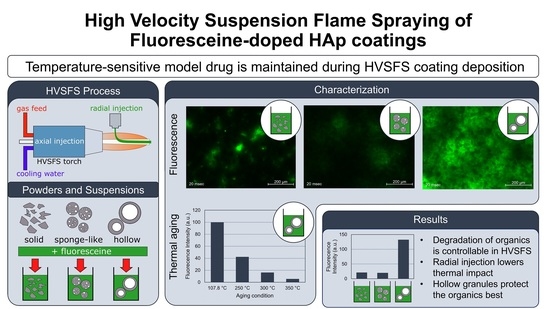
Deposition of Fluoresceine-Doped HAp Coatings via High-Velocity Suspension Flame Spraying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Suspension Preparation
2.1.1. HAp Suspension for Axial Injection
2.1.2. Fluoresceine-Doped HAp Suspensions for Radial Injection
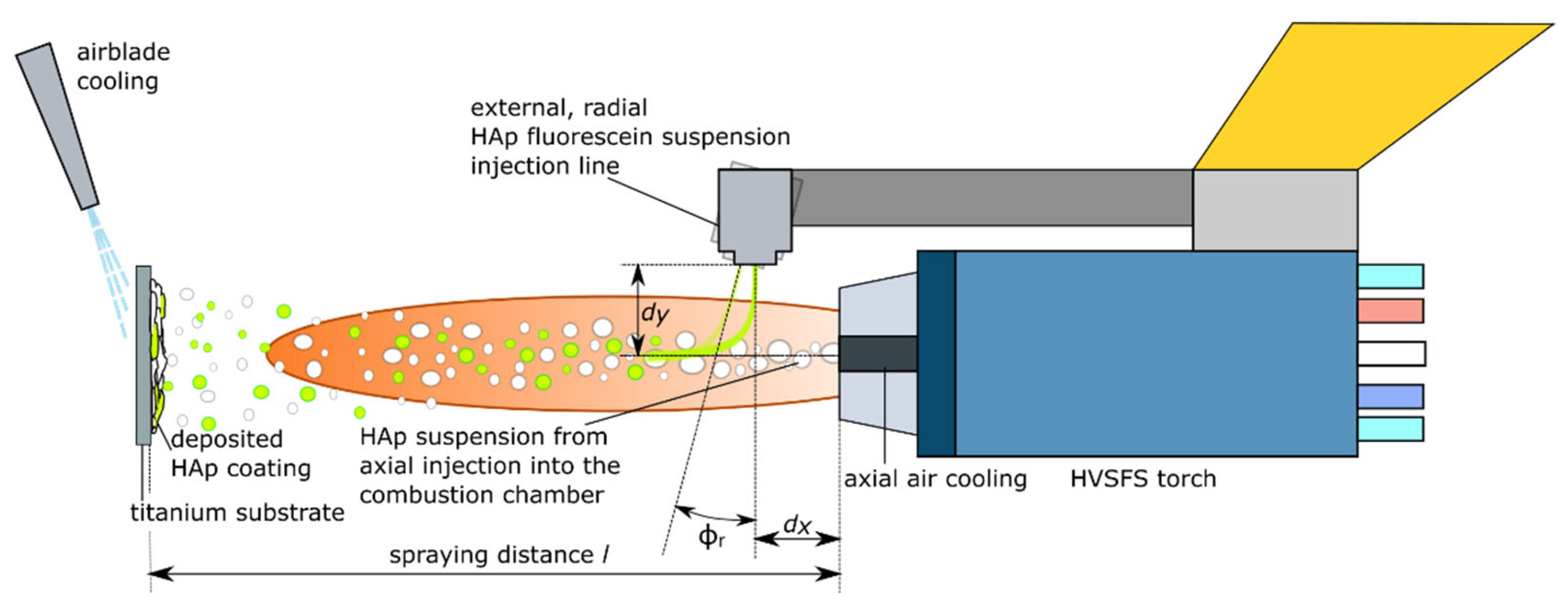
2.2. Coating Deposition
2.3. Feedstock and Coating Characterization
3. Results
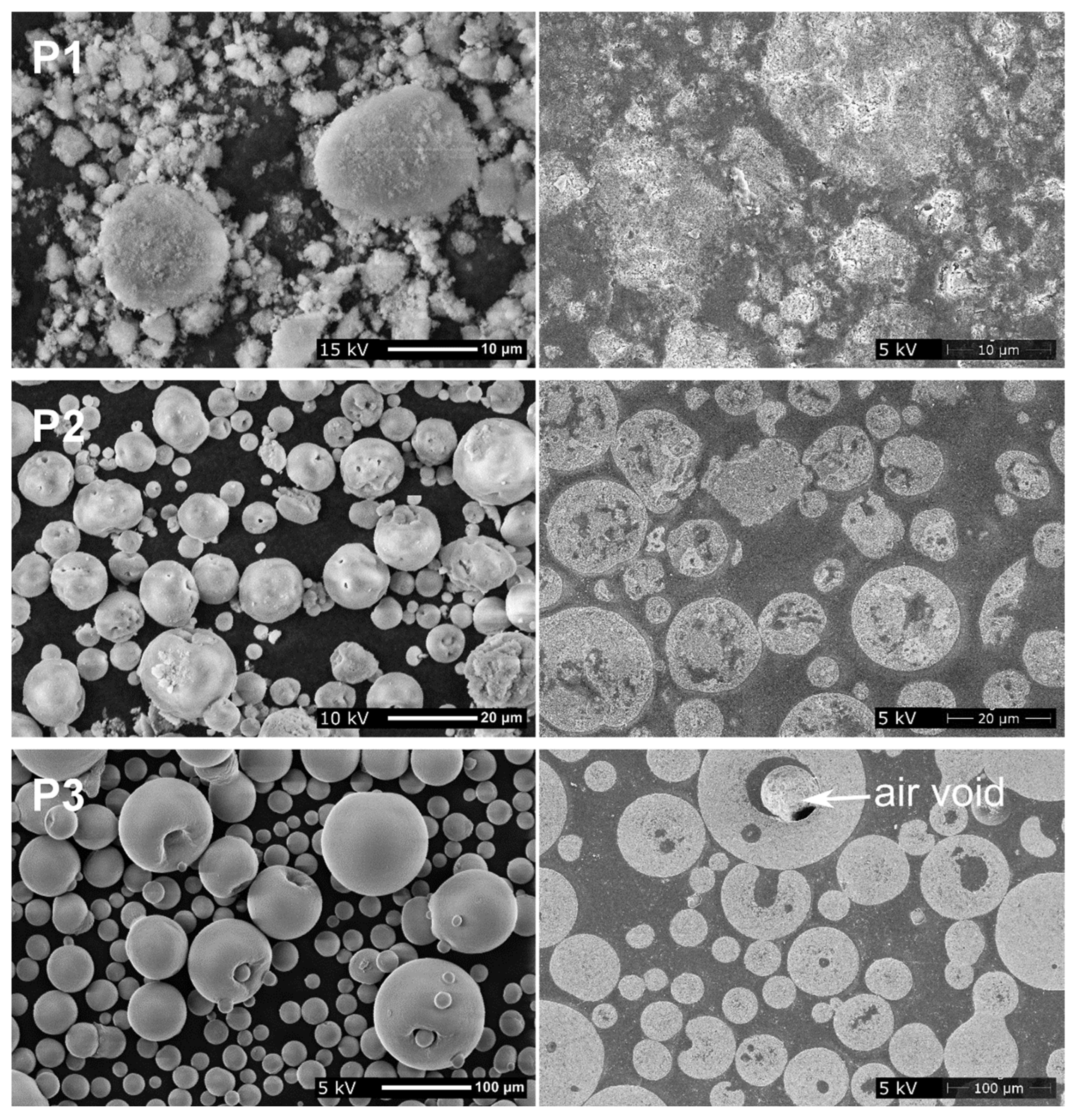
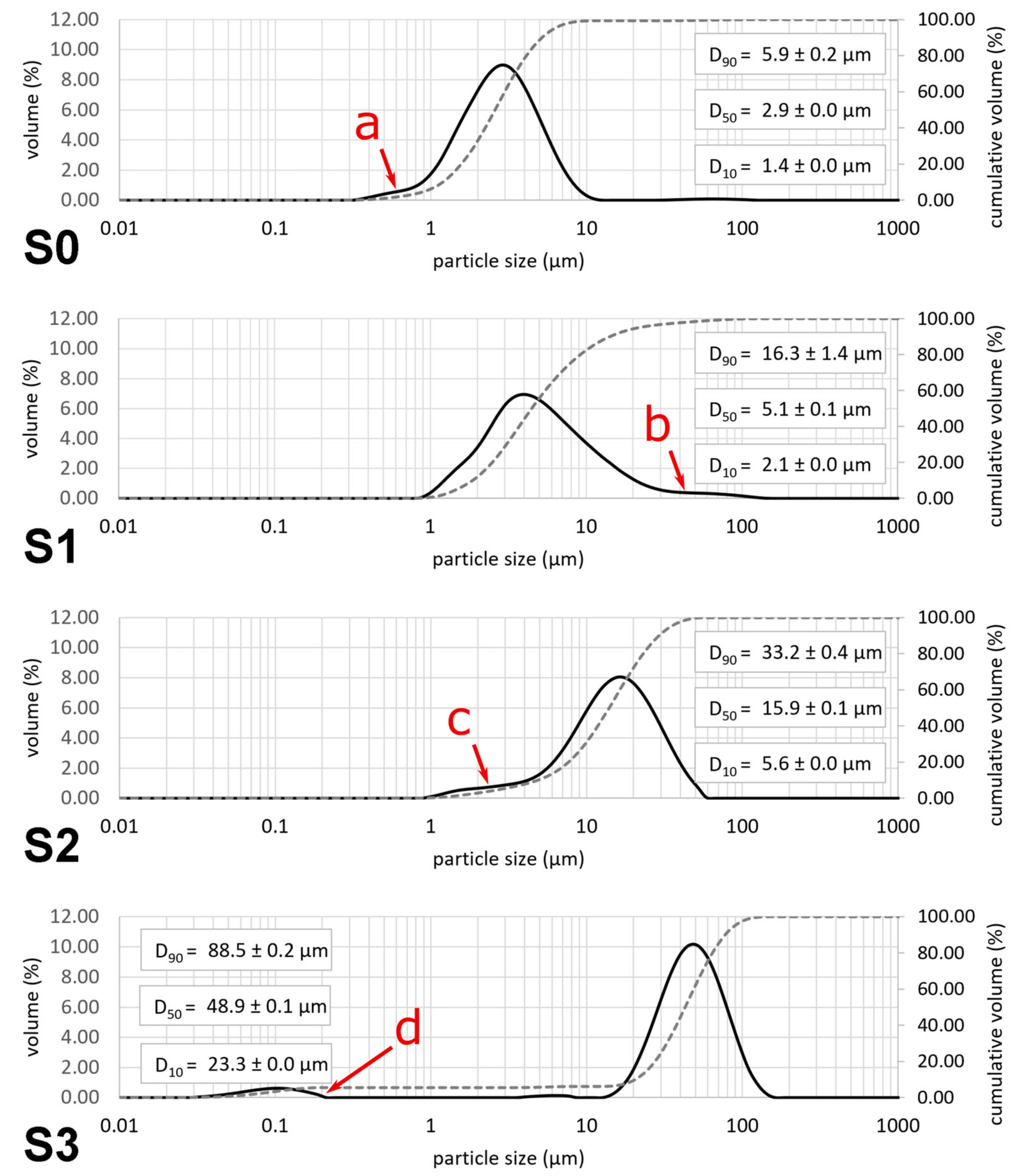
3.1. Powders
3.2. Suspensions Used for Coating Experiments
3.3. Properties of the Deposited Coatings
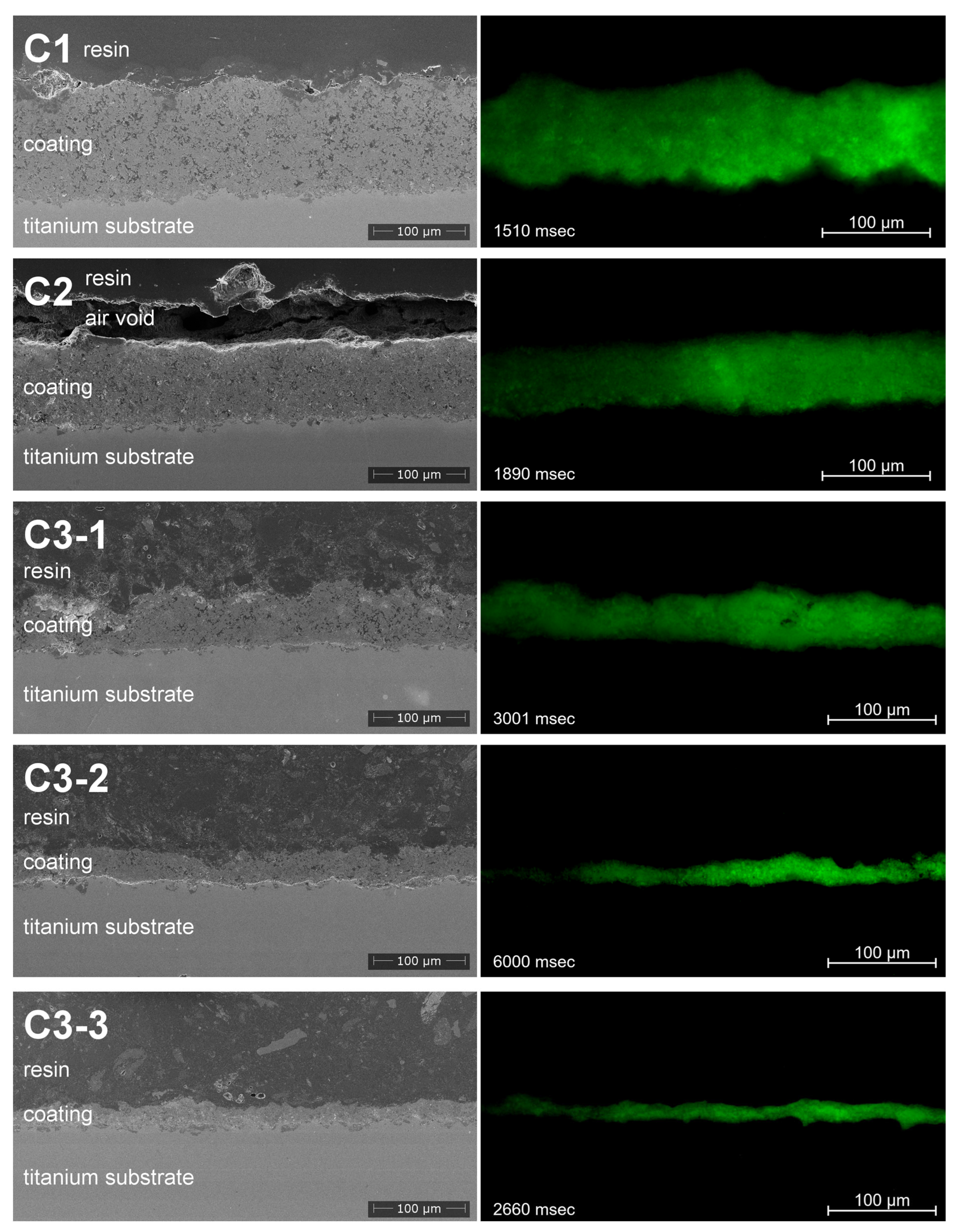
3.4. Coating Morphology
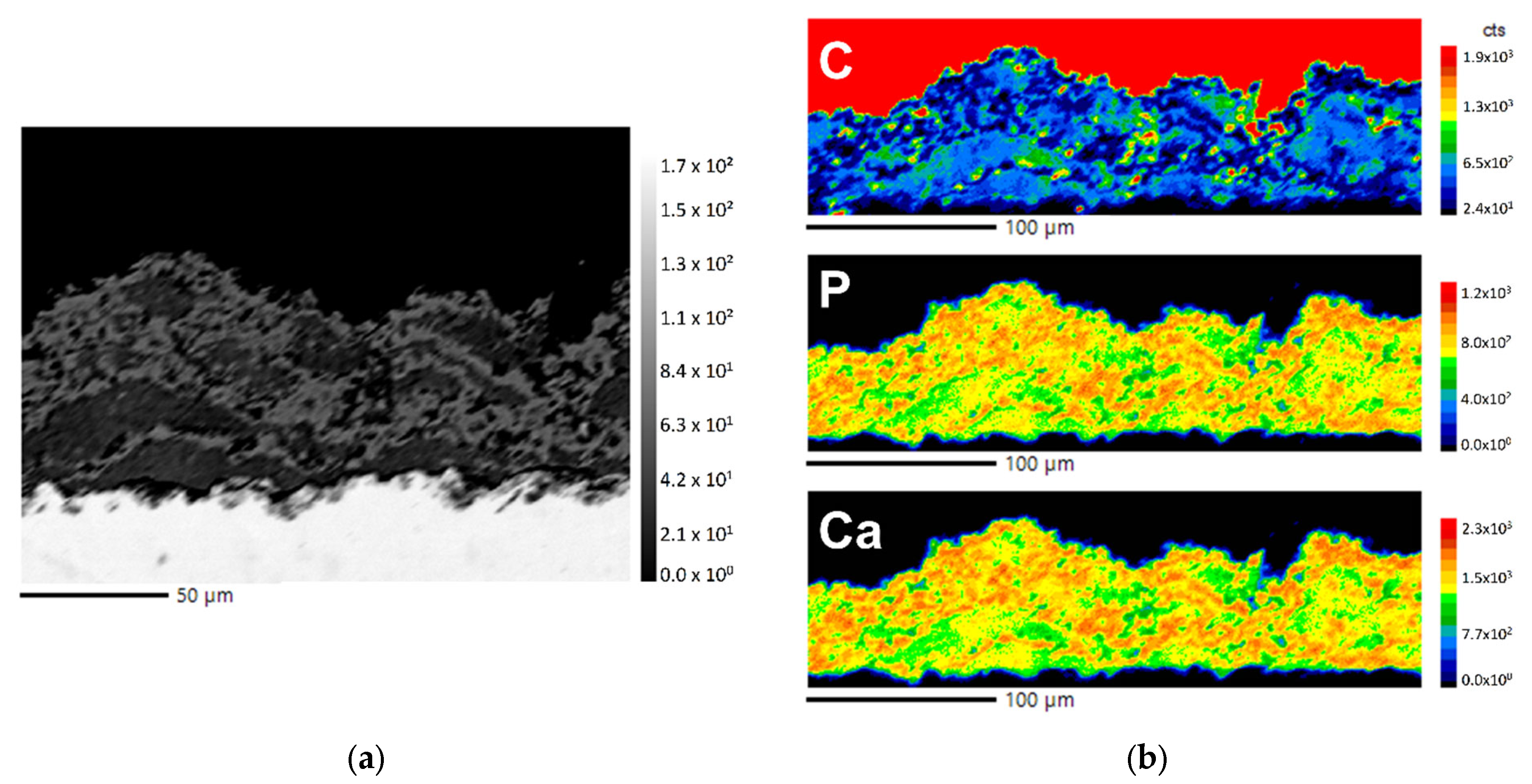
3.5. Element Analysis
3.6. Fluorescence Properties in Correlation to Suspension Formulation and Process Parameters
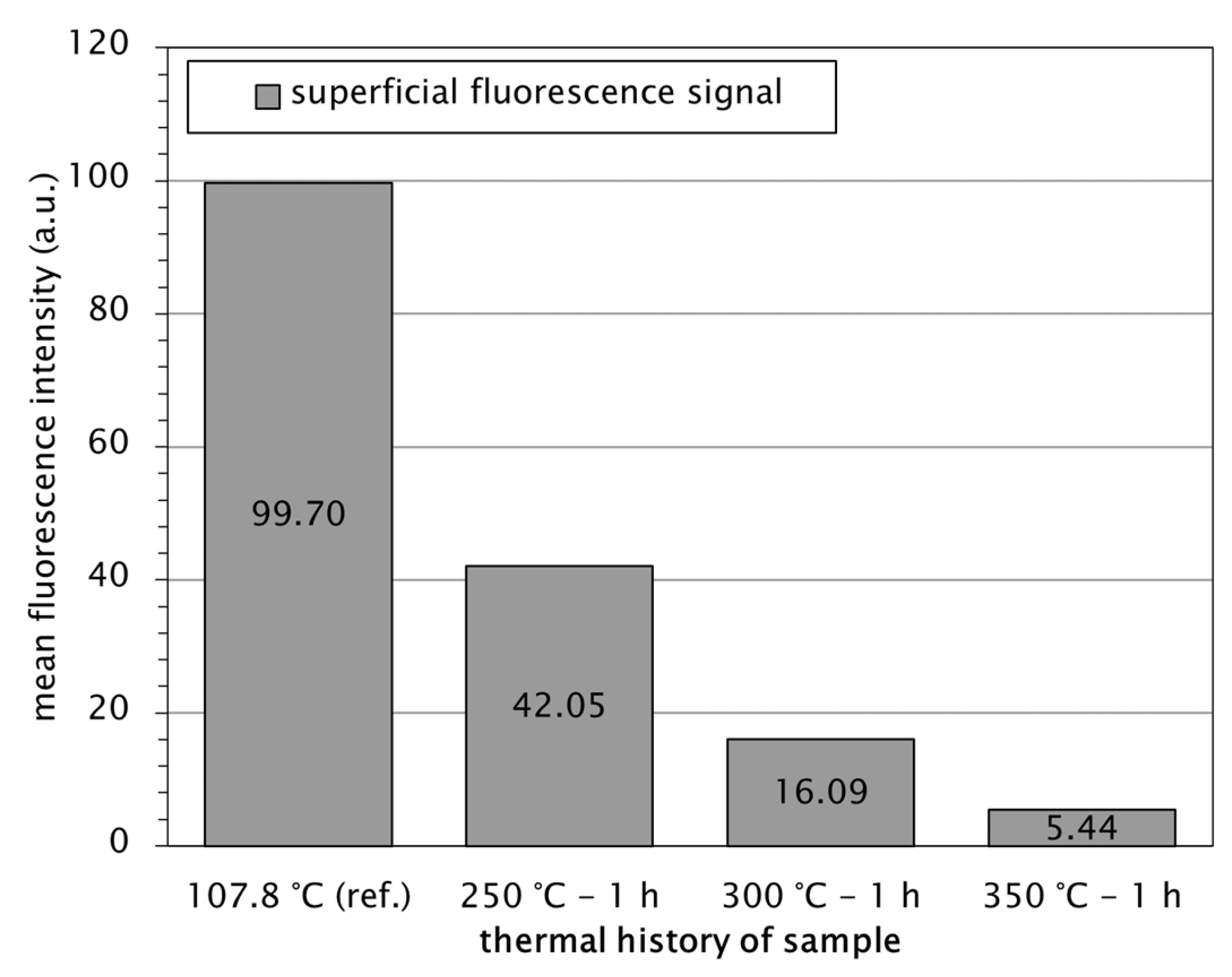
3.7. Thermal Degradation Behaviour of Fluoresceine
4. Discussion
4.1. Suspension Preparation
4.2. Coating Process Characteristics
4.3. Hardness of the Coatings
4.4. Coating Microstructure and Composition
4.5. Fluorescence Imaging of Cross-Sections
4.6. Decomposition of Fluoresceine and Correlation with Powder Morphology and Process Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto-Lambertz, C.; Yagdiran, A.; Wallscheid, F.; Eysel, P.; Jung, N. Periprosthetic Infection in Joint Replacement. Dtsch. Arztebl. Int. 2017, 114, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Institut für Medizinische Dokumentation und Information. Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik) Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern. Statistisches Bundesamt (Destatis): Wiesbaden, Germany, 2019. [Google Scholar]
- Haaker, R.; Senge, A.; Krämer, J.; Rubenthaler, F. Osteomyelitis nach Endoprothesen. Orthopade 2004, 33, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Uchida, A.; Yamakawa, T.; Shinto, Y.; Araki, N.; Kato, K. Treatment of chronic osteomyelitis using calcium hydroxyapatite ceramic implants impregnated with antibiotic. Int. Orthop. 1998, 22, 247–251. [Google Scholar] [CrossRef] [PubMed]
- de Groot, K.; Klein, C.; Wolke, J.; de Blieck-Hogervorst, J. Plasma-sprayed coatings of calcium phosphate. In Handbook of Bioactive Ceramics; Yamamuro, T., Hench, L.L., Wilson, J., Eds.; CRC Press: Boca Raton, FL, USA; Ann Arbor, MI, USA; Boston, MA, USA, 1990. [Google Scholar]
- Ha, S.W.; Gisep, A.; Mayer, J.; Wintermantel, E.; Gruner, H.; Wieland, M. Topographical characterization and microstructural interface analysis of vacuum-plasma-sprayed titanium and hydroxyapatite coatings on carbon fibre-reinforced poly (etheretherketone). J. Mater. Sci. Mater. Med. 1997, 8, 891–896. [Google Scholar] [CrossRef]
- McPherson, E.J.; Dorr, L.D.; Gruen, T.A.; Saberi, M.T. Hydroxyapatite-coated proximal ingrowth femoral stems. A matched pair control study. Clin. Orthop. Relat. Res. 1995, 315, 223–230. [Google Scholar]
- Klein, C.; Patka, P.; Wolke, J.; de Blieck-Hogervorst, J.; de Groot, K. Long-term in vivo study of plasma-sprayed coatings on titanium alloys of tetracalcium phosphate, hydroxyapatite and α-tricalcium phosphate. Biomaterials 1994, 15, 146–150. [Google Scholar] [CrossRef]
- Gruner, H. Thermal Spray Coatings on Titanium. In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 375–416. ISBN 978-3-642-63119-1. [Google Scholar]
- Heimann, R.B. Recent trends towards improved plasma-sprayed advanced bioceramic coatings on Ti6Al4V implants. Mater. und Werkst. 1999, 30, 775–782. [Google Scholar] [CrossRef]
- Jaworski, R.; Pierlot, C.; Pawlowski, L.; Bigan, M.; Quivrin, M. Synthesis and Preliminary Tests of Suspension Plasma Spraying of Fine Hydroxyapatite Powder. J. Therm. Spray Technol. 2008, 17, 679–684. [Google Scholar] [CrossRef]
- Bouyer, E.; Gitzhofer, F.; Boulos, M.I. Suspension plasma spraying for hydroxyapatite powder preparation by RF plasma. IEEE Trans. Plasma Sci. 1997, 25, 1066–1072. [Google Scholar] [CrossRef]
- Bouyer, E.; Gitzhofer, F.; Boulos, M.I. Suspension Plasma Spraying of Hydroxyapatite. In Hydroxyapatite Proceedings of the 12th International Symposium of Plasma Chemistry, II.; Minneapolis, MN, USA, 1995. [Google Scholar]
- Gitzhofer, F.; Bouyer, E.; Boulos, M.I. Suspension plasma spray. JOM 1997, 49, 58–62. [Google Scholar]
- Oberste Berghaus, J.; Legoux, J.-G.; Moreau, C.; Tarasi, F.; Chráska, T. Mechanical and Thermal Transport Properties of Suspension Thermal-Sprayed Alumina-Zirconia Composite Coatings. J. Therm. Spray Technol. 2008, 17, 91–104. [Google Scholar] [CrossRef]
- Killinger, A.; Kuhn, M.; Gadow, R. High-Velocity Suspension Flame Spraying (HVSFS), a new approach for spraying nanoparticles with hypersonic speed. Surf. Coat. Technol. 2006, 201, 1922–1929. [Google Scholar] [CrossRef]
- Smith, W.; Kelley, K.; Coy, D.; Roberts, W. Thermal Spray Coating Process with Nano-Sized Materials. U.S. Patent Application 10/154,199, 27 November 2003. [Google Scholar]
- Bellucci, D.; Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A.; Stiegler, N. High velocity suspension flame sprayed (HVSFS) potassium-based bioactive glass coatings with and without TiO2 bond coat. Surf. Coat. Technol. 2012, 206, 3857–3868. [Google Scholar] [CrossRef]
- Bernstein, A.; Suedkamp, N.; Mayr, H.O.; Gadow, R.; Killinger, A.; Stiegler, N. Investigations on Vitro Behaviour of Ceramic Coatings Deposited by High Velocity Suspension Flame Spraying. KEM 2011, 493–494, 530–534. [Google Scholar] [CrossRef]
- Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Rauch, J. Microstructural and in vitro characterisation of high-velocity suspension flame sprayed (HVSFS) bioactive glass coatings. J. Eur. Ceram. Soc. 2009, 29, 2249–2257. [Google Scholar] [CrossRef]
- Burtscher, S.; Krieg, P.; Killinger, A.; Al-Ahmad, A.; Seidenstücker, M.; Latorre, S.H.; Bernstein, A. Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis. Materials 2019, 12, 3495. [Google Scholar] [CrossRef]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef]
- Alves, R.; Da Reis, T.V.S.; Da Silva, L.C.C.; Storpírtis, S.; Mercuri, L.P.; Matos, J.d.R. Thermal behavior and decomposition kinetics of rifampicin polymorphs under isothermal and non-isothermal conditions. Braz. J. Pharm. Sci. 2010, 46, 343–351. [Google Scholar] [CrossRef]
- Venturi, F.; Hussain, T. Radial Injection in Suspension High Velocity Oxy-Fuel (S-HVOF) Thermal Spray of Graphene Nanoplatelets for Tribology. J. Therm. Spray Technol. 2020, 29, 255–269. [Google Scholar] [CrossRef]
- Blum, M.; Gyoktepeliler-Akin, E.; Killinger, A. High velocity suspension flame spraying of AlN/Al2O3 composite coatings. Surf. Coat. Technol. 2022, 441, 128588. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Arshad, M.; Masud, K.; Saeed, A.; Qureshi, A.H.; Shabir, G. Thermogravimetric and differential thermal analyses of fluorescein dye in inert and static air atmosphere. J. Therm. Anal. Calorim. 2018, 131, 1385–1390. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Sprayed Hydroxylapatite-Based Coatings: Chemical, Mechanical, Microstructural, and Biomedical Properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Chen, J.P.; Isa, K. Thermal Decomposition of Urea and Urea Derivatives by Simultaneous TG/(DTA)/MS. J. Mass Spectrom. Soc. Jpn. 1998, 46, 299–303. [Google Scholar] [CrossRef]
- Turhanen, P.A.; Demadis, K.D.; Kafarski, P. Editorial: Phosphonate Chemistry in Drug Design and Development. Front. Chem. 2021, 9, 695128. [Google Scholar] [CrossRef]
- Papapoulos, S.E. The role of bisphosphonates in the prevention and treatment of osteoporosis. Am. J. Med. 1993, 95, S48–S52. [Google Scholar] [CrossRef]
- Morks, M.F.; Kobayashi, A. Influence of spray parameters on the microstructure and mechanical properties of gas-tunnel plasma sprayed hydroxyapatite coatings. Mater. Sci. Eng. B 2007, 139, 209–215. [Google Scholar] [CrossRef]
- Hasan, M.F.; Wang, J.; Berndt, C. Evaluation of the mechanical properties of plasma sprayed hydroxyapatite coatings. Appl. Surf. Sci. 2014, 303, 155–162. [Google Scholar] [CrossRef]
- Mancini, C.E.; Berndt, C.C.; Sun, L.; Kucuk, A. Porosity determinations in thermally sprayed hydroxyapatite coatings. J. Mater. Sci. 2001, 36, 3891–3896. [Google Scholar] [CrossRef]
- Krieg, P.; Killinger, A.; Gadow, R.; Burtscher, S.; Bernstein, A. High velocity suspension flame spraying (HVSFS) of metal doped bioceramic coatings. Bioact. Mater. 2017, 2, 162–169. [Google Scholar] [CrossRef] [PubMed]

| Name | Solid Content [%] | Phosphonate [wt.%] | Modified Urea [wt.%] | Fluoresceine [wt.%] |
|---|---|---|---|---|
| S0 | 10 | 3 | 0 | 0 |
| S1 | 10 | 8 | 3 | 0.25 |
| S2 | 10 | 8 | 3 | 0.25 |
| S3 | 10 | 8 | 3 | 0.25 |
| Coating Denotation | Axially Injected Suspension | Radially Injected Suspension | Total Gas Flow [slpm] | C2H4 [slpm] | O2 [slpm] |
|---|---|---|---|---|---|
| R0 | S0 | - | 230 | 80 | 150 |
| C1 | S0 | S1 | 230 | 80 | 150 |
| C2 | S0 | S2 | 230 | 80 | 150 |
| C3-1 | S0 | S3 | 230 | 80 | 150 |
| C3-2 | S0 | S3 | 184 | 64 | 120 |
| C3-3 | S0 | S3 | 145 | 50 | 95 |
| Powder Type | d10 [μm] | d50 [μm] | d90 [μm] |
|---|---|---|---|
| P1 (raw material) | 2.2 ± 0.0 | 5.2 ± 0.0 | 14.4 ± 0.3 |
| P2 (sponge-like granules, calcined) | 5.3 ± 0.0 | 15.5 ± 0.1 | 33.2 ± 0.9 |
| P3 (hollow granules, calcined, sieved) | 28.6 ± 0.0 | 51.5 ± 0.0 | 92.2 ± 0.2 |
| Sample | Radially Injected Feedstock | Gas Flow C2H4/O2 [slpm] | Hardness (n = 10) [HV0.01] | Deposition Efficiency [%] | Coating Thickness [μm] |
|---|---|---|---|---|---|
| R0 | - | 80/150 | 246.1 ± 16.9 | 66.2 | 86.1± 4.8 |
| C1 | S1 | 80/150 | 94.8 ± 30.5 | 41.6 | 122.6 ± 8.7 |
| C2 | S2 | 80/150 | 165.1 ± 7.0 | 29.2 | 90.7 ± 3.4 |
| C3-1 | S3 | 80/150 | 159.1 ± 19.5 | 19.7 | 58.8 ± 10.7 |
| C3-2 | S3 | 64/120 | 129.1 ± 16.8 | 11.7 | 38.6 ± 4.6 |
| C3-3 | S3 | 50/95 | 118.9 ± 6.3 | 8.8 | 24.6 ± 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, M.; Derad, L.; Killinger, A. Deposition of Fluoresceine-Doped HAp Coatings via High-Velocity Suspension Flame Spraying. Coatings 2022, 12, 1251. https://doi.org/10.3390/coatings12091251
Blum M, Derad L, Killinger A. Deposition of Fluoresceine-Doped HAp Coatings via High-Velocity Suspension Flame Spraying. Coatings. 2022; 12(9):1251. https://doi.org/10.3390/coatings12091251
Chicago/Turabian StyleBlum, Matthias, Lukas Derad, and Andreas Killinger. 2022. "Deposition of Fluoresceine-Doped HAp Coatings via High-Velocity Suspension Flame Spraying" Coatings 12, no. 9: 1251. https://doi.org/10.3390/coatings12091251