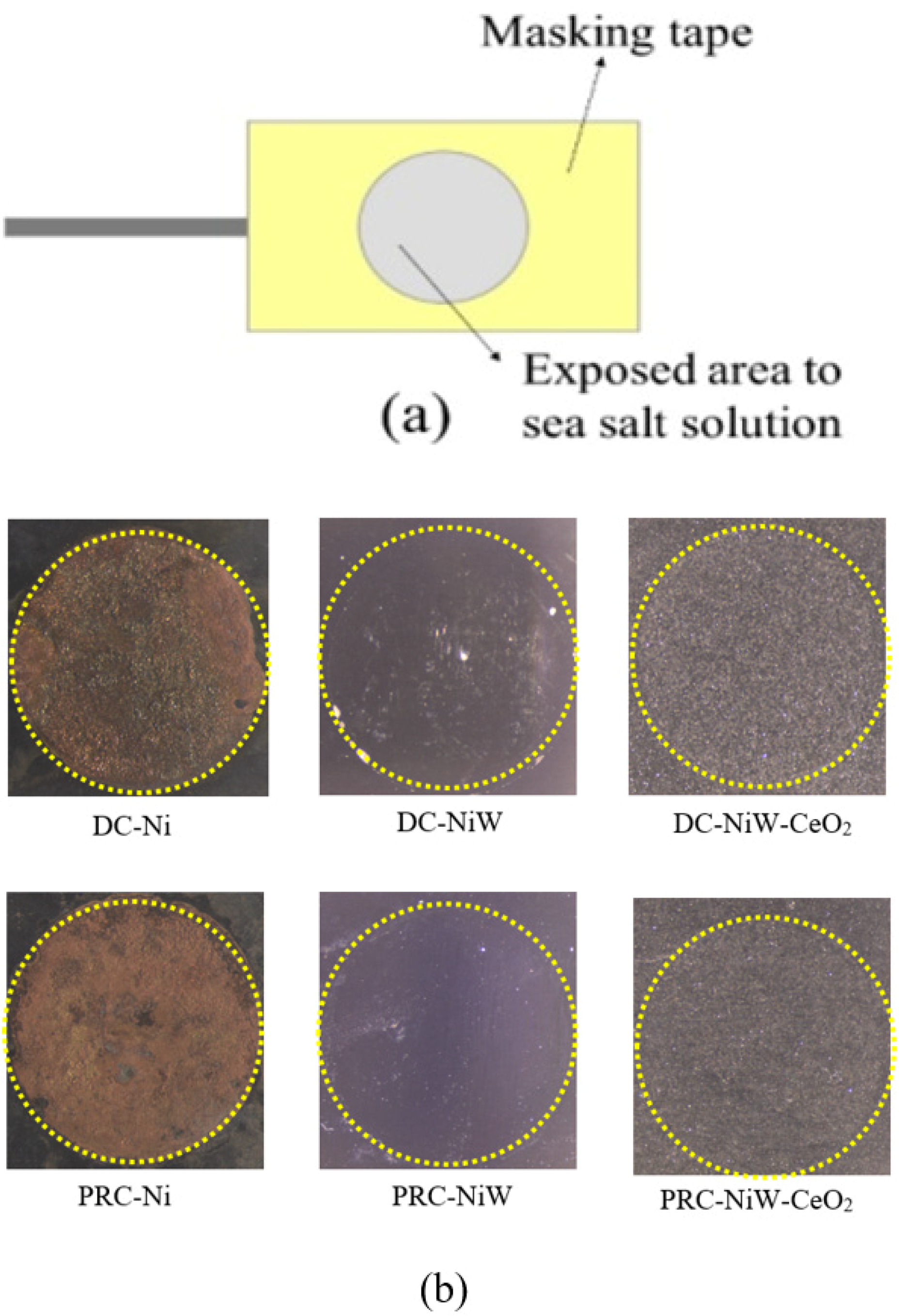
2.2. Substrate Preparation
2.3. Electrodeposition Setup
2.4. Optimization of PRC Waveform
2.5. Characterization of Deposits
3. Results and Discussion
3.1. Corrosion Analysis
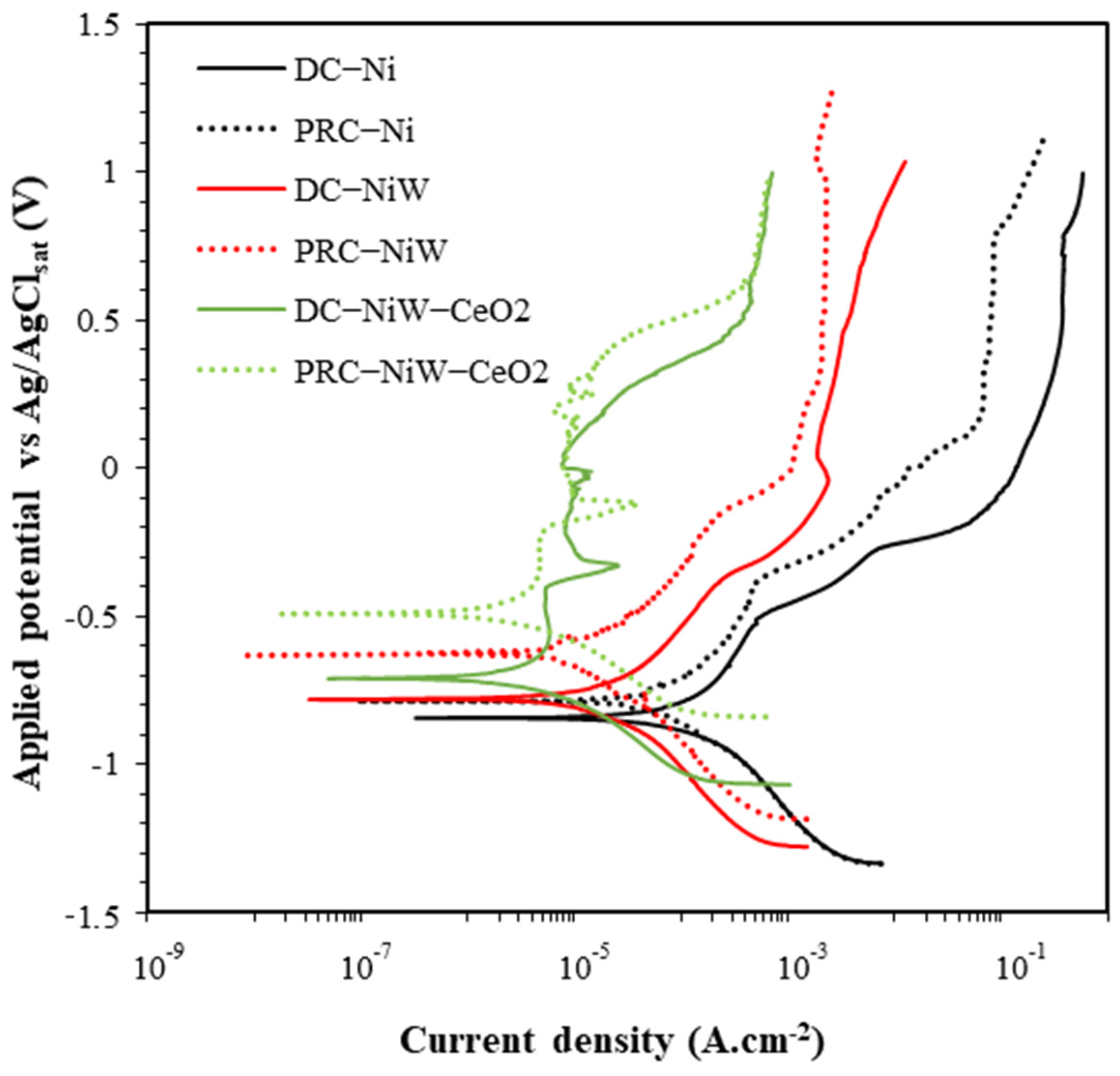
3.1.1. Cyclic Potentiodynamic Polarization (CPP)
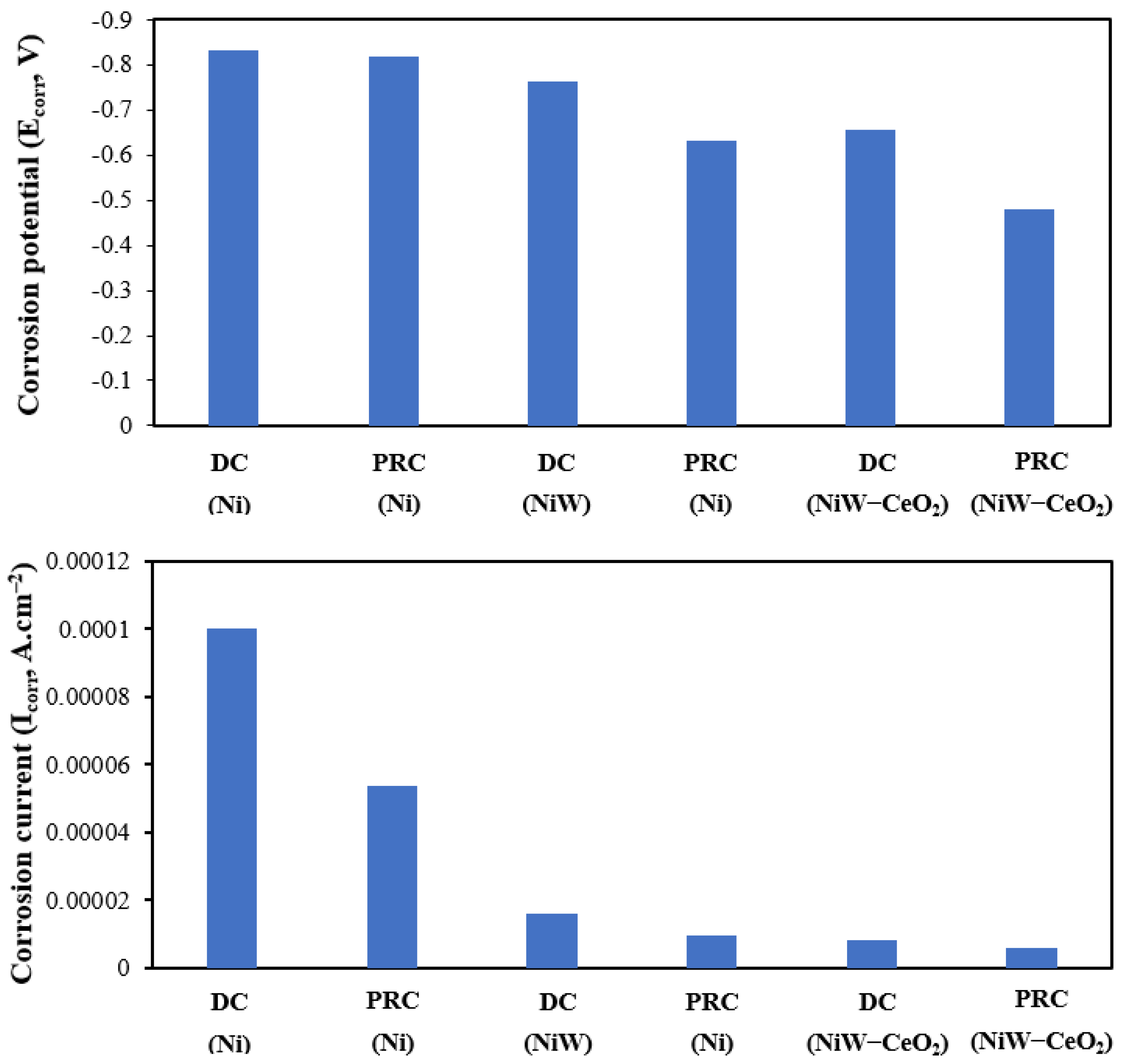
3.1.2. Potentiodynamic Polarization (PP)
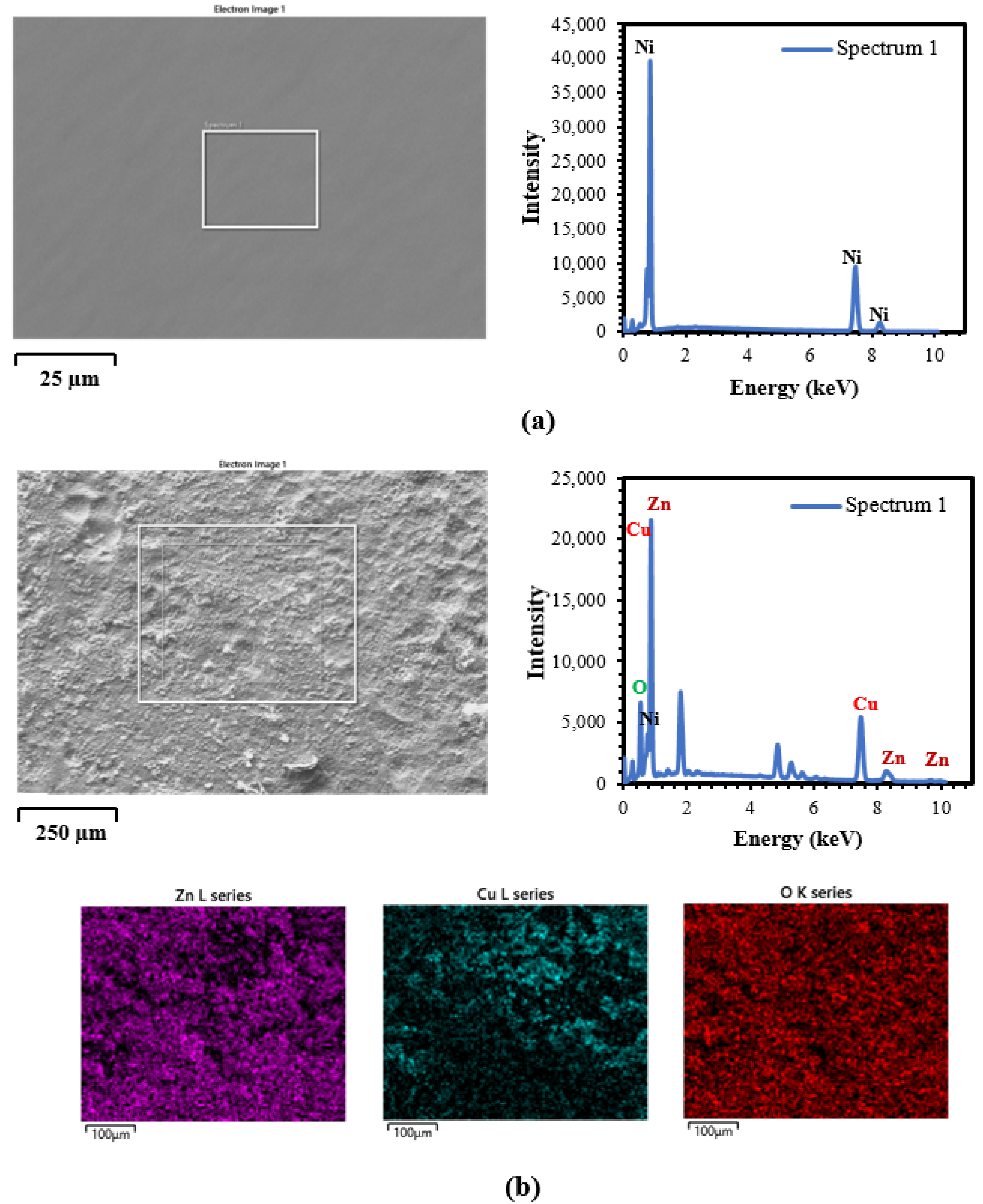
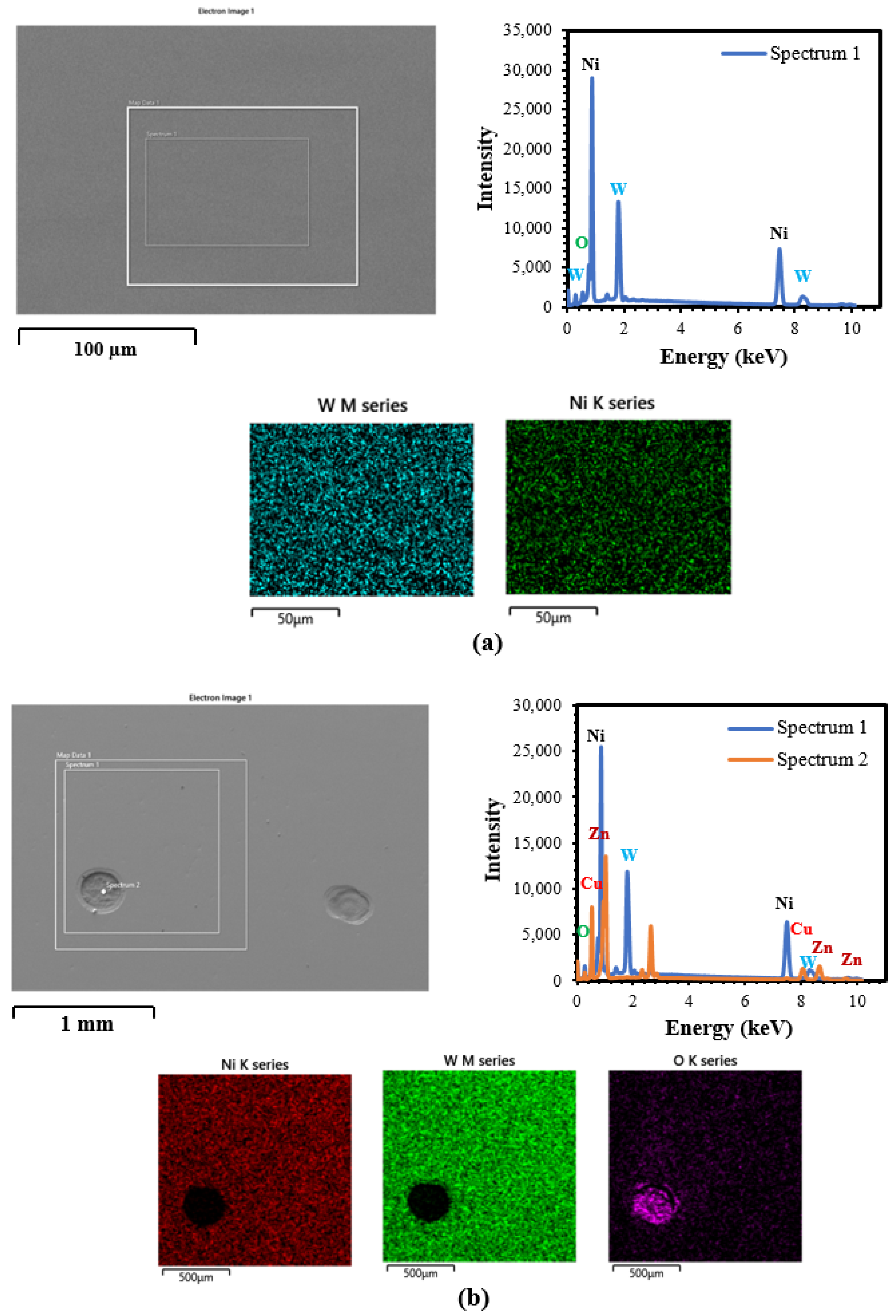
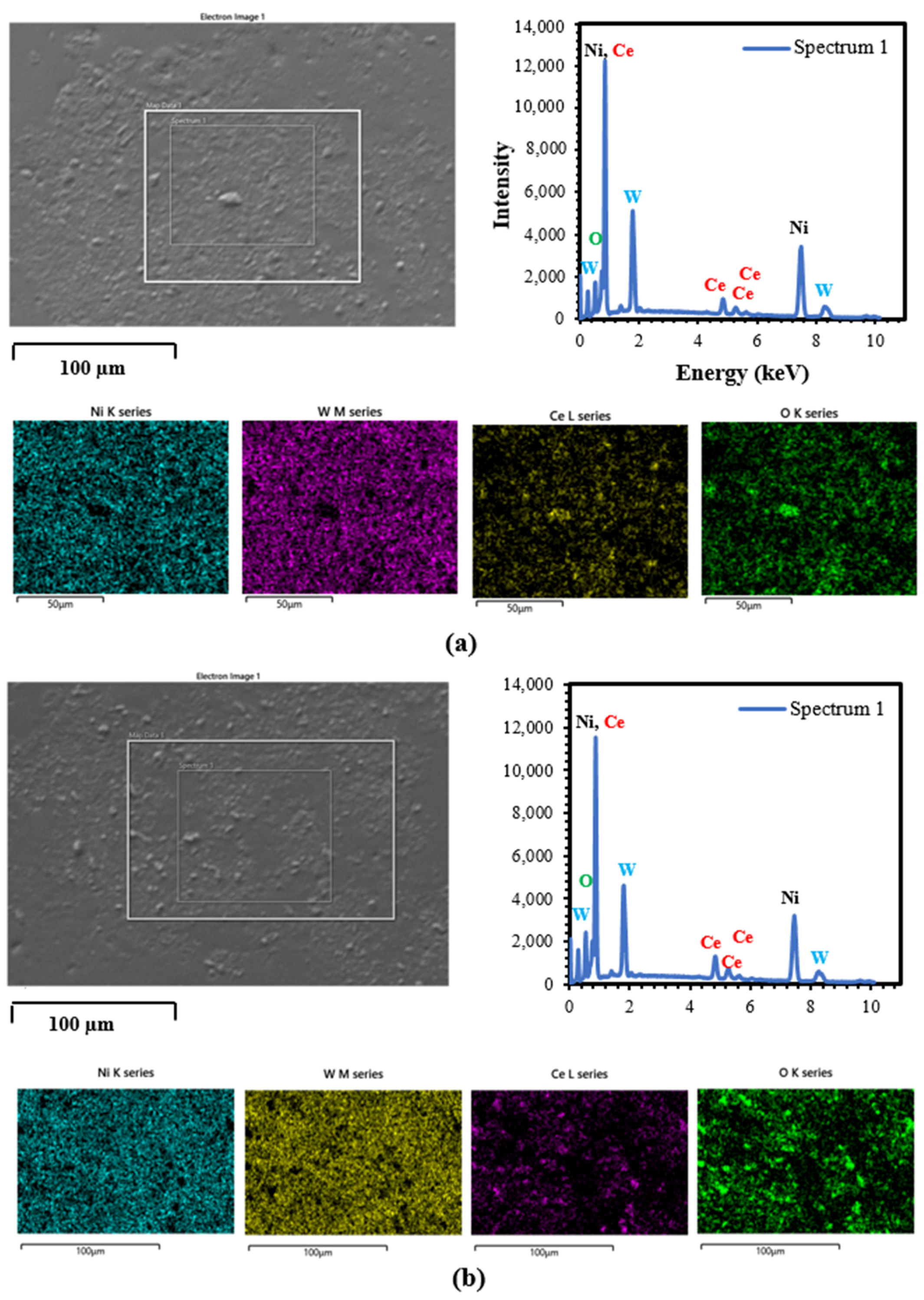
3.2. SEM/EDS Analysis
3.2.1. DC and PRC Deposited Ni
3.2.2. DC and PRC Deposited Ni–W
3.2.3. DC and PRC Deposited NiW–CeO2 Composite
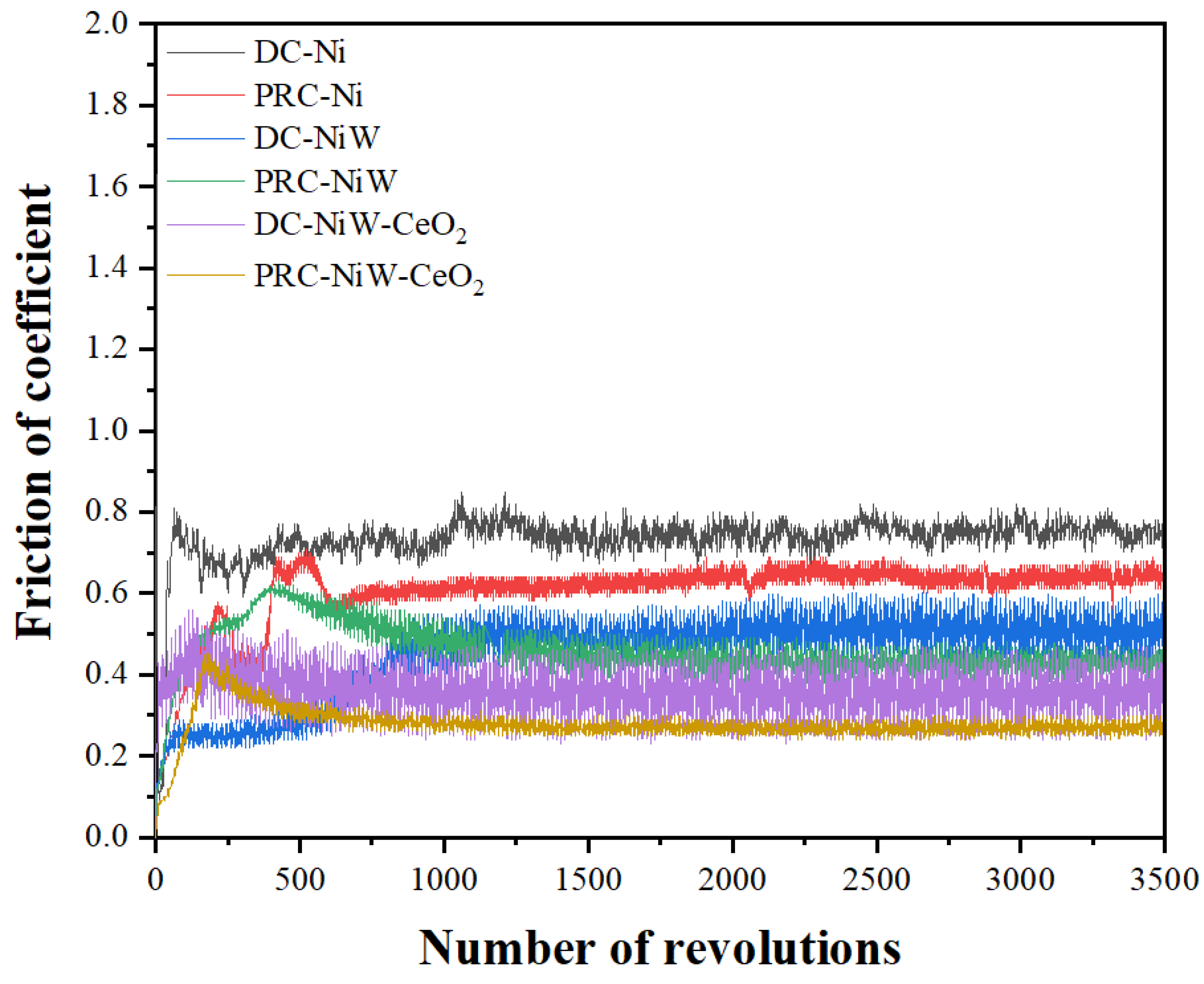
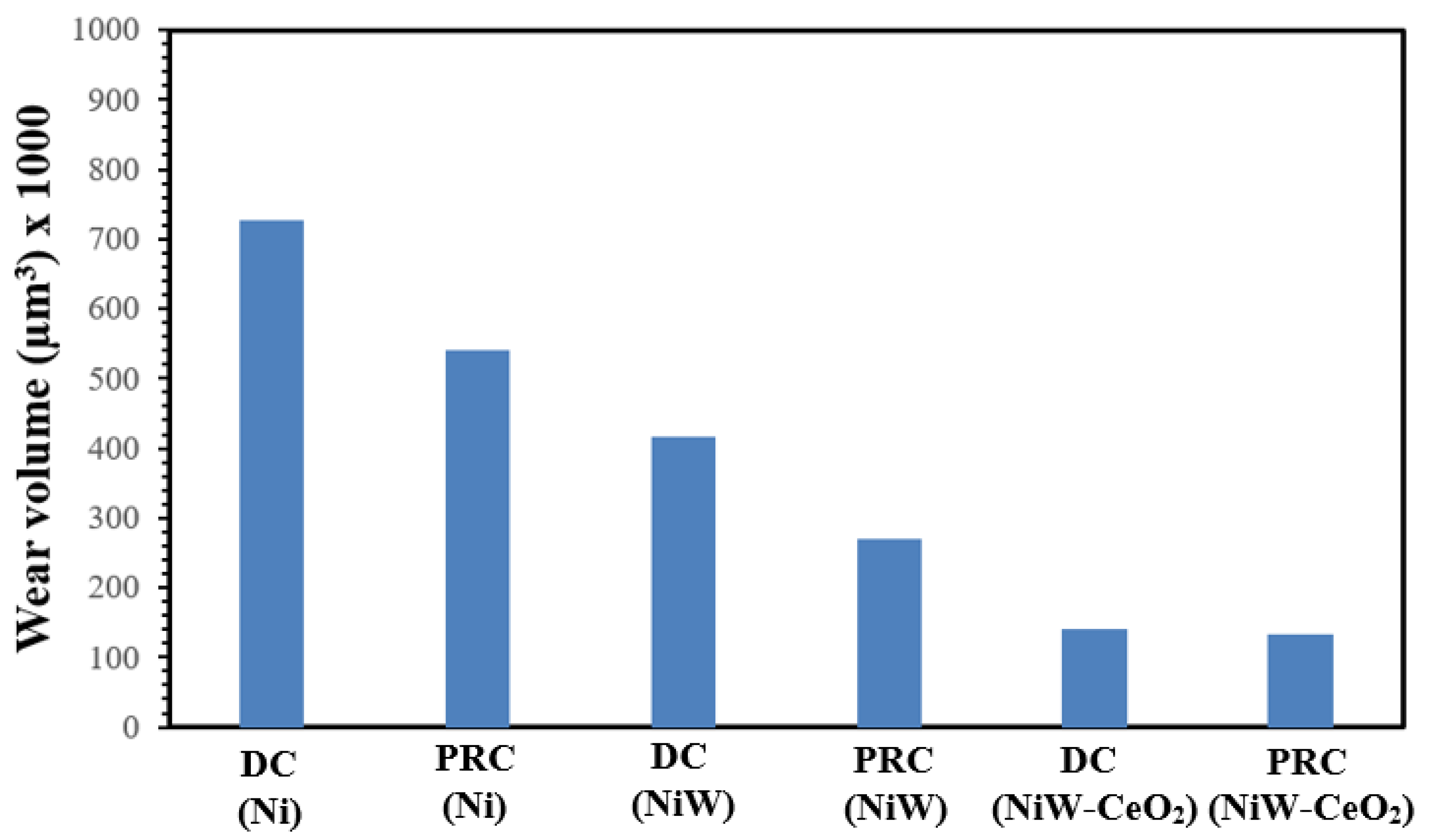
3.3. Tribological Analysis (Coefficient of Friction and Wear Rate)
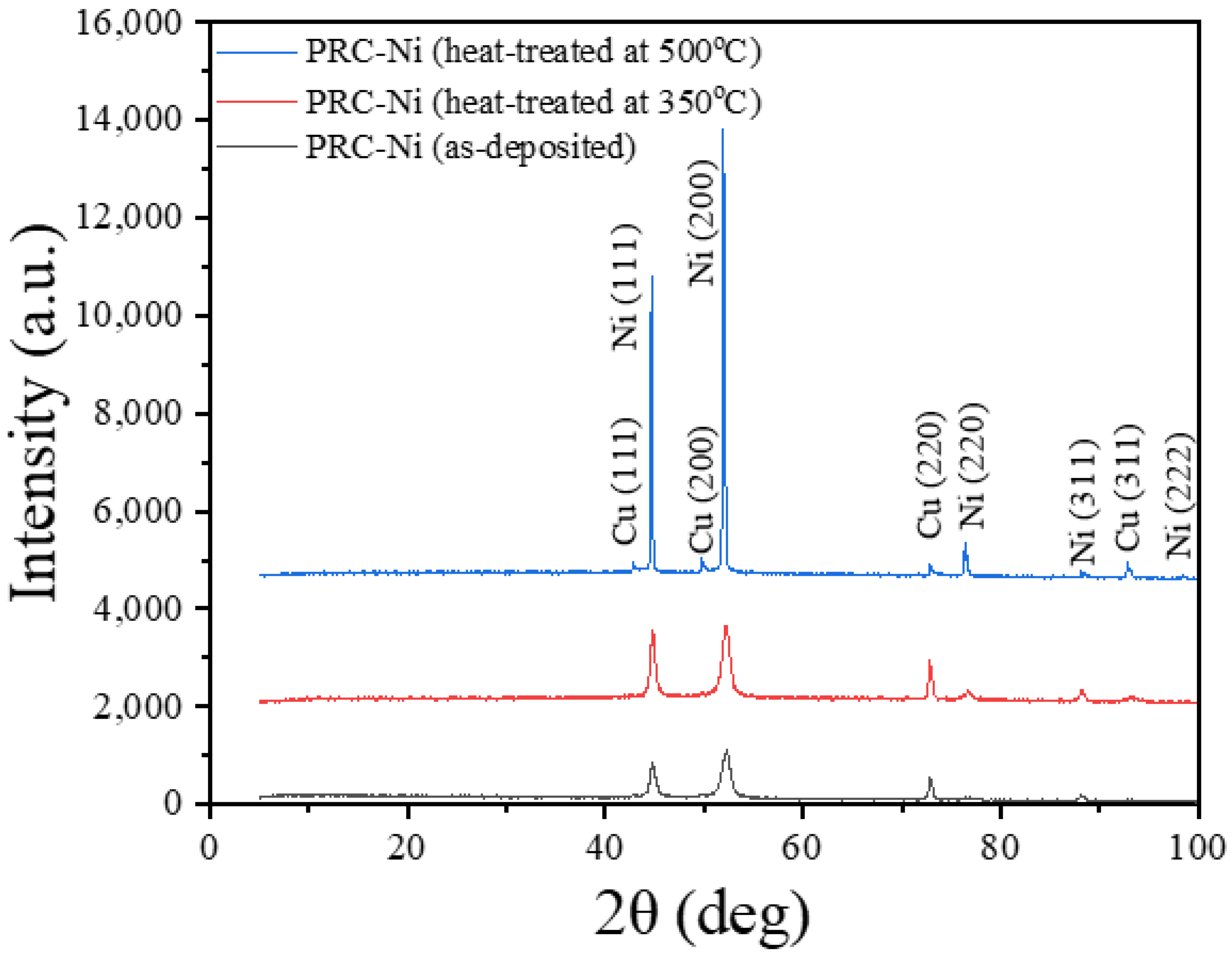
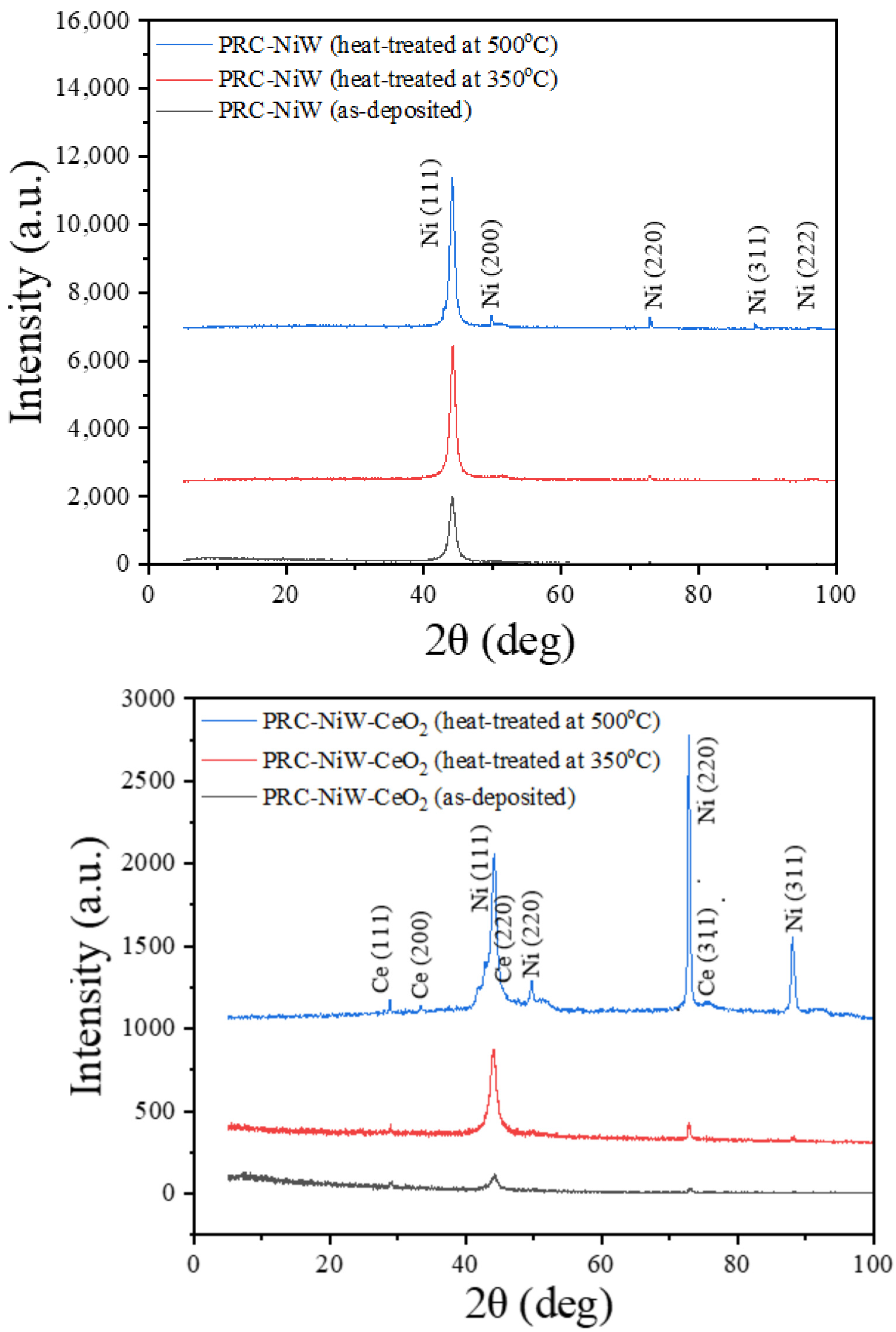
3.4. XRD Analysis (Effect of Heat Treatment on Crystallite Sizes of PRC deposited Ni, NiW and NiW–CeO2)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, A.; Fu, X.-Q.; Chen, X.; Lin, J.; Cao, H. Effect of Current Density on the Properties of Ni–CeO2 Composite Coatings prepared using Magnetic Field–Assisted Jet Electrodeposition. Int. J. Electrochem. Sci. 2021, 16, 210658. [Google Scholar]
- Guo, S.; Wang, L.; Jin, Y.; Piao, N.; Chen, Z.; Tian, G.; Li, J.; Zhao, C.; He, X. A polymeric composite protective layer for stable Li metal anodes. Nano Converg. 2020, 7, 21. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; He, X.; Fang, M.; Gao, J.; Li, J.; Deng, L.; Chen, H.; Tian, G.; Wang, J.; et al. In situ prepared nano–crystalline TiO2–poly(methyl methacrylate) hybrid enhanced composite polymer electrolyte for Li–ion batteries. J. Mater. Chem. A 2013, 1, 5955–5961. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, H.; Jiao, L.; Yu, N.; He, L. Preparation and electrocatalytic property of PbO2–CeO2 nanocomposite electrodes by pulse reverse electrodeposition methods. J. Electrochem. Soc. 2016, 163, D179–D184. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Xue, Y.J.; Yang, F.; Liu, C.Y.; Li, J.S. Preparation of Ni–ZrO2–CeO2 Nanocomposite Coatings by Pulse Electrodeposition. Appl. Mech. Mater. 2013, 395–396, 174–178. [Google Scholar] [CrossRef]
- Wang, L.; Xing, S.; Liu, H.; Jiang, C.; Ji, V. Improved wear properties of Ni Ti nanocomposite coating with tailored spatial microstructures by extra adding CeO2 nanoparticles. Surf. Coat. Technol. 2020, 399, 126119. [Google Scholar] [CrossRef]
- Uhm, S.; Yi, Y.; Lee, J. Electrocatalytic Activity of Pd–CeO2 Nanobundle in an Alkaline Ethanol Oxidation. Catal. Lett. 2010, 138, 46–49. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y. A comparative study of pure nickel and the Ni–CeO2 nanocrystalline coatings: Microstructural evolution, oxidation behavior, and thermodynamic stability. J. Mater. Sci. 2014, 49, 3755–3774. [Google Scholar] [CrossRef]
- Xue, Y.J.; Jia, X.Z.; Zhou, Y.W.; Ma, W.; Li, J.S. Tribological performance of Ni–CeO2 composite coatings by electrodeposition. Surf. Coat. Technol. 2006, 200, 5677–5681. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoo, H.J. Formation of bilayer Ni–SiC composite coatings by electrodeposition. Surf. Coat. Technol. 1998, 108–109, 564–569. [Google Scholar] [CrossRef]
- Garcia, I.; Fransaer, J.; Celis, J.-P. Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Wang, W.; Guo, H.T.; Gao, J.P.; Dong, X.H.; Qin, Q.X. XPS, UPS and ESR studies on the interfacial interaction in Ni–ZrO2 composite plating. J. Mater. Sci. 2000, 35, 1495–1499. [Google Scholar] [CrossRef]
- Banovic, S.W.; Barmak, K.; Marder, A.R. Characterization of single and discretely–stepped electro–composite coatings of nickel–alumina. J. Mater. Sci. 1999, 34, 3203–3211. [Google Scholar] [CrossRef]
- Wu, G.; Li, N.; Zhou, D.; Mitsuo, K. Electrodeposited Co–Ni–Al2O3 composite coatings. Surf. Coat. Technol. 2004, 176, 157–164. [Google Scholar] [CrossRef]
- Surviliene, S.; Orlovskaja, L.; Bikulcius, G.; Biallozor, S. Effect of MoO2 and TiO2 on electrodeposition and properties of chromium coating. Surf. Coat. Technol. 2001, 137, 230–234. [Google Scholar] [CrossRef]
- Losiewicz, B.; Stępień, A.; Gierlotka, D.; Budniok, A. Composite layers in Ni–P system containing TiO2 and PTFE. Thin Solid Films 1999, 349, 43–50. [Google Scholar] [CrossRef]
- Levin, B.; DuPont, J.; Marder, A. The effect of second phase volume fraction on the erosion resistance of metal–matrix composites. Wear 2000, 238, 160–167. [Google Scholar] [CrossRef]
- Jun, L.; Yiyong, W.; Dianlong, W.; Xinguo, H. The microstructure and wear resistance characteristics of electroformed nickel and partially stabilized zirconia composite coatings. J. Mater. Sci. 2000, 35, 1751–1758. [Google Scholar] [CrossRef]
- Hou, K.; Ger, M.; Wang, L.; Ke, S. The wear behaviour of electro–codeposited Ni–SiC composites. Wear 2002, 253, 994–1003. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, F.; Wu, X.; Zhao, W.; Chen, X. A novel plating apparatus for electrodeposition of Ni–SiC composite coatings using circulating–solution co–deposition technique. J. Alloys Compd. 2017, 699, 366–377. [Google Scholar] [CrossRef]
- Han, B.; Lu, X. Tribological and anti–corrosion properties of Ni–W–CeO2 coatings against molten glass. Surface and Coatings Technology. Surf. Coat. Technol. 2008, 202, 3251–3256. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Xue, Q.J.; Liu, W.-M.; Shen, W.C. Effect of rare earth compounds as fillers on friction and wear behaviors of PTFE–based composites. J. Appl. Polym. 1999, 72, 361–369. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Haghshenas, S.M.S.; Badr, P.; Setoudeh, N.; Hosseinpour, S. Wear and tribological characterization of nickel matrix electrodeposited composites: A review. Wear 2021, 486–487, 204098. [Google Scholar] [CrossRef]
- Cârâc, G.; Benea, L.; Iticescu, C.; Lampke, T.; Steinhäuser, S.; Wielage, B. Codeposition of Cerium Oxide with Nickel and Cobalt: Correlation between Microstructure and Microhardness. Surf. Eng. 2004, 20, 353–359. [Google Scholar] [CrossRef]
- Tarkowski, L.; Indyka, P.; Bełtowska-Lehman, E. XRD characterisation of composite Ni–based coatings prepared by electrodeposition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 284, 40–43. [Google Scholar] [CrossRef]
- Atanassov, N.; Gencheva, K.; Bratoeva, M. Properties of nickel–tungsten alloys electrodeposited from sulfamate electrolyte. Plat. Surf. Finish. 1997, 84, 67–71. [Google Scholar]
- Jinlong, L.; Zhuqing, W.; Tongxiang, L.; Suzuki, K.; Hideo, M. Effect of tungsten on microstructures of annealed electrodeposited Ni–W alloy and its corrosion resistance. Surf. Coat. Technol. 2018, 337, 516–524. [Google Scholar] [CrossRef]
- Younes, O.; Gileadi, E. Electroplating of Ni/W alloys: I. Ammoniacal citrate baths. J. Electrochem. Soc. 2002, 149, C100. [Google Scholar] [CrossRef]
- Klimenkov, M.; Haseeb, A.; Bade, K. Structural investigations on nanocrystalline Ni–W alloy films by transmission electron microscopy. Thin Solid Films 2009, 517, 6593–6598. [Google Scholar] [CrossRef]
- Sriraman, K.; Raman, S.G.S.; Seshadri, S. Synthesis and evaluation of hardness and sliding wear resistance of electrodeposited nanocrystalline Ni–W alloys. Mater. Sci. Eng. A 2006, 418, 303–311. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Zare, H.R. Improving the Corrosion Resistance of the Nickel–Tungsten Alloy by Optimization of the Electroplating Conditions. Trans. Indian Inst. Met. 2020, 73, 937–944. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Latha, S.M.; Ramakrishna, M.; Rao, D.; Sundararajan, G. Pulsed electrodeposition and mechanical properties of Ni–W/SiC nano–composite coatings. Mater. Des. 2016, 112, 140–150. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, M.; Luo, H.; Qiao, Q.; Xiao, Z.; Zhao, Y.; Zhao, L.; Sun, H.; Xu, Z.; Matsugi, K.; et al. Effect of Saccharin on the Structure and Properties of Electrodeposition NiWP Alloy Coatings. J. Mater. Eng. Perform. 2016, 25, 4402–4407. [Google Scholar] [CrossRef]
- Cameron, J.C. Acetylenic Compositions and Nickel Plating Baths Containing Same. U.S. Patent No. 4,421,6111983, 20 December 1983. [Google Scholar]
- Rahman, O.A.; Wasekar, N.P.; Sundararajan, G.; Keshri, A.K. Experimental investigation of grain boundaries misorientations and nano twinning induced strengthening on addition of silicon carbide in pulse electrodeposited nickel tungsten composite coating. Mater. Charact. 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Liaw, P.K. Corrosion–Resistant High–Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Ren, Y.-Y.; Zhang, Y.-H.; Wang, S.; Li, L. Direct Correlations among the Grain Size, Texture, and Indentation Behavior of Nanocrystalline Nickel Coatings. Metals 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.F.; Luo, H.; Xiao, K.; Li, X.G.; Cheng, Y.F. In Situ Characterization of Pitting Corrosion of Stainless Steel by a Scanning Electrochemical Microscopy. J. Mater. Eng. Perform. 2011, 21, 406–410. [Google Scholar] [CrossRef]
- Ahadian, M.; Zad, A.I.; Nouri, E.; Ranjbar, M.; Dolati, A. Diffusion and segregation of substrate copper in electrodeposited Ni–Fe thin films. J. Alloys Compd. 2007, 443, 81–86. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Influence of grain size on the corrosion behavior of a Ni–based superalloy nanocrystalline coating in NaCl acidic solution. Electrochimica Acta 2008, 53, 2453–2462. [Google Scholar] [CrossRef]
- Palumbo, G.; Dunikowski, D.; Wirecka, R.; Mazur, T.; Lelek-Borkowska, U.; Wawer, K.; Banaś, J. Effect of Grain Size on the Corrosion Behavior of Fe–3wt.%Si–1wt.%Al Electrical Steels in Pure Water Saturated with CO2. Materials 2021, 14, 5084. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, M.; Li, C.; Liu, N.; Wei, Y. The influence of grain size on the corrosion resistance of nanocrystalline zirconium metal. Mater. Sci. Eng. A 2007, 448, 259–263. [Google Scholar] [CrossRef]
- Nyambura, S.M.; Kang, M.; Zhu, J.; Liu, Y.; Zhang, Y.; Ndiithi, N.J. Synthesis and Characterization of Ni–W/Cr2O3 Nanocomposite Coatings Using Electrochemical Deposition Technique. Coatings 2019, 9, 815. [Google Scholar] [CrossRef] [Green Version]
- Sriraman, K.; Raman, S.G.S.; Seshadri, S. Corrosion behaviour of electrodeposited nanocrystalline Ni–W and Ni–Fe–W alloys. Mater. Sci. Eng. A 2007, 460–461, 39–45. [Google Scholar] [CrossRef]
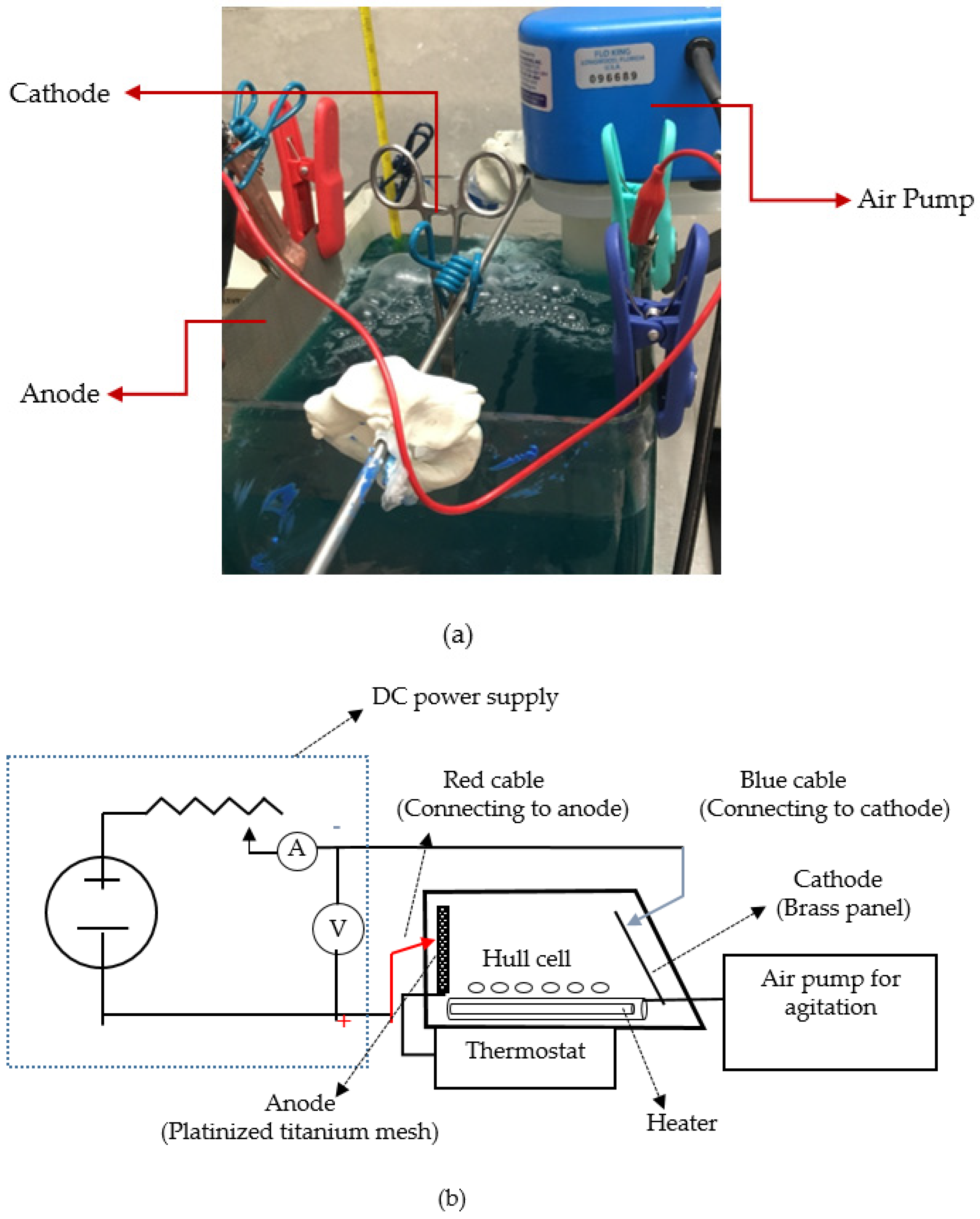
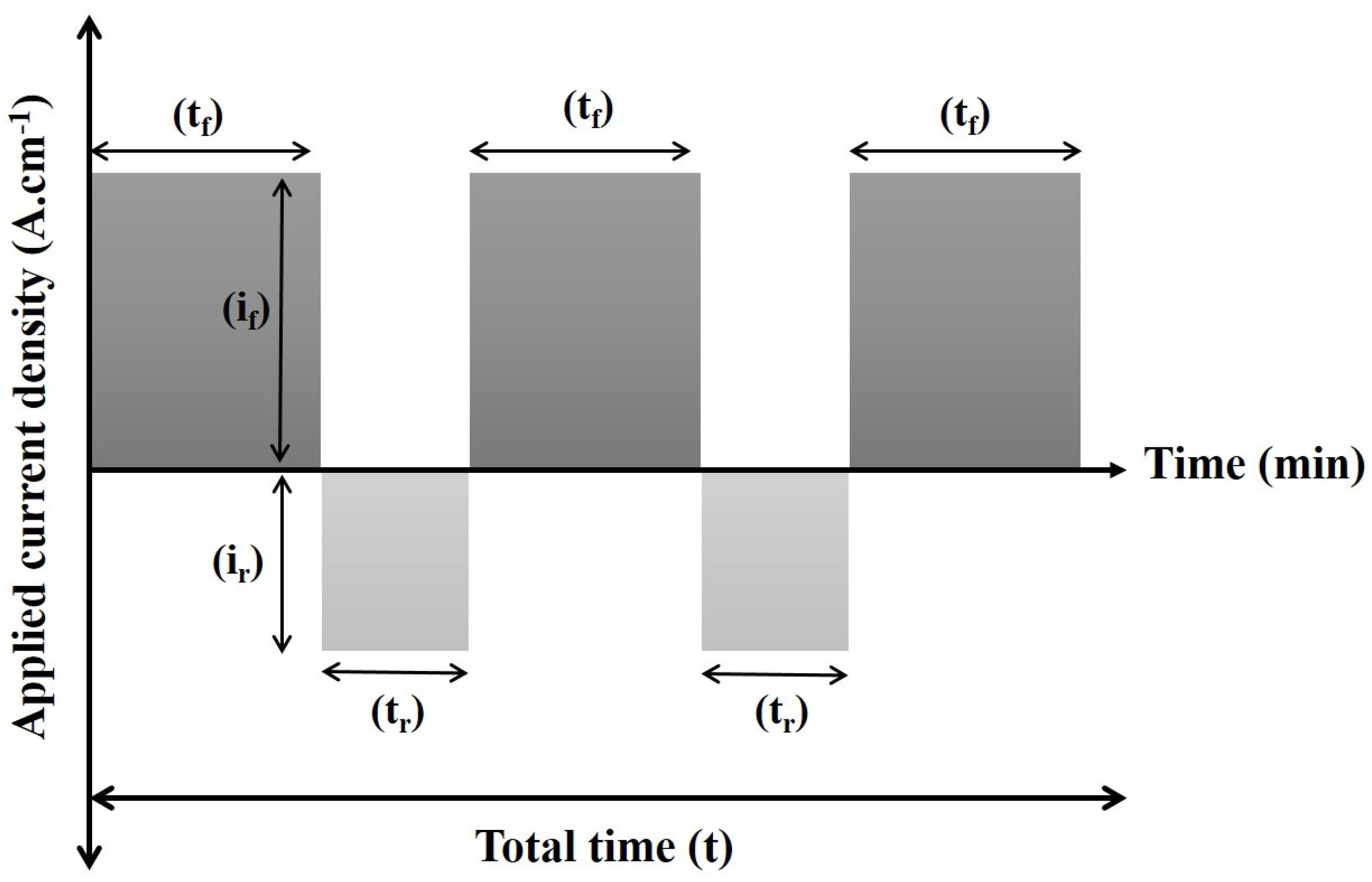





| Name of Chemicals | Concentration |
|---|---|
| Nickel sulfate | 29.5–30 (g·L−1) |
| Sodium tungstate | 58–60 (g·L−1) |
| Citric acid | 63–67 (g·L−1) |
| Ammonia | 58 (mL·L−1) |
| Sulfuric acid | as needed |
| Propargyl–oxo–propane–2,3–dihydroxy (POPDH) | 0.9–1 (g·L−1) |
| DuPont™ Capstone® Fluoro–surfactant FS–63 | 1.8–2 (g·L−1) |
| Sodium saccharin | 0.5–1 (g·L−1) |
| Experimental Parameters | |
| pH | 7.8–8.0 |
| Temperature | 58–61 °C |
| Duration of electrodeposition | 30 min |
| Ingredients | Concentrations (wt%) |
|---|---|
| NaCl | 58.49 |
| Na2SO4 | 9.75 |
| CaCl2 | 2.765 |
| KCl | 1.645 |
| NaHCO3 | 0.477 |
| KBr | 0.238 |
| H3BO3 | 0.071 |
| SrCl2.6H2O | 0.095 |
| NaF | 0.007 |
| MgCl2 | 26.46 |
| Coatings | Peak Position of (111) [°2Th] | FWHM [°2Th] | Crystallite Size [Å] |
|---|---|---|---|
| PRC–Ni (as–deposited) | 44.712 | 0.443 | 197 |
| PRC–Ni (heat–treated at 350 °C) | 44.810 | 0.315 | 280 |
| PRC–Ni (heat–treated at 500 °C) | 44.651 | 0.079 | 1210 |
| PRC–NiW (as–deposited) | 44.220 | 0.630 | 138 |
| PRC–NiW (heat–treated at 350 °C) | 44.319 | 0.433 | 202 |
| PRC–NiW (heat–treated at 500 °C) | 44.130 | 0.386 | 222 |
| PRC–NiW–CeO2 (as–deposited) | 44.261 | 0.779 | 110 |
| PRC–NiW–CeO2 (heat–treated at 350 °C) | 44.075 | 0.692 | 124 |
| PRC–NiW–CeO2 (heat–treated at 500 °C) | 44.214 | 0.543 | 158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadvand, M.; Savadogo, O. Synthesis and Characterization of Novel NiW–CeO2 Composite Coating with Enhanced Corrosion and Wear Resistance. Coatings 2022, 12, 878. https://doi.org/10.3390/coatings12070878
Dadvand M, Savadogo O. Synthesis and Characterization of Novel NiW–CeO2 Composite Coating with Enhanced Corrosion and Wear Resistance. Coatings. 2022; 12(7):878. https://doi.org/10.3390/coatings12070878
Chicago/Turabian StyleDadvand, Mina, and Oumarou Savadogo. 2022. "Synthesis and Characterization of Novel NiW–CeO2 Composite Coating with Enhanced Corrosion and Wear Resistance" Coatings 12, no. 7: 878. https://doi.org/10.3390/coatings12070878





