The Microwave Facile Synthesis of NiOx@graphene Nanocomposites for Application in Supercapacitors: Insights into the Formation and Storage Mechanisms
Abstract
:1. Introduction
2. Experimental
2.1. Material Characterization
2.2. Materials
2.3. Preparation of the NiOx/Graphene Nanocomposite
2.4. Electrochemical Measurements
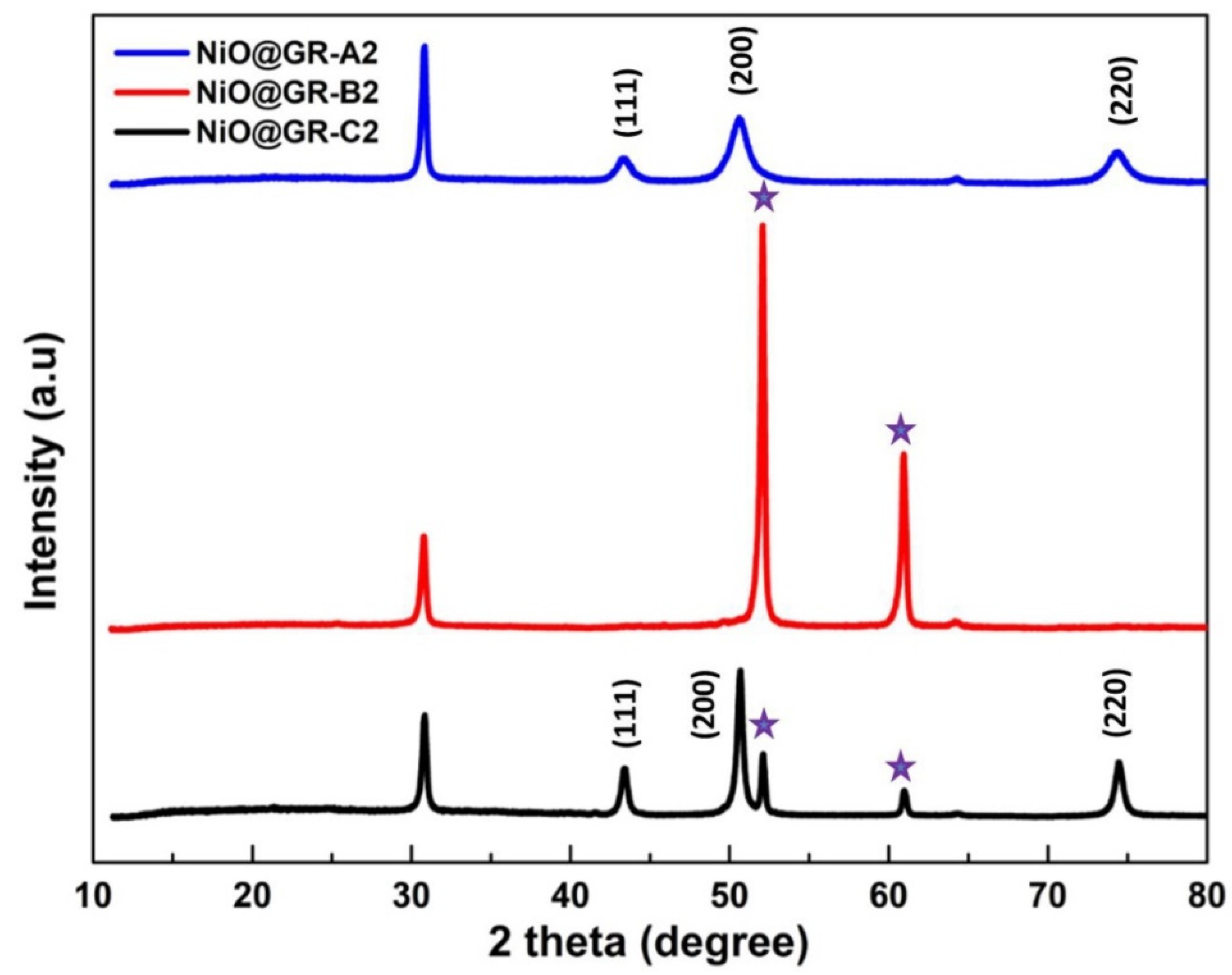
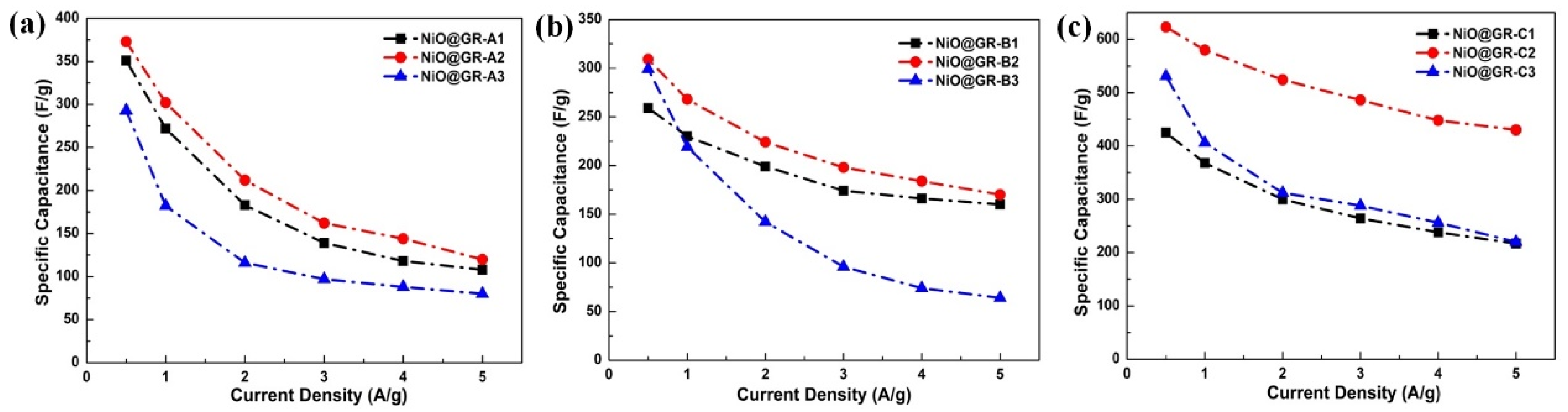
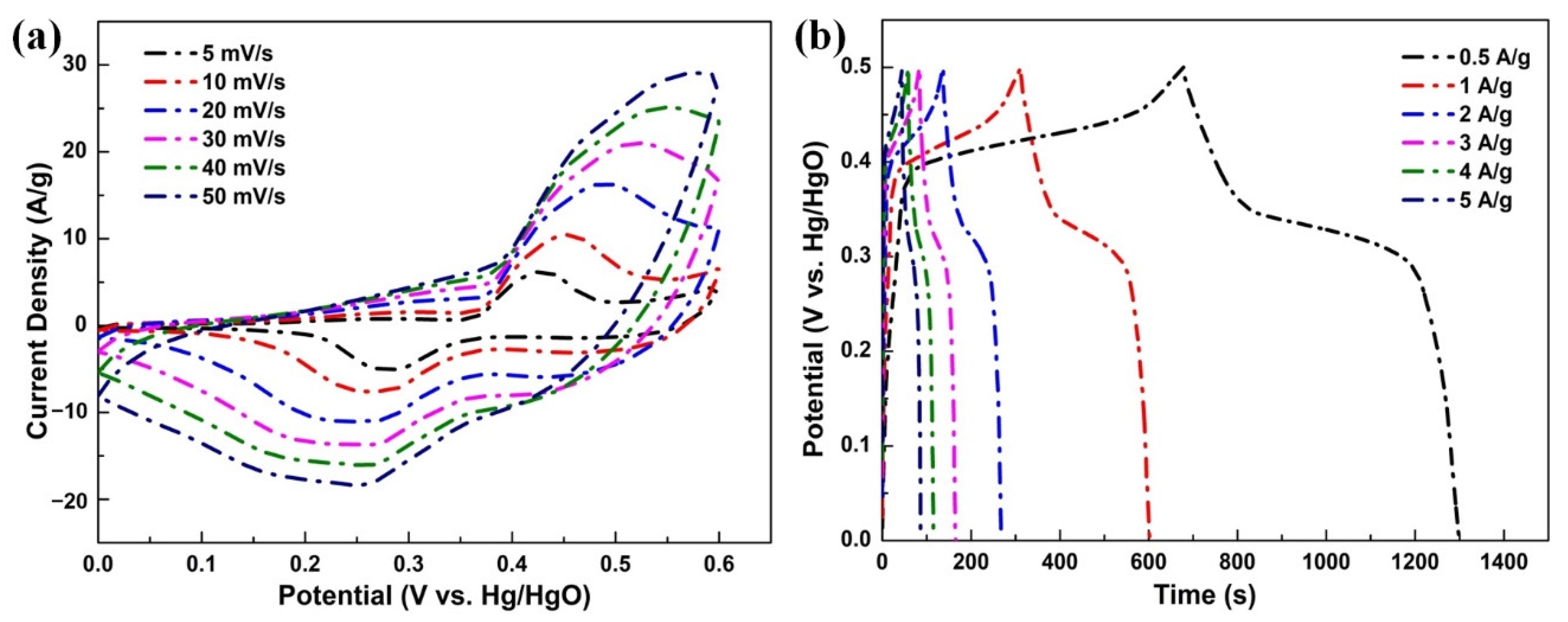
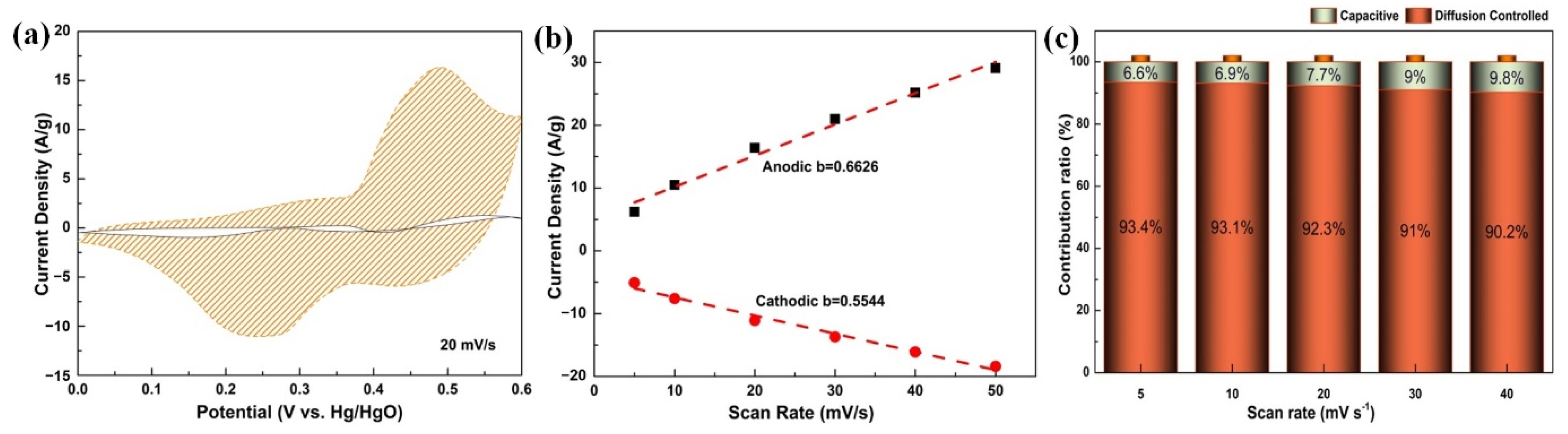
3. Results and Discussion
| Method | Specific Capacitance | Stability | Reaction Time | Ref |
|---|---|---|---|---|
| Thermal decomposition | 417 F/g, 13 A/g | 85.5%, 3000 cycles | 10 min | [48] |
| Hydrothermal and thermal decomposition | 430 F/g, 0.2 A/g | 86.1%, 2000 cycles | 4 h | [44] |
| Solvothermal | 587 F/g, 1 A/g | 98%, 1000 cycles | 12 h | [49] |
| Sol-gel | 628 F/g, 1 A/g | 82.4%, 3000 cycles | 24 h | [34] |
| Hydrothermal | 500 F/g, 5 mV/s | 84%, 3000 cycles | 2 h | [43] |
| Microwave heating | 623 F/g, 0.5 A/g | 62.2%, 4000 cycles | 5 min | Our work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloys Compd. 2018, 734, 89. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Wutthiprom, J.; Chiochan, P.; Suktha, P.; Suksomboon, M.; Kalasina, S.; Sawangphruk, M. Turning conductive carbon nanospheres into nanosheets for high-performance supercapacitors of MnO2 nanorods. Chem. Commun. 2016, 52, 2585. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Yu, L.; Liu, X.; Yang, J.; Wei, M. A new promising Ni-MOF superstructure for high-performance supercapacitors. Chem. Commun. 2020, 56, 1803. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Facile sonochemical assisted synthesis of a hybrid red-black phosphorus/sulfonated porous carbon composite for high-performance supercapacitors. Chem. Commun. 2020, 56, 7096. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.H.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage-a review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Satpati, B.; Nandi, M.; Das, S. NiO-CNT composite for high performance supercapacitor electrode and oxygen evolution reaction. Electrochim. Acta 2018, 283, 327. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Li, Y.; Liu, J. Construction of high-capacitance 3D CoO@polypyrrole nanowire array electrode for aqueous asymmetric supercapacitor. Nano Lett. 2013, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Song, J.; Liu, P.; Xu, W.; Fang, T.; Jiao, Z.; Zhang, H.; Jiang, Y. Monolayer graphene/NiO nanosheets with two-dimension structure for supercapacitors. J. Mater. Chem. 2011, 21, 18792. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Jiao, L.; Mao, Y.; Wang, T.; Duan, X.; Zheng, W. NiO nanomaterials: Controlled fabrication, formation mechanism and the application in lithium-ion battery. CrystEngComm 2012, 14, 453–459. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Zhu, J.; Zhao, W.; Ma, J.; Mhaisalkar, S.; Yan, Q. Synthesis of porous NiO nanocrystals with controllable surface area and their application as supercapacitor electrodes. Nano Res. 2010, 3, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Hotovy, I.; Huran, J.; Siciliano, P.; Capone, S.; Spiess, L.; Rehacek, V. The influences of preparation parameters on NiO thin film properties for gas-sensing application. Sens. Actuators B Chem. 2001, 78, 126–132. [Google Scholar] [CrossRef]
- Motahari, F.; Mozdianfard, M.R.; Soofivand, F.; Salavati-Niasari, M. NiO nanostructures: Synthesis, characterization and photocatalyst application in dye wastewater treatment. RSC Adv. 2014, 4, 27654–27660. [Google Scholar] [CrossRef]
- Lang, J.W.; Kong, L.B.; Wu, W.J.; Luo, Y.C.; Kang, L. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors. Chem. Commun. 2008, 35, 4213–4215. [Google Scholar] [CrossRef] [PubMed]
- Justin, P.; Meher, S.K.; Rao, G.R. Tuning of capacitance behavior of NiO using anionic, cationic, and nonionic surfactants by hydrothermal synthesis. J. Phys. Chem. C 2010, 114, 5203–5210. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, Y.G.; Wang, S.; Zhang, Z.; Fei, L.; Qian, Y. High-yield synthesis of NiO nanoplatelets and their excellent electrochemical performance. Cryst. Growth Des. 2006, 6, 2163–2165. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Liu, J.; Qiao, S.; Ahn, H. Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J. Mater. Chem. A 2011, 21, 3046–3052. [Google Scholar] [CrossRef]
- Liu, M.; Chang, J.; Sun, J.; Gao, L. A facile preparation of NiO/Ni composites as high-performance pseudocapacitor materials. RSC Adv. 2013, 3, 8003–8008. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarkar, D.; Khan, G.G.; Mandal, K. Unique hydrogenated Ni/NiO core/shell 1D nano-heterostructures with superior electrochemical performance as supercapacitors. J. Mater. Chem. A 2013, 1, 12759–12767. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Li, W.; Fang, L.; Wang, Y. Targeted synthesis of novel hierarchical sandwiched NiO/C arrays as high-efficiency lithium ion batteries anode. J. Power Sources 2016, 301, 78–86. [Google Scholar] [CrossRef]
- Hakamada, M.; Abe, T.; Mabuchi, M. Electrodes from carbon nanotubes/NiO nanocomposites synthesized in modified Watts bath for supercapacitors. J. Power Sources 2016, 325, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gao, C.; Li, Q.; Pang, H. Nickel oxide/graphene composites: Synthesis and applications. Chem. Eur. J. 2019, 25, 2141–2160. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, S.; Jin, H.; Zhang, W.; Lin, J.; Wang, J. Synthesis of porous NiO/reduced graphene oxide composites for supercapacitors. J. Electrochem. Soc. 2012, 159, A990. [Google Scholar] [CrossRef]
- Hui, X.; Qian, L.; Harris, G.; Wang, T.; Che, J. Fast fabrication of NiO@graphene composites for supercapacitor electrodes: Combination of reduction and deposition. Mater. Des. 2016, 109, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Li, G.H.; Li, L.H.; Liu, L.; Xu, Y.; Ding, H.Y.; Zhang, T. Large-scale self-assembly of 3D flower-like hierarchical Ni/Co-LDHs microspheres for high-performance flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 2562–2572. [Google Scholar] [CrossRef]
- Nie, L.; Wang, H.; Chai, Y.; Liu, S.; Yuan, R. In situ formation of flower-like CuCo2S4 nanosheets/graphene composites with enhanced lithium storage properties. RSC Adv. 2016, 6, 38321–38327. [Google Scholar] [CrossRef]
- Wu, M.S.; Huang, Y.A.; Yang, C.H. Capacitive behavior of porous nickel oxide/hydroxide electrodes with interconnected nanoflakes synthesized by anodic electrodeposition. J. Electrochem. Soc 2008, 155, A798. [Google Scholar] [CrossRef]
- Michalska, M.; Lipińska, L.; Mirkowska, M.; Aksienionek, M.; Diduszko, R.; Wasiucionek, M. Nanocrystalline lithium-manganese oxide spinels for Li-ion batteries-Sol-gel synthesis and characterization of their structure and selected physical properties. Solid State Ion. 2011, 188, 160–164. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Št’astná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case study of polyvinylidene fluoride doping by carbon nanotubes. Materials 2021, 14, 1428. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.-S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuat. B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Yin, C.; Wan, W.; Xie, H.; Weng, W.; Li, G.; Li, B.; Wang, Y.; Wu, X.; Sun, W. Supercapacitance Performances of Electrodeposited Nickel Oxide/Graphene Nanocomposite. Int. J. Electrochem. Sci. 2019, 14, 4185. [Google Scholar] [CrossRef]
- Lin, J.; Jia, H.; Liang, H.; Chen, S.; Cai, Y.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. In situ synthesis of vertical standing nanosized NiO encapsulated in graphene as electrodes for high-performance supercapacitors. Adv. Sci. 2018, 5, 1700687. [Google Scholar] [CrossRef] [PubMed]
- Dam, D.T.; Wang, X.; Lee, J.-M. Graphene/NiO nanowires: Controllable one-pot synthesis and enhanced pseudocapacitive behavior. ACS Appl. Mater. Inter. 2014, 6, 8246. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.-A.; Lau, J.; Lin, T.; Tolbert, S.H.; Dunn, B.S.; Pilon, L. Physical interpretations of electrochemical impedance spectroscopy of redox active electrodes for electrical energy storage. J. Phys. Chem. C 2018, 122, 24499. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J.; Wang, R. Surface Engineered Polar CeO2-based Cathode Host Materials for Immobilizing Lithium Polysulfides in High-performance Li-S Batteries. Appl. Surf. Sci. 2021, 580, 152237. [Google Scholar] [CrossRef]
- Lenz, M.; Zabel, J.; Franzreb, M. New approach for investigating diffusion kinetics within capacitive deionization electrodes using electrochemical impedance spectroscopy. Front. Mater. 2020, 7, 229. [Google Scholar] [CrossRef]
- Liu, P.; Yang, M.; Zhou, S.; Huang, Y.; Zhu, Y. Hierarchical shell-core structures of concave spherical NiO nanospines@carbon for high performance supercapacitor electrodes. Electrochim. Acta 2019, 294, 383. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced energy storage devices: Basic principles, analytical methods, and rational materials design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Vijeth, H.; Ashokkumar, S.P.; Yesappa, L.; Vandana, M.; Devendrappa, H. Hybrid core-shell nanostructure made of chitosan incorporated polypyrrole nanotubes decorated with NiO for all-solid-state symmetric supercapacitor application. Electrochim. Acta 2020, 354, 136651. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl. Mater. Inter. 2013, 5, 2188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhuang, H.; Fang, T.; Jiao, Z.; Liu, R.; Ling, X.; Lu, B.; Jiang, Y. Self-assembly of NiO/graphene with three-dimension hierarchical structure as high performance electrode material for supercapacitors. J. Alloys Compd. 2014, 597, 291. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, J.; Wang, L.; Li, Y.; Huang, Z.; Chen, S.; Ma, Y.; Wang, L.; Yan, X. Synthesis of shape-controlled NiO-graphene nanocomposites with enhanced supercapacitive properties. New J. Chem. 2015, 39, 4026. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, D.; Song, J.; Jiao, Z.; Ma, Q.; Zhang, H.; Cheng, L.; Zhao, B.; Chu, Y. A facile hydrothermal synthesis of graphene porous NiO nanocomposite and its application in electrochemical capacitors. Electrochim. Acta 2013, 91, 173. [Google Scholar] [CrossRef]
- Su, X.; Chai, H.; Jia, D.; Bao, S.; Zhou, W.; Zhou, M. Effective microwave-assisted synthesis of graphene nanosheets/NiO composite for high-performance supercapacitors. New J. Chem. 2013, 37, 439. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Huo, J.; Zhang, P.; Strømme, M.; Nyholm, L. Solution-processed poly (3,4-ethylenedioxythiophene) nanocomposite paper electrodes for high-capacitance flexible supercapacitors. J. Mater. Chem. A 2016, 4, 1714. [Google Scholar] [CrossRef]
- Chen, W.; Gui, D.; Liu, J. Nickel oxide/graphene aerogel nanocomposite as a supercapacitor electrode material with extremely wide working potential window. Electrochim. Acta 2016, 222, 1424. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Wei, Z.; Wang, R.; Zhang, X. The Microwave Facile Synthesis of NiOx@graphene Nanocomposites for Application in Supercapacitors: Insights into the Formation and Storage Mechanisms. Coatings 2022, 12, 1060. https://doi.org/10.3390/coatings12081060
Liang Y, Wei Z, Wang R, Zhang X. The Microwave Facile Synthesis of NiOx@graphene Nanocomposites for Application in Supercapacitors: Insights into the Formation and Storage Mechanisms. Coatings. 2022; 12(8):1060. https://doi.org/10.3390/coatings12081060
Chicago/Turabian StyleLiang, Yue, Zhen Wei, Ruigang Wang, and Xinyu Zhang. 2022. "The Microwave Facile Synthesis of NiOx@graphene Nanocomposites for Application in Supercapacitors: Insights into the Formation and Storage Mechanisms" Coatings 12, no. 8: 1060. https://doi.org/10.3390/coatings12081060






