Microstructure and Properties in Simulated Seawater of Copper-Doped Micro-arc Coatings on TC4 Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Structure and Performance Characterization
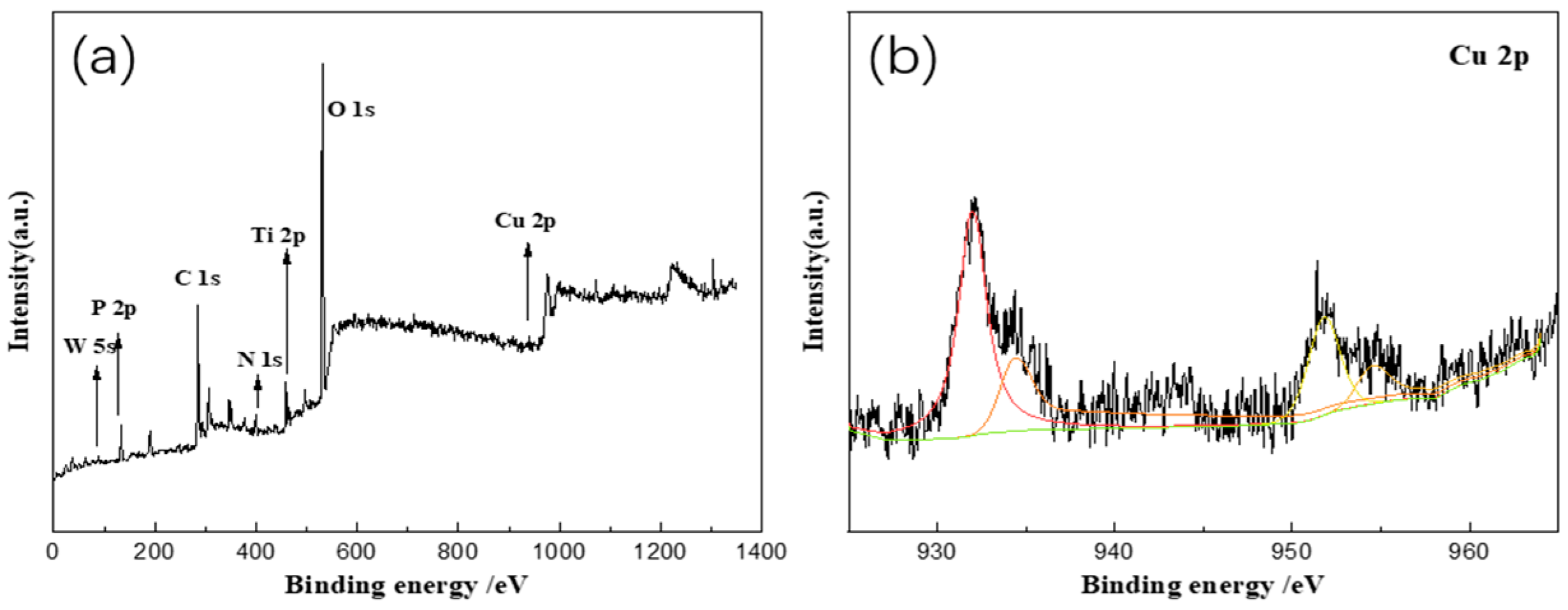
3. Results and Discussion
4. Conclusions
- (1)
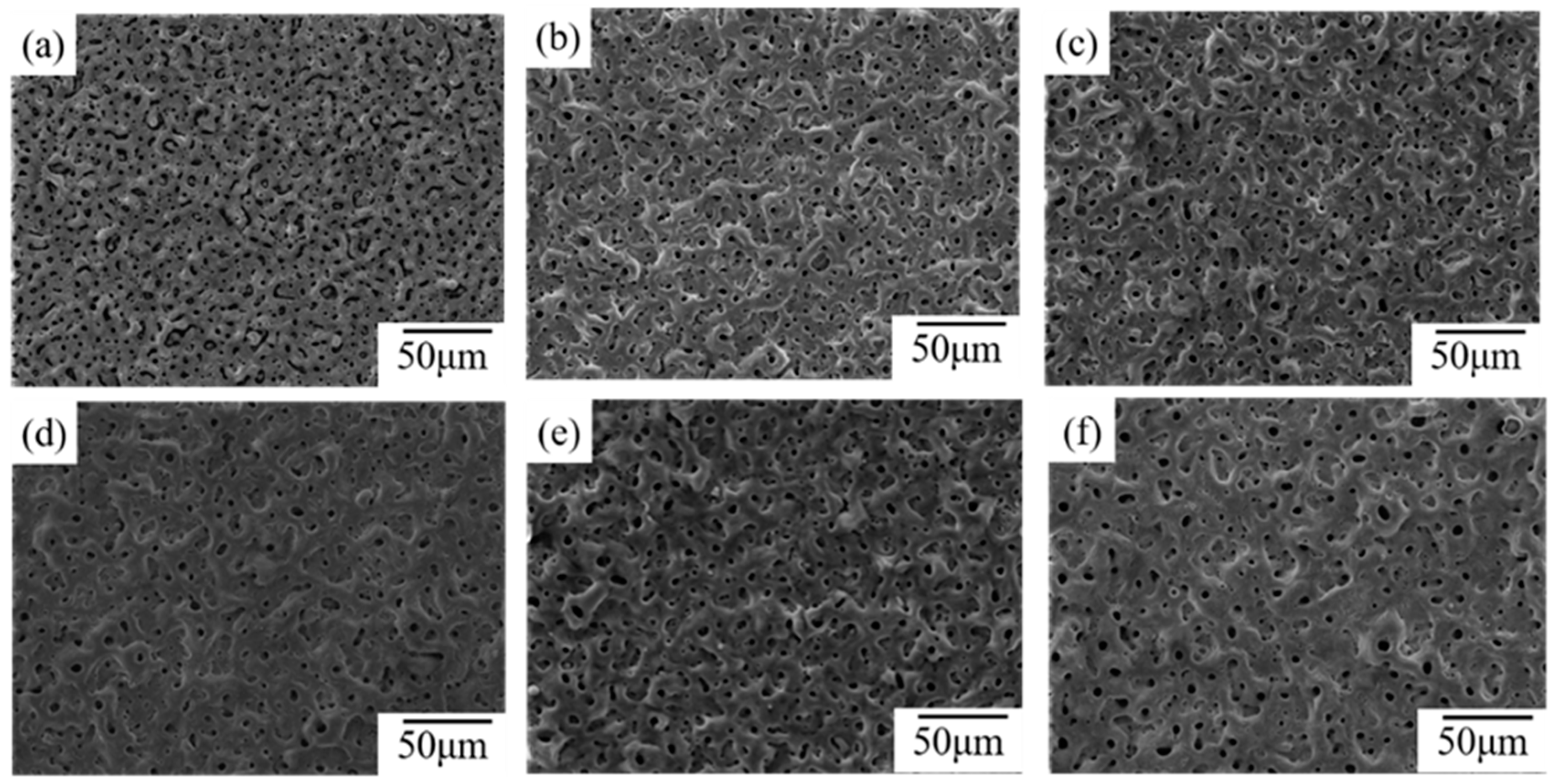
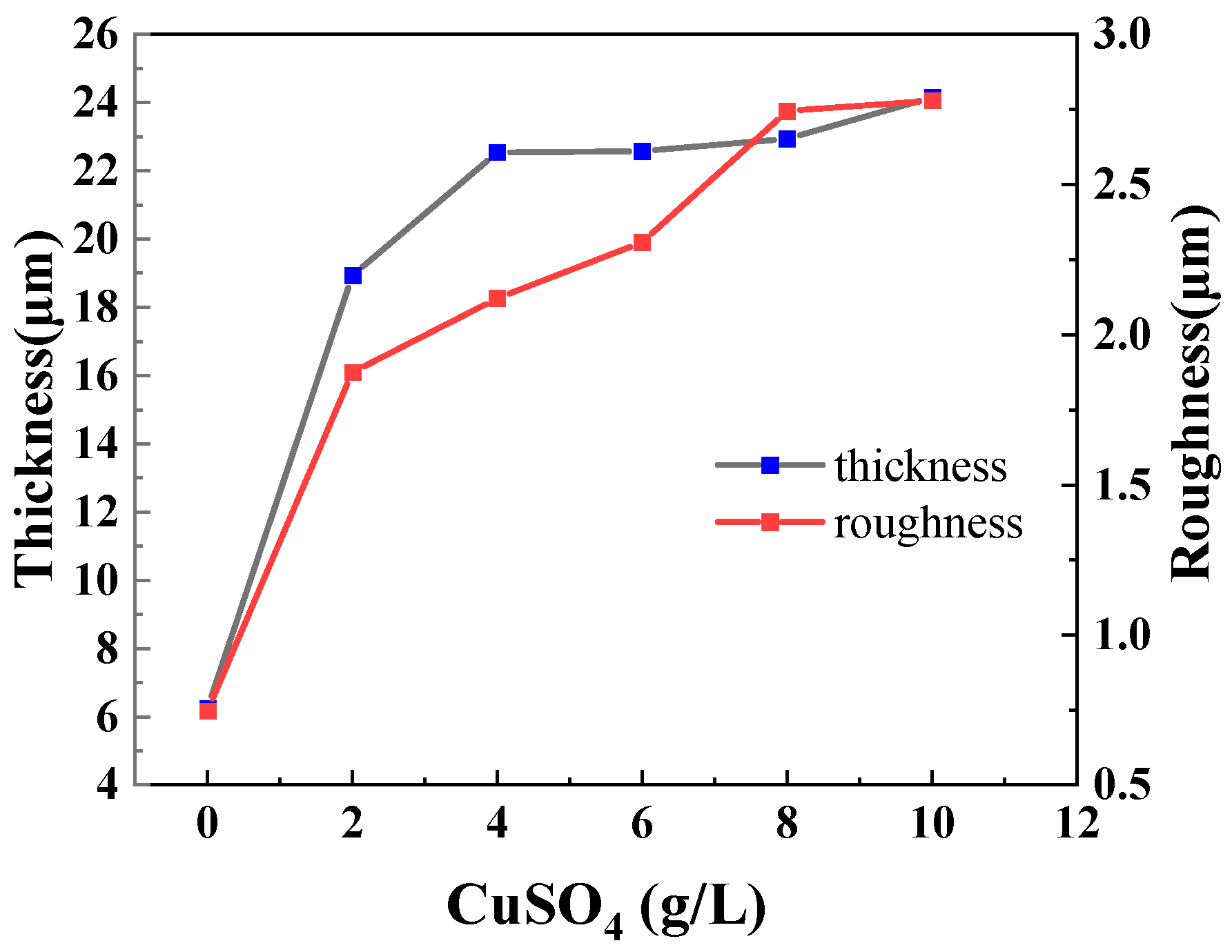
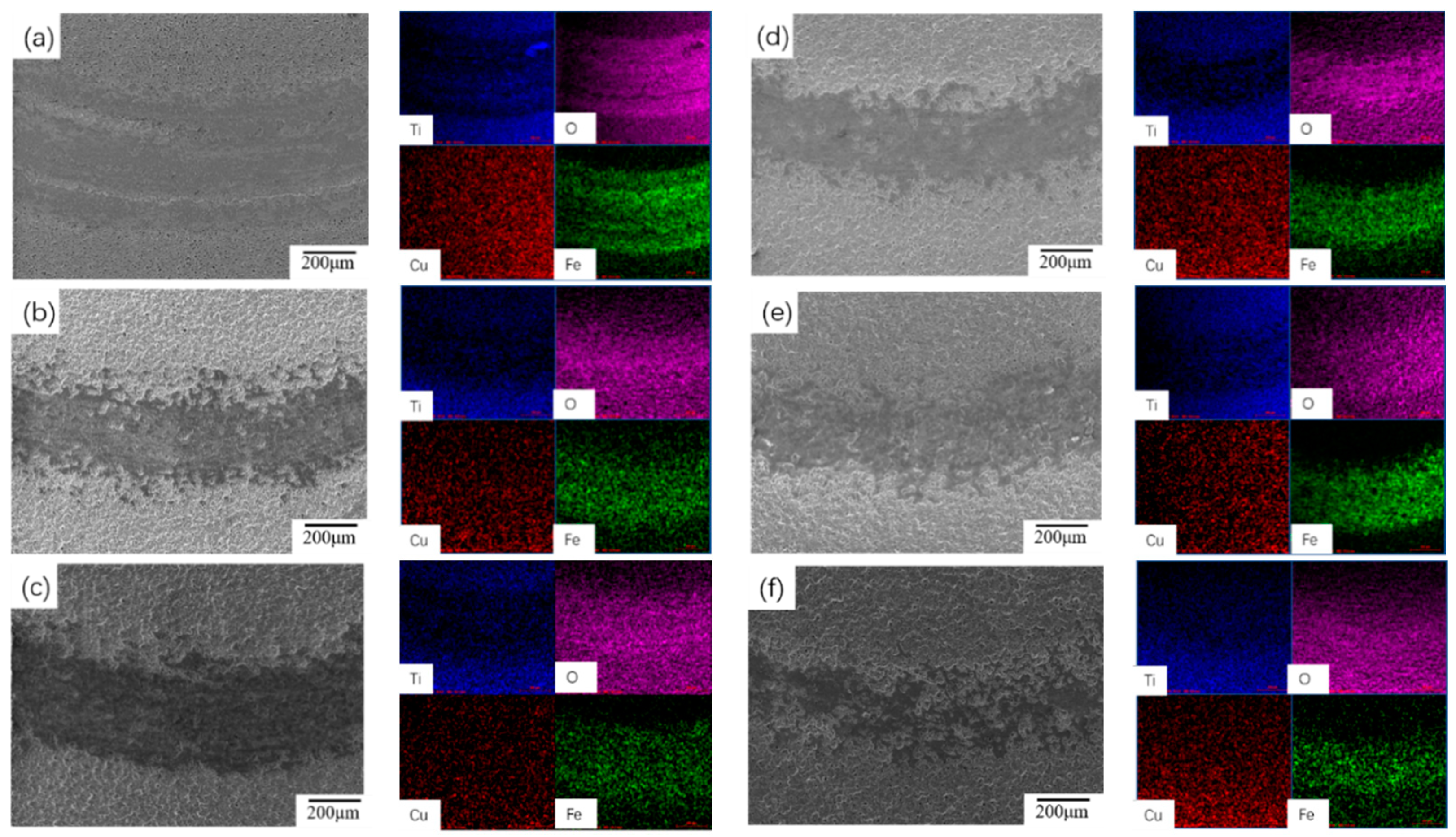
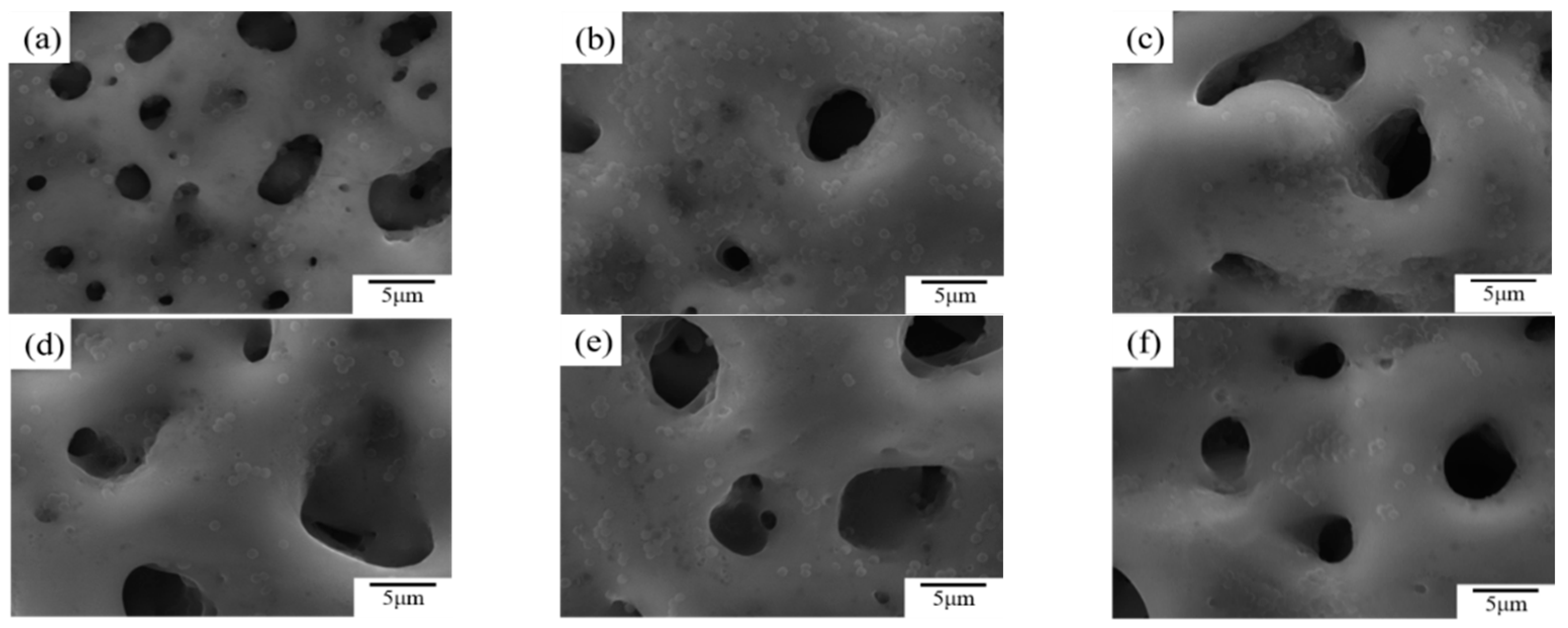
- Incorporation of CuSO4 significantly improved the surface morphology of the MAO coating and enhanced the coating thickness; a concentration of 6 g/L unified the surface morphology of the coating and minimized the number and diameter of micro-pores.
- (2)
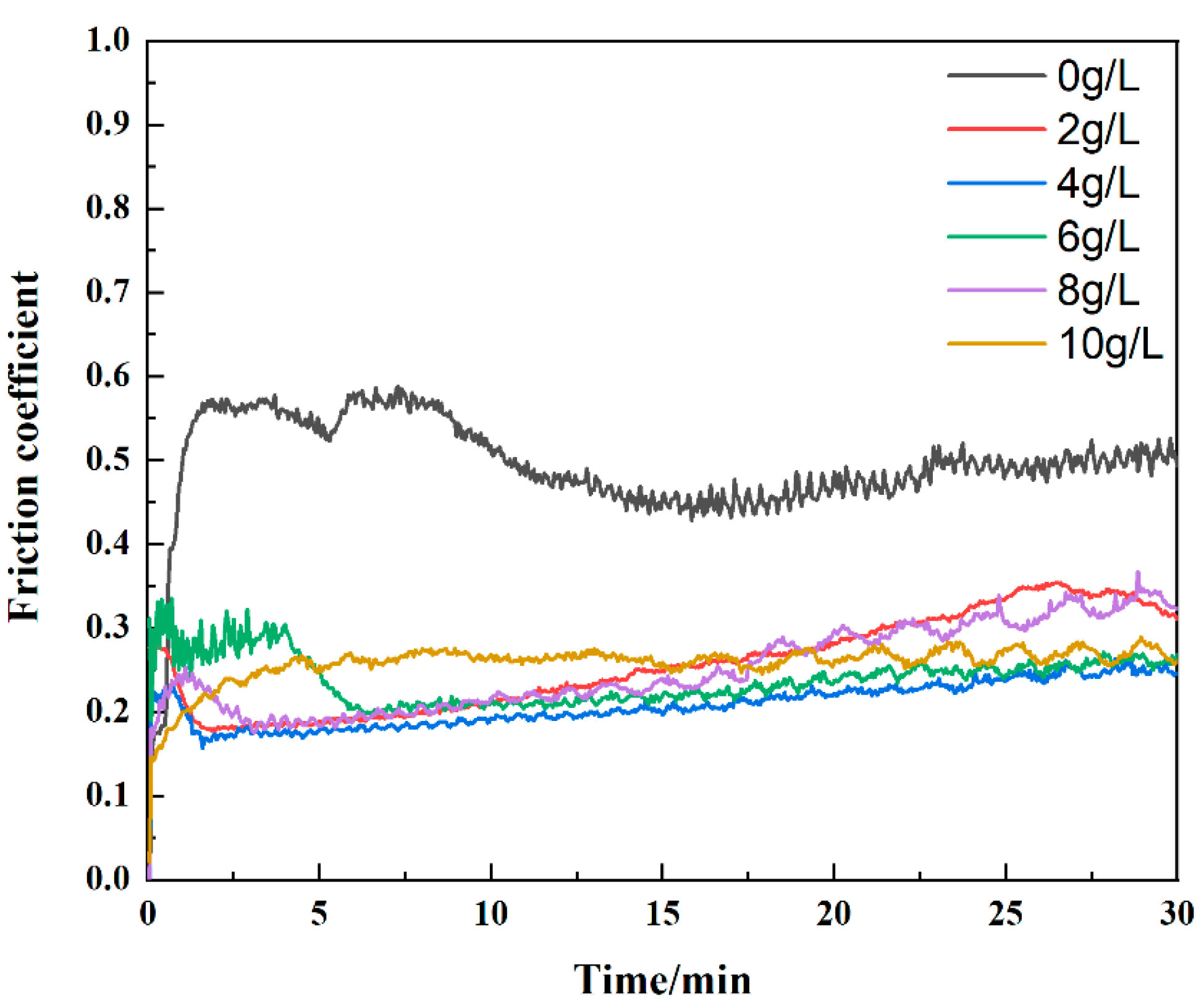
- The addition of CuSO4 significantly reduced the friction coefficient of the coating. As the concentration of CuSO4 increased, the friction coefficient fluctuation decreased, the width of the abrasion marks decreased, and the wear dopped. The MAO coating with 4 g/L CuSO4 had excellent wear resistance with a small friction coefficient of 0.2.
- (3)
- The addition of CuSO4 lowered the corrosion resistance of the coating. However, there was a significant inhibitory effect on the proliferation of S. aureus, and the number of bacteria attached to the surface of the coating was significantly reduced, with a minimum OD value of 0.526 at a CuSO4 concentration of 6 g/L.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lu, J.; Zhang, Y.; Huo, W.; Zhang, W.; Zhao, Y.; Zhang, Y. Electrochemical corrosion characteristics and biocompatibility of nanostructured titanium for implants. Appl. Surf. Sci. 2018, 434, 63–72. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Q.; Han, Y. Zn and Ag Co-doped Anti-microbial TiO2 Coatings on Ti by Micro-arc Oxidation. J. Mater. Sci. Technol. 2016, 32, 919–924. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H.; Liao, K.; Li, X. Improvement on corrosion resistance of micro-arc oxidized AZ91D magnesium alloy by a pore-sealing coating. J. Alloys Compd. 2022, 889, 161460. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Y.; Xu, D.; Zhao, J.; Ke, P.; Wang, A. Bactericidal abilities and in vitro properties of diamond-like carbon films deposited onto MAO-treated titanium. Mater. Lett. 2019, 244, 155–158. [Google Scholar] [CrossRef]
- Ren, S.; Du, C.; Liu, Z.; Li, X.; Xiong, J.; Li, S. Effect of fluoride ions on corrosion behaviour of commercial pure titanium in artificial seawater environment. Appl. Surf. Sci. 2020, 506, 144759. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Shamanian, M. Improvement of Corrosion and Tribocorrosion Behavior of Pure Titanium by Subzero Anodic Spark Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 15281–15287. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Chu, C.-H.; Zhang, M.-X.; Wang, H.-B.; Xue, S.-X. Wear resistance of alumina-coated oil casing steel N80 via MAO with rare earth additive. Ceram. Int. 2017, 43, 6397–6402. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Lv, Y.; Yang, L.; Zhang, E.; Dong, Z. Enhancement of Corrosion Resistance and Biological Performances of Cu-Incorporated Hydrox-yapatite/TiO2 Coating by Adjusting Cu Chemical Configuration and Hydroxyapatite Contents. ACS Appl. Bio Mater. 2021, 4, 903–917. [Google Scholar] [CrossRef]
- Aydogan, D.T.; Muhaffel, F.; Acar, O.K.; Topcuoglu, E.N.; Kulekci, H.G.; Kose, G.T.; Baydogan, M.; Cimenoglu, H. Surface modification of Ti6Al4V by micro-arc oxidation in AgC2H3O2-containing electrolyte. Surf. Innov. 2018, 6, 277–285. [Google Scholar] [CrossRef]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.-M.; Descamps, S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta Biomater. 2020, 117, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, X.; Wu, H.; Tian, L.; Ma, Y.; Tang, B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014, 292, 944–947. [Google Scholar] [CrossRef]
- Zhao, Q.; Yi, L.; Hu, A.; Jiang, L.; Hong, L.; Dong, J. Antibacterial and osteogenic activity of a multifunctional microporous coating codoped with Mg, Cu and F on titanium. J. Mater. Chem. B 2019, 7, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matýsek, D.; Dudek, L.; et al. Characterization of porous phosphate coatings enriched with calcium, magnesium, zinc and copper created on CP titanium grade 2 by plasma electrolytic oxidation. Metals 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, T.; Lv, Y.; Zhang, Y.; Lu, X.; Xiao, J.; Ma, C.; Li, Z.; Dong, Z. Enhanced uniformity, corrosion resistance and biological performance of Cu-incorporated TiO2 coating produced by ultrasound-auxiliary micro-arc oxidation. Appl. Surf. Sci. 2021, 569, 150932. [Google Scholar] [CrossRef]
- He, R.; Wang, B.; Xiang, J.; Pan, T. Effect of copper additive on microstructure and anti-corrosion performance of black MAO films grown on AZ91 alloy and coloration mechanism. J. Alloys Compd. 2021, 889, 161501. [Google Scholar] [CrossRef]
- Gao, W.; Liu, J.; Wei, J.; Yao, Y.; Ma, X.; Yang, W. Enhanced properties of micro arc oxidation coating with Cu addition on TC4 alloy in marine environment. Coatings 2021, 11, 1168. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, M.; Li, L.; Dong, L.; Wu, J.; Li, D. Co-regulation of Cu/Zn contents enhanced the biological and mechanical properties of TiN coated Ti-6Al-4V alloy. Surf. Coat. Technol. 2020, 395, 125943. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Z.; Lu, X.; Lv, Y.; Cai, G.; Yang, L.; Dong, Z. Microstructural evolution and biological performance of Cu-incorporated TiO2 coating fabricated through one-step micro-arc oxidation. Appl. Surf. Sci. 2020, 508, 144766. [Google Scholar] [CrossRef]
- Schreckenbach, J.P.; Marx, G.; Schlottig, F.; Textor, M.; Spencer, N.D. Characterization of anodic spark-converted titanium surfaces for biomedical applications. J. Mater. Sci. Mater. Med. 1999, 10, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, srep44458. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Li, G.; Zhang, S.; Zhao, R.; Dong, A.; Zhang, R. Preparation and in vitro antibacterial properties of anodic coatings co-doped with Cu, Zn, and P on a Ti-6Al-4V alloy. Mater. Chem. Phys. 2020, 241, 122360. [Google Scholar] [CrossRef]
- Liang, T.; Wang, Y.; Zeng, L.; Liu, Y.; Qiao, L.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Xiang, J.; et al. Copper-doped 3D porous coating developed on Ti-6Al-4V alloys and its in vitro long-term antibacterial ability. Appl. Surf. Sci. 2020, 509, 144717. [Google Scholar] [CrossRef]
- Chen, L.; Jin, X.; Qu, Y.; Wei, K.; Zhang, Y.; Liao, B.; Xue, W. High temperature tribological behavior of microarc oxidation film on Ti-39Nb-6Zr alloy. Surf. Coat. Technol. 2018, 347, 29–37. [Google Scholar] [CrossRef]
- Lan, N.; Yang, W.; Gao, W.; Guo, P.; Zhao, C.; Chen, J. Characterization of ta-C film on micro arc oxidation coated titanium alloy in simulated seawater. Diam. Relat. Mater. 2021, 117, 108483. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Song, L.; Zeng, R.; Li, S.; Han, E. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, M.C.; Yang, Y.; Tan, L.; Zhao, Y.C.; Yin, D.F.; Yang, K.; Atrens, A. Improvement of biodegradable and antibacterial properties by solution treatment and mi-cro-arc oxidation (MAO) of a magnesium alloy with a trace of copper. Corros. Sci. 2019, 156, 125–138. [Google Scholar] [CrossRef]


| Samples | S0 | S2 | S4 | S6 | S8 | S10 | Saline |
|---|---|---|---|---|---|---|---|
| OD value | 1.160 | 0.834 | 0.792 | 0.526 | 0.546 | 0.559 | 0.500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, W.; Yu, S.; Wang, L.; Ma, X.; Gao, W.; Lan, N.; Shao, W.; Chen, J. Microstructure and Properties in Simulated Seawater of Copper-Doped Micro-arc Coatings on TC4 Alloy. Coatings 2022, 12, 883. https://doi.org/10.3390/coatings12070883
Zhang Y, Yang W, Yu S, Wang L, Ma X, Gao W, Lan N, Shao W, Chen J. Microstructure and Properties in Simulated Seawater of Copper-Doped Micro-arc Coatings on TC4 Alloy. Coatings. 2022; 12(7):883. https://doi.org/10.3390/coatings12070883
Chicago/Turabian StyleZhang, Yong, Wei Yang, Sen Yu, Liqun Wang, Xiqun Ma, Wei Gao, Nan Lan, Wenting Shao, and Jian Chen. 2022. "Microstructure and Properties in Simulated Seawater of Copper-Doped Micro-arc Coatings on TC4 Alloy" Coatings 12, no. 7: 883. https://doi.org/10.3390/coatings12070883
APA StyleZhang, Y., Yang, W., Yu, S., Wang, L., Ma, X., Gao, W., Lan, N., Shao, W., & Chen, J. (2022). Microstructure and Properties in Simulated Seawater of Copper-Doped Micro-arc Coatings on TC4 Alloy. Coatings, 12(7), 883. https://doi.org/10.3390/coatings12070883





