Effect of Humidity on the Thermal Properties of Aluminum Nanopowders with Different Surface Coatings
Abstract
:1. Introduction
2. Experiment
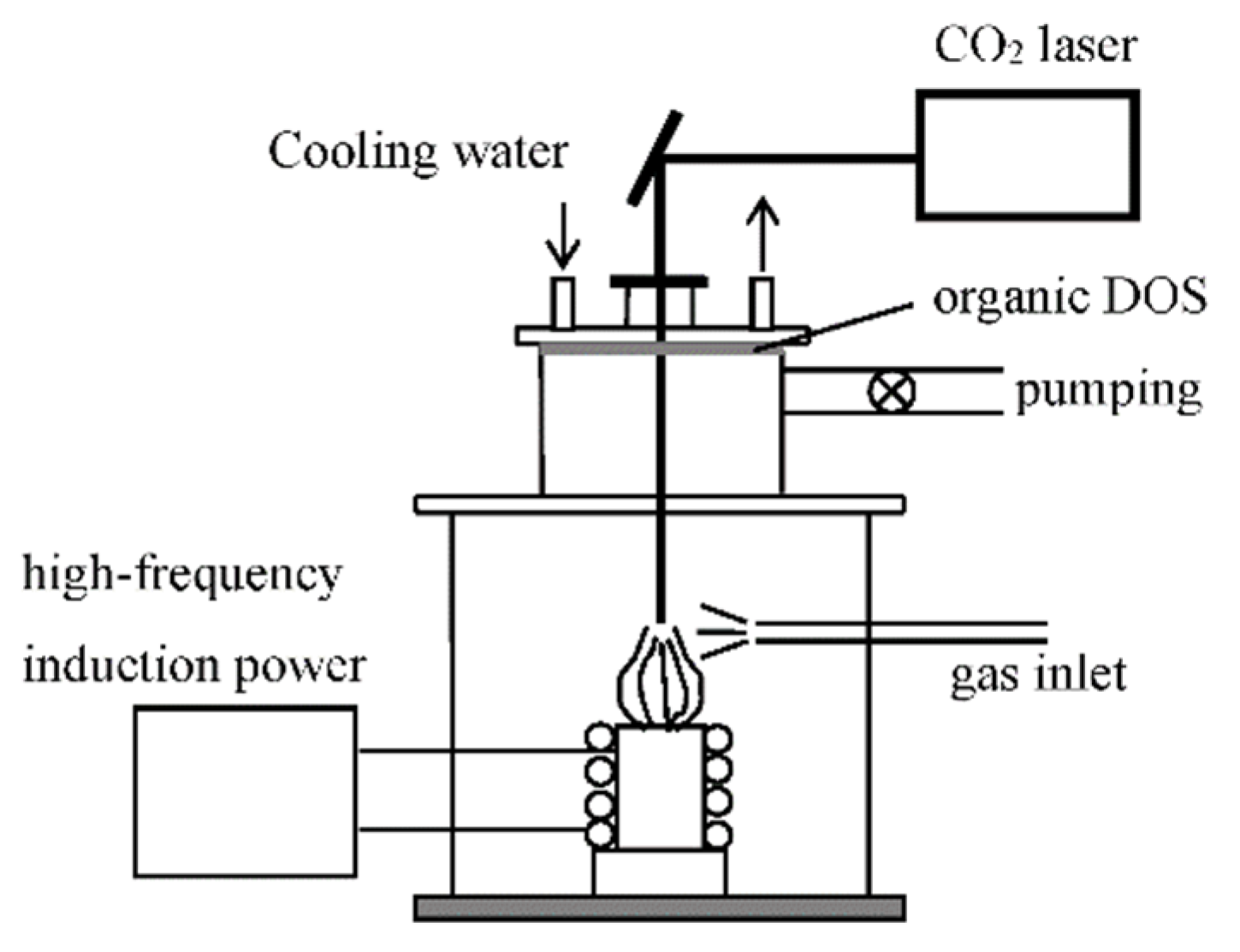
2.1. Materials Preparations
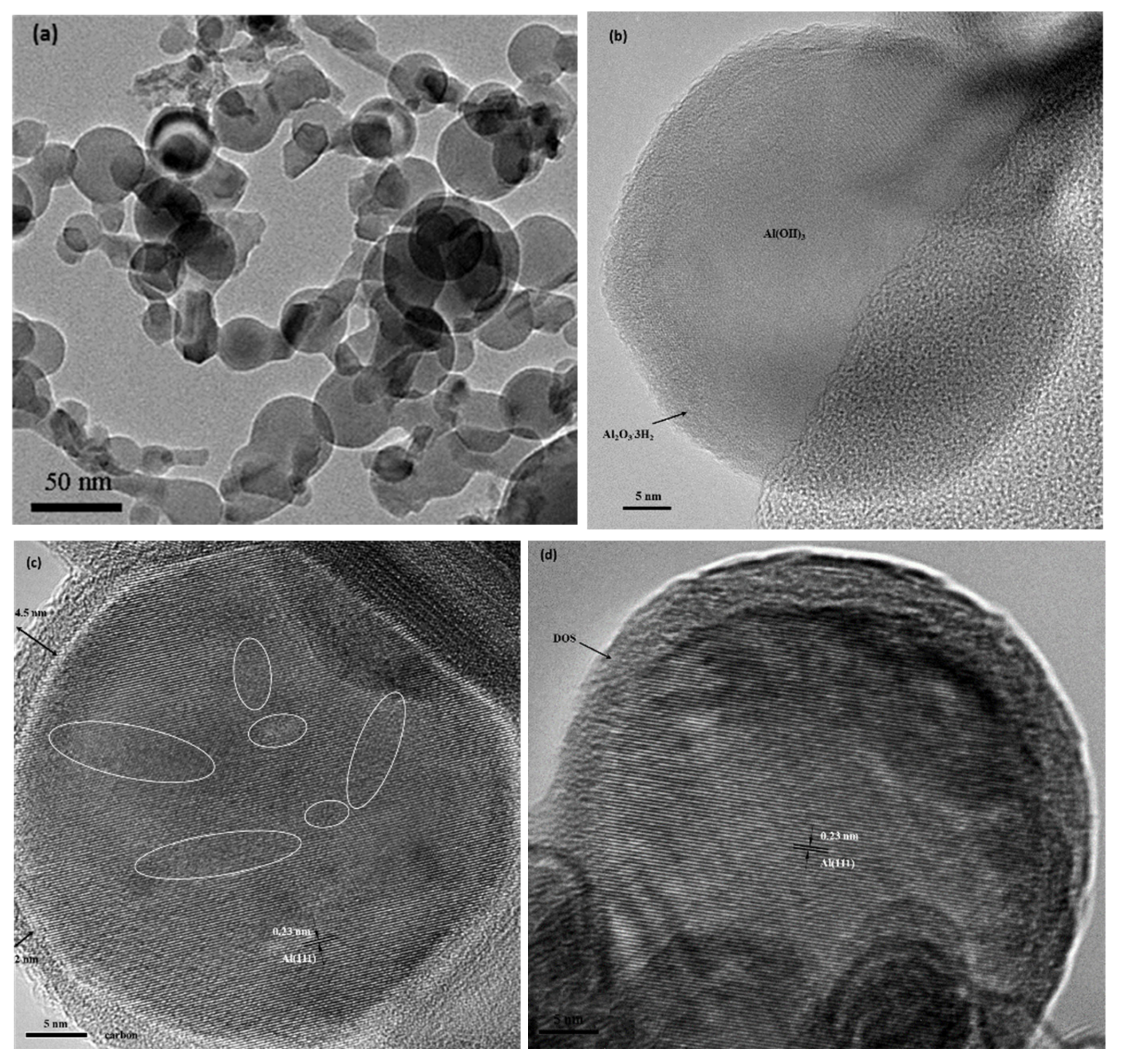
2.2. Materials Characterizations and Measurements
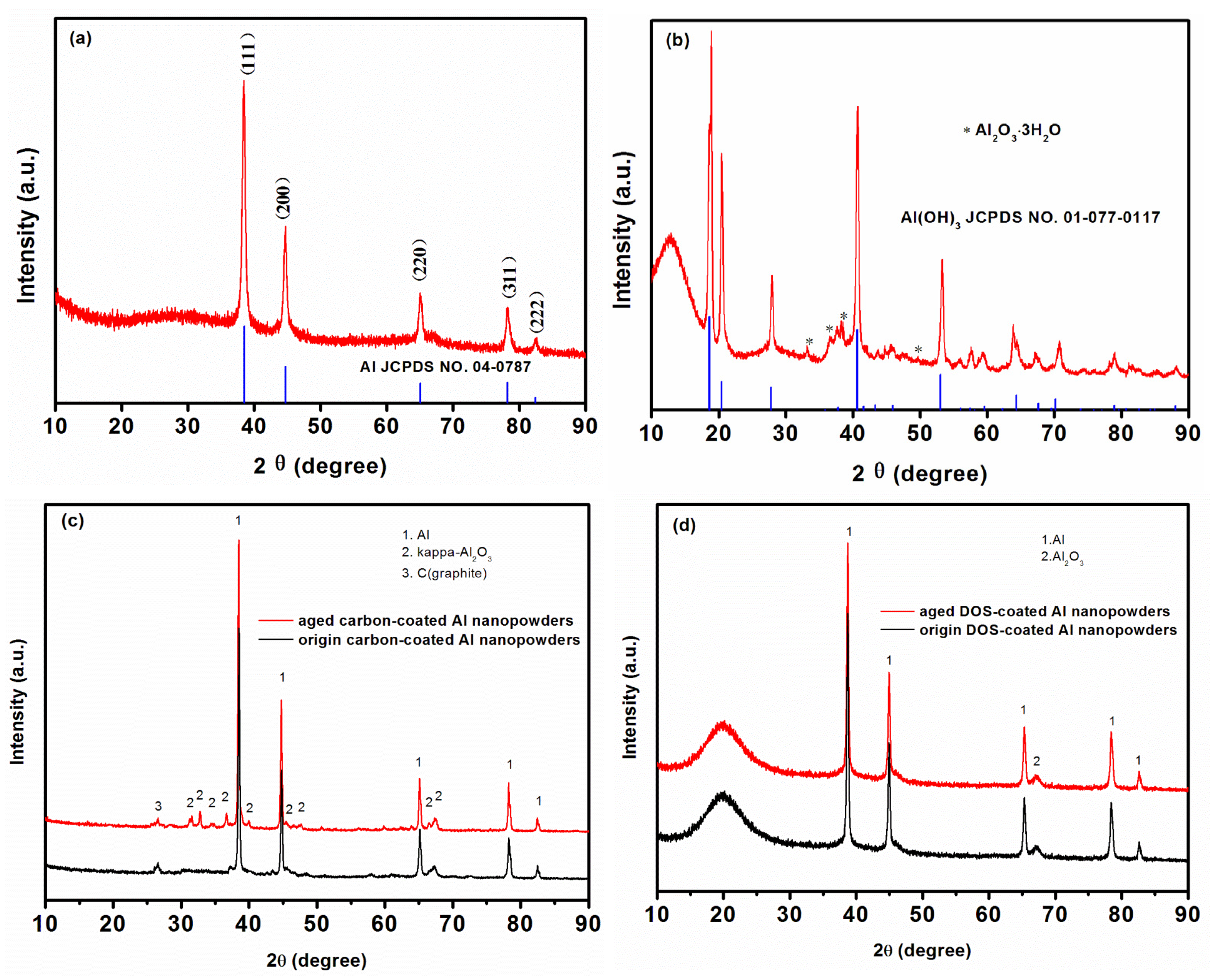
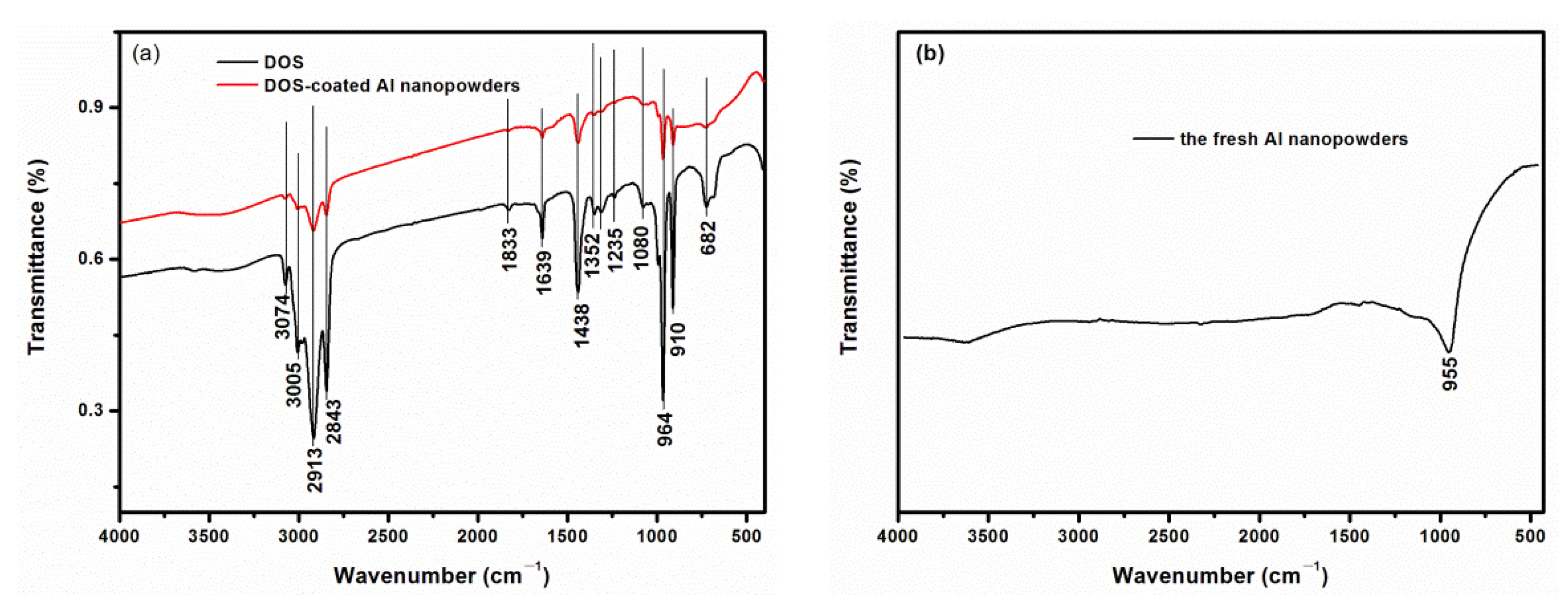
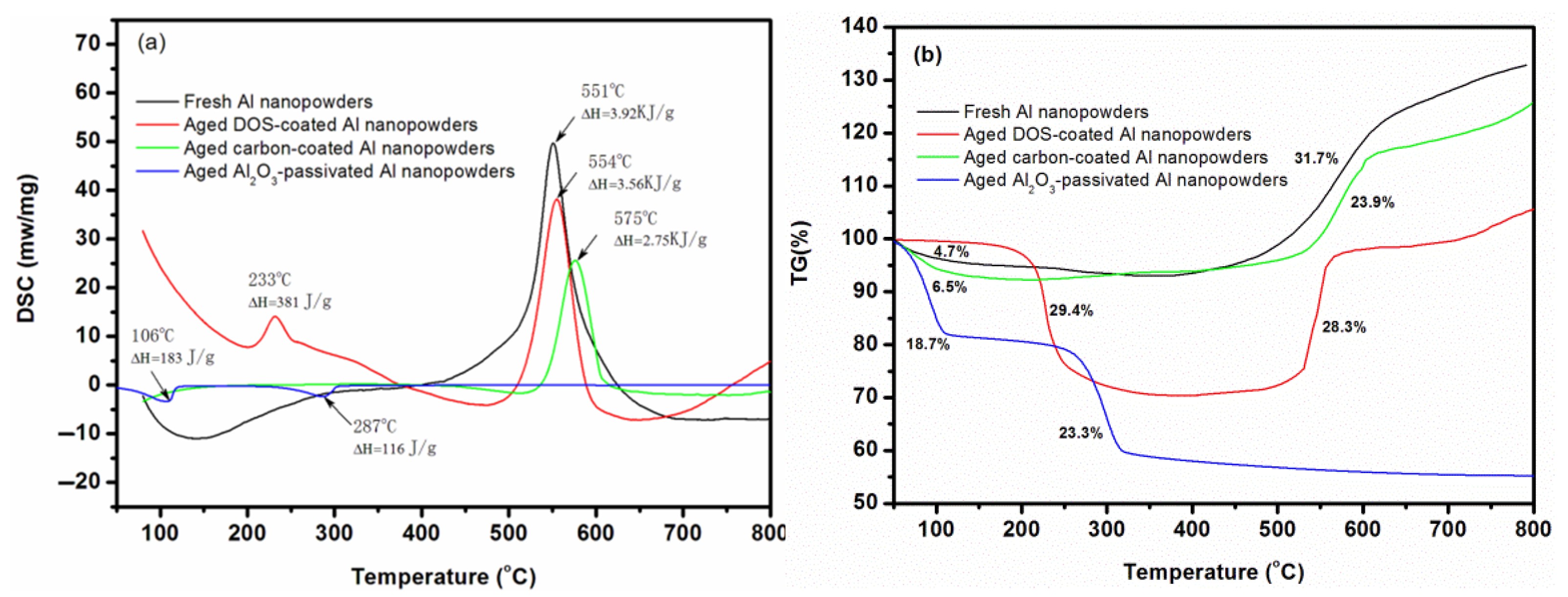
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, F.; Yao, E.; Xu, S.; Xu, H.; Hao, H. Laser Ignition of Different Aluminum Nanopowders for Solid Rocket Propulsion. In Chemical Rocket Propulsion; Springer Aerospace Technology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–295. [Google Scholar] [CrossRef]
- Ding, A.; Hao, J.; Dong, X.; Huang, J.; Qian, K.; Ji, S.; Zhang, Y.; Li, M. Production, characterization, and flowability of assemblies consisting of highly active aluminum nanoparticles. J. Propuls. Power 2017, 33, 1–6. [Google Scholar] [CrossRef]
- Lade, R.; Wasewar, K.; Sangtyani, R.; Kumar, A.; Shende, D.; Peshwe, D. Effect of aluminum nanoparticles on rheological behavior of HTPB-based composite rocket propellant. J. Energ. Mater. 2018, 37, 125–140. [Google Scholar] [CrossRef]
- Brousseau, P.; Anderson, C.J. Nanometric aluminum in explosives. Propellants Explos. Pyrotech. 2002, 27, 300–306. [Google Scholar] [CrossRef]
- E, X.-T.-F.; Zhang, L.; Wang, F.; Zhang, X.; Zou, J.-J. Synthesis of aluminum nanoparticles as additive to enhance ignition and combustion of high energy density fuels. Front. Chem. Sci. Eng. 2018, 12, 358–366. [Google Scholar] [CrossRef]
- Laboureur, D.; Glabeke, G.; Gouriet, J.B. Aging behavior of aluminum nanopowders: Accelerated aging experiments and modeling of the influence of temperature and humidity on the aluminum content. J. Nanopart. Res. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.J.; Yang, Y.H.; Yang, Y.J.; Huang, X.F. Effect of heating rate on ignition characteristics of newly prepared and aged aluminum nanoparticles. Propellants Explos. Pyrotech. 2020, 45, 1428–1435. [Google Scholar] [CrossRef]
- Younes, P.A.; Sayegh, S.; Nada, A.A.; Weber, M.; Iatsunskyi, I.; Coy, E.; Abboud, N.; Bechelany, M. Elaboration of porous alumina nanofibers by electrospinning and molecular layer deposition for organic pollutant removal. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127274. [Google Scholar] [CrossRef]
- Kwok, Q.S.M.; Fouchard, R.C.; Turcotte, A.-M.; Lightfoot, P.D.; Bowes, R.; Jones, D.E.G. Characterization of aluminum nanopowder compositions. Propellants Explos. Pyrotech. 2002, 27, 229–240. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Xie, C.; Zeng, D.; Wang, A.; Hu, M. Influence of humidity on the thermal behavior of aluminum nanopowders. Mater. Chem. Phys. 2006, 97, 127–131. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; A Gromov, A.; Ilyin, A.P. Reactivity of superfine aluminum powders stabilized by aluminum diboride. Combust. Flame 2002, 131, 349–352. [Google Scholar] [CrossRef]
- Foley, T.J.; Johnson, A.C.E.; Higa, K.T. Inhibition of oxide formation on aluminum nanoparticles by transition metal coating. Chem. Mater. 2005, 17, 4086–4091. [Google Scholar] [CrossRef]
- Singh, M.; Naspoori, S.K.; Arghode, V.K.; Kumar, R. Study of nickel-coated aluminum nanoparticles using molecular dynamic simulations and thermodynamic modeling. J. Nanopart. Res. 2020, 22, 1–16. [Google Scholar] [CrossRef]
- Jouet, R.J.; Warren, A.D.; Rosenberg, D.M.; Bellitto, V.J.; Park, K.; Zachariah, M.R. Surface passivation of bare aluminum nanoparticles using perfluoroalkyl carboxylic acids. Chem. Mater. 2005, 17, 2987–2996. [Google Scholar] [CrossRef]
- Gromov, A.A.; Forter-Barth, U.; Teipel, U. Aluminum nanopowders produced by electrical explosion of wires and passivated by non-inert coatings: Characterization and reactivity with air and water. Powder Technol. 2006, 164, 111–115. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Alexander, A.G.; Julia, I.S. Passivation of the surface of aluminum nanopowders by protective coatings of the different chemical origin. Appl. Surf. Sci. 2007, 253, 5558–5564. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, J.; He, G.; Huang, C.; Yang, Z.; Liu, S.; Gong, F. Enhanced water resistance and energy performance of core–shell aluminum nanoparticles via in situ grafting of energetic glycidyl azide polymer. J. Mater. Sci. 2018, 53, 12091–12102. [Google Scholar] [CrossRef]
- Miller, K.K.; Gottfried, J.; Walck, S.D.; Pantoya, M.L.; Wu, C.-C. Plasma surface treatment of aluminum nanoparticles for energetic material applications. Combust. Flame 2019, 206, 211–213. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Rodkevich, N.G.; Vorozhtsov, A.B.; Lerner, M.I. Oxidation and oxidation products of encapsulated aluminum nanopowders. J. Nanopart. Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Ao, W.; Gao, Y.; Zhou, S.; Li, L.K.; He, W.; Liu, P.; Yan, Q.-L. Enhancing the stability and combustion of a nanofluid fuel with polydopamine-coated aluminum nanoparticles. Chem. Eng. J. 2021, 418, 129527. [Google Scholar] [CrossRef]
- Zhao, W.; Jiao, Q.; Ou, Y.; Yang, R.; Zhu, Y.; Wang, F. Perfluoroalkyl acid-functionalized aluminum nanoparticles for fluorine fixation and energy generation. ACS Appl. Nano Mater. 2021, 4, 6337–6344. [Google Scholar] [CrossRef]
- Ju, Z.; An, J.; Guo, C.; Li, T.-R.; Jia, Z.-Y.; Wu, R.-F. The oxidation reaction and sensitivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene. J. Energ. Mater. 2020, 39, 299–312. [Google Scholar] [CrossRef]
- Song, L.; Zhao, F.-Q.; Xu, S.-Y.; Ju, X.-H. Encapsulating aluminum nanoparticles into carbon nanotubes for combustion: A molecular dynamics study. Phys. Chem. Chem. Phys. 2021, 23, 11886–11892. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Liu, J.Z.; Shan, S.Q.; Yang, W.J. Adsorption mechanism of oleic acid on the surface of aluminum nanoparticle: ReaxFF molecular dynamics simulation and experimental study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126500. [Google Scholar] [CrossRef]
- Weeks, N.J.; Gazmin, E.; Iacono, S.T. Optimizing the interfaces of energetic textiles with perfluorinated oligomer-coated aluminum nanoparticles: Implications for metastable intermolecular composites. ACS Appl. Nano Mater. 2021, 4, 6002–6011. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Baek, J.; Wang, H.; Gottfried, J.L.; Wu, C.-C.; Shi, X.; Zachariah, M.R.; Zheng, X. Ignition and combustion of Perfluoroalkyl-functionalized aluminum nanoparticles and nanothermite. Combust. Flame 2022, 242, 112170. [Google Scholar] [CrossRef]
- Biswas, P.; Xu, F.; Ghildiyal, P.; Zachariah, M.R. In-situ thermochemical shock-induced stress at the metal/oxide interface enhances reactivity of aluminum nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 26782–26790. [Google Scholar] [CrossRef]
- Guo, L.; Song, W.; Xie, C.; Zhang, X.; Hu, M. Characterization and thermal properties of carbon-coated aluminum nanopowders prepared by laser-induction complex heating in methane. Mater. Lett. 2007, 61, 3211–3214. [Google Scholar] [CrossRef]
- Guo, L.; Song, W.; Hu, M.; Xie, C.; Chen, X. Preparation and reactivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene (HTPB). Appl. Surf. Sci. 2008, 254, 2413–2417. [Google Scholar] [CrossRef]
- Chung, S.W.; Guliants, E.A.; Bunker, C.E.; Jelliss, P.A.; Buckner, S.W. Size-dependent nanoparticle reaction enthalpy: Oxidation of aluminum nanoparticles. J. Phys. Chem. Solids 2011, 72, 719–724. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Li, Y.; Song, W.; He, B.; Yang, M.; Zhu, L. Effect of Humidity on the Thermal Properties of Aluminum Nanopowders with Different Surface Coatings. Coatings 2022, 12, 1147. https://doi.org/10.3390/coatings12081147
Guo L, Li Y, Song W, He B, Yang M, Zhu L. Effect of Humidity on the Thermal Properties of Aluminum Nanopowders with Different Surface Coatings. Coatings. 2022; 12(8):1147. https://doi.org/10.3390/coatings12081147
Chicago/Turabian StyleGuo, Liangui, Yulin Li, Wulin Song, Bianyang He, Mengli Yang, and Lei Zhu. 2022. "Effect of Humidity on the Thermal Properties of Aluminum Nanopowders with Different Surface Coatings" Coatings 12, no. 8: 1147. https://doi.org/10.3390/coatings12081147
APA StyleGuo, L., Li, Y., Song, W., He, B., Yang, M., & Zhu, L. (2022). Effect of Humidity on the Thermal Properties of Aluminum Nanopowders with Different Surface Coatings. Coatings, 12(8), 1147. https://doi.org/10.3390/coatings12081147





