1. Introduction
Atomic layer deposition (ALD) process technology was first developed in the 1970s. In 1977, Dr. Tuomo Suntola of Finland officially applied for the first patent related to ALD technology [
1,
2,
3]. Between 1983 and 1998, ALD technology was applied to the production of electronic displays at Helsinki Airport, Finland. In the late 1990s, as the semiconductor industry began to introduce ALD processes, a large amount of research and development funding and manpower was invested into the rapid growth of ALD process technology. In the year 2007, Intel used ALD process technology to grow a hafnium oxide (HfO
2) gate-oxide layer for a metal-oxide-semiconductor field-effect transistor on a 45 nm microprocessor, further establishing the importance of ALD process technology in the semiconductor industry [
4].
ALD is a surface-chemical reaction-based technology featuring excellent atomic thickness accuracy, large area uniformity, and conformity of the film on high-aspect-ratio structures [
5].
The coating of thermally fragile substrates by ALD may provide exciting new applications in diverse areas ranging from packaging to microelectronics. For example, thin ALD films may serve as an efficient hole-blocking layer in a complementary metal-oxide-semiconductor image sensor to enhance the signal to noise ratio [
6,
7]. ALD coatings on polymers may also be important as gas diffusion barriers for flexible electronic devices or organic light-emitting diodes [
8,
9]. Moreover, applications of ALD processes have been demonstrated at low temperatures of <100 °C [
10,
11,
12,
13,
14,
15,
16,
17,
18]. Room-temperature catalytic SiO
2 ALD has been realized using SiCl
4 and H
2O plus pyridine or NH
3 as the catalyst [
19,
20]. The ALD of ZnSe and CdS has also been demonstrated at room temperature [
21]. The ALD technique is widely used in the electronic industry [
22]. However, few studies have addressed the advantages of deposition on a packaged chip. Most ALD processes occur at temperatures >100 °C that would degrade organic, polymeric, or biological material. The material normally used for the packaging of semiconductor chips is organic polyimide. As the technology advances, the performance in terms of reliability becomes critical, and sensitivity to moisture in harsh environments becomes a factor when using the chip. In this study, a 14 nm high-performance computing application specific integrated circuit (ASIC) was selected to verify the effect of changing the thickness of the ALD coating on performance in terms of reliability, which was proposed for the first time. An open/short test, standby current measurement, interface input/output performance test, and phase-locked loops (PLL) function test were used to verify the chip yield. The lowest operating voltage (LOV) was used as the performance index for reliability after wear-out tests and unbiased highly accelerated temperature and humidity stress test (uHAST). The ASIC with a 15 nm Al
2O
3 ALD coating showed the best performance in both the uHAST and the 72 h wear-out test. The reasons for failure of the 25 nm thick coating remain unclear and are worthy of further investigation. The results of this study provide an interesting evaluation of the relationship between ALD coating thickness and performance in terms of reliability, which can pave the way for a new package scheme in advanced semiconductor processes.
The aim of this study is to establish a protective nanoscale thin film using the ALD technology for electronic components in a prepackaged high-performance computing chip, which can improve the electrical performance and reliability after high-temperature and high-humidity stress tests.
2. Materials and Methods
The ALD technique was applied using the Lunamia model LA-1T from Pure Metallica Co., Ltd., Taipei City, Taiwan. The Lunamia is a type of capacitive plasma-enhanced ALD equipment. To apply the plasma ALD coating of the Al2O3 layer, the reaction conditions were trimethylaluminum exposure (from PentaPro Materials Inc., Hsinchu County, Taiwan, purity: 6N), argon purge (from Jing De Gases Co., Ltd., Kaohsiung City, Taiwan, purity: 5N), and oxygen (from Jing De Gases Co., Ltd., purity: 6N) with a plasma power of 100 W. Each step was controlled by the automatic processes described below.
First, the inert gas was introduced into the deionized water tank, as well as water vapor, using a plasma power of 100 W, which covered the substrate with hydroxyl groups, thereby forming a reactive layer (i.e., prereaction seasoning). The precursor was introduced into the chamber, along with the carrier gas Ar, at a flow rate of 500 sccm to form a surface-adsorbed monolayer for later reaction.
Second, the precursor and other byproducts were removed by introducing the inert gas Ar, followed by pumping the chamber to a base pressure of 10−6 Torr. Third, the reaction gas O2 was then introduced, supplemented by plasma. This O2/plasma mixture allowed oxygen radicals to react with the surface-adsorbed Al precursor, thereby forming an alumina monolayer, i.e., the desired material for the ALD process.
The fourth and final step of the ALD reaction cycle involved removal of the excess reaction gas and byproducts by again introducing the inert gas Ar, followed by pumping the chamber to the base pressure.
Table 1 lists the ALD process parameters. The O
2 process was to ensure the complete oxidation of the trimethylaluminum. The radio frequency (RF) power of 100 W was selected by considering the degree of oxidation with the minimum of substrate reflected power. The soak time was considered for the full diffusion of the precursor for better process uniformity. The Ar purge time was to ensure no aluminum precursor remained forming gas-phase reaction-generating particles.
The thickness and the quality of the ALD coatings were determined by ellipsometry. The desired thickness could be controlled by the cycle number, whereby one ALD cycle deposited 0.24 nm of Al2O3 thin film. The ALD thicknesses of 5–20 nm were selected to study their effect on performance in terms of reliability.
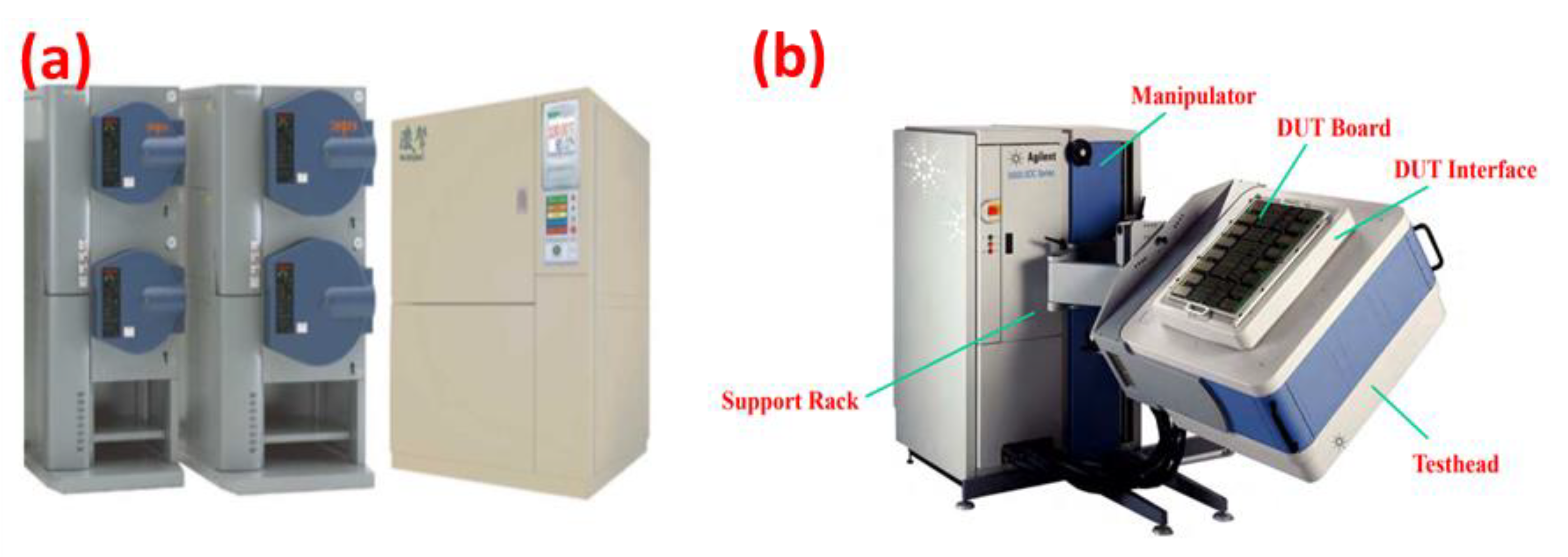
The uHAST was carried out at a temperature of 130 °C and 85% relative humidity (RH) for 96 h at a relative pressure of 2.3 atm. The stressed duration was 96 h under the JESD22-A118 regulation. After an ambient temperature test, various electrical measurements were performed, including an open/short test, power short test, ISB (power standby current test), direct current (DC) characterization test, and lowest operation voltage functional test (0.62–0.78 V) using an Agilent 9300 SOC series (Santa Clara, CA, USA). The uHAST and automatic testing equipment is displayed in
Figure 1.
3. Results
3.1. Background of the 14 nm HPC ASIC
The cryptocurrency mining chip is a 14 nm ASIC. This chip operates in harsh conditions when used in high-performance computing. Thus, its reliability is crucial, as cryptocurrency mining machines require reliable computing performance to ensure their profitability. A 14 nm ASIC can provide lower power consumption, along with high computing performance, making it suitable for mining applications.
Figure 2 shows a block diagram illustrating the functions of the 14 nm ASIC. The ASIC consists of five main functional circuits, including the PLL which fundamentally provides the clock source used for controlling the calculation via an automatic feedback design. The PLL clock serves as a gateway to the calculation hash core, which features translated algorithms used to directly operate the software to generate cryptocurrency. The control unit rechecks the calculation result and outputs the data through the IO_OUT pin.
3.2. Yield and Reliability of the ALD-Coated ASIC Chip
Figure 3 illustrates the conditions of the coating structure and the thermal dispatch pathway of the flip-chip land grid array (FCLGA). Two exposed heat sinks were used as the main heat dispatch material. Inside the package, the chip was connected via a copper pillar bump. During high-speed operation, the chip generates tremendous heat and becomes vulnerable to moisture in the environment. In order to prevent its penetration into the chip, different ALD Al
2O
3 coatings thicknesses were applied, and we studied their effect on performance in terms of reliability.
Figure 4 shows the cross-section FIB image of the 50 nm ALD-coated thin film. The ALD-coated thin film established a uniform and continuous protection layer of the integrated chip. The diagram in
Figure 5 (top panel) schematically demonstrates the ALD coating on the 14 nm ASIC. The results showed that the 15 nm thick ALD alumina coating exhibited the lowest operation voltage performance after a 1000 h uHAST, whereby the operation voltage was reduced to 0.62 V, thus indicating its ability to resist harsh conditions. The similar results of a 72 h wear-out test were also performed (not shown here). On the other hand, the chip with a 20 nm thick coating failed the DC 72 h wear-out stress test. The reason for failure was not clear and is worthy of further investigation. It is suspected that the chlorine ion may have reacted with the alumina coating, thereby causing interconnect path damage. The previous work in corrosion research indicated that the existence of Al
2O
3 accelerated the penetration of the chlorine ion (Cl
−) and thus corrosion occurred [
24]. The mechanism of how the corrosion induced by moisture and Cl
− occurred was also proposed by Fu et al. [
25].
Figure 5 shows how the ALD coating protected the printed circuit board (PCB) structure; the alumina layer prevented moisture from penetrating into the main chip, while also facilitating good heat dispatch. The ALD layer established a capped circuit structure, allowing the thermal stress of the surface and the chip to be alleviated. Otherwise, moisture could penetrate through the land grid metal pad and the adjacent plastic substrate due to the difference in thermal expansion coefficient between the metal pad and epoxy polymer molding, thus leading to thermal stress accumulation and thermal strain distribution problems. In our study, the ALD coating alleviated the stress-induced electronic performance decay. The effect of an epoxy molding compound on thermal stresses in IC packages during the manufacturing process were carefully discussed [
26,
27]. In the study, the failure of the chip was caused by the thermal deformations of the die/EMC bimaterial. The experimental results also showed that the ability to resist the harsh environment varied with the tested thicknesses of 0, 5, 10, 15, and 20 nm, according to 1000 h of an uHAST and 72 h of a wear-out salt-spray test.
Figure 6 illustrates the open/short testing method. A current of 100 mA was collected from the input/output (IO) pad, and the voltage was measured. The ALD coating with a thickness of 20 nm failed the open/short test, according to the criteria listed in the figure (−0.2 to 0.7 V). This may have been due to the thickness of the Al
2O
3 coating, which caused poor pad connection after the 72 h wear-out test. The sheet resistance test was performed with different ALD thicknesses on the conducting substrate. The sheet resistance was 1.6 × 10
7 ohm/sq with an Al
2O
3 thickness of 16 nm.
Table 2 lists the uHAST results with different Al
2O
3 thicknesses. The chip with a 15 nm thick protection layer demonstrated the lowest operation voltage (0.66 V). It was also discovered that a thinner protection layer led to a higher operation voltage shift. The yield was only 50% with an operation voltage of 0.78 V when the ALD coating thickness was below 10 nm.
Figure 7 shows the operation voltage results as a function of ALD thickness before and after the uHAST. Each sample was evaluated under the same stress conditions, thus demonstrating the correlation of voltage shift with thickness. The samples with ALD thicknesses of 15 and 20 nm both surpassed the lowest operation voltage shift after the stress test.
4. Future Aspects
In this study, an ALD fabricated Al
2O
3 thin film was used to establish a protection layer on the high-performance computing chip, which can also be applied to a bonding wire to form an insulating and heat dispatch layer. Detailed diagrams of these applications are shown in
Figure 8. The figure shows the patent design. The original bonding wire is marked “31”, while the functional layers are marked “411” and “412”, i.e., the electromagnetic interference shielding layer or heat dispatch layer. The outer insulating layer is marked “42”. The methodology can also be used in 3D inkjet flexible printed touch sensors to enhance the sensor lifetime [
28].
5. Conclusions
In this study, the overall protective layer using the atomic layer deposition technology established after encapsulation with electronic components obviously improved the lifetime in harsh environments. The excavator integrated circuit tested in this paper is prone to generate high heat during operation as a packaging technology in a flip-chip land grid array package type, whereby the stress caused by high temperature can easily destroy the interface between plastic substrates, solder, and metal mats, thereby reducing its service lifetime. The creation of an Al2O3 protective thin film layer with a thickness of 15 nm through atomic layer deposition technology significantly improved the component damage caused by thermal stress. The protective film with a thickness of 15 nm showed the same lowest operation voltage at 0.66 V before and after the 1000 h unbiased highly accelerated temperature and humidity stress test. This research showed that atomic layer deposition can be effectively used to block moisture and oxygen from entering electronic devices, to avoid their rapid deterioration. The protection layer can also improve the mechanical protection and reduce the damage caused by moving collisions, which has potential applications in organic light-emitting diodes, micro light-emitting diodes, and semiconductor packages for resisting moisture. In addition, the atomic layer deposition can be used to fabricate luminescent material layers, biomedicine sensor components, and water-resistant thin films for use in energy engineering and other applications related to coating technology.
6. Patents
The related patents described in this research are pending.
Author Contributions
Methodology, P.-C.C. and S.-M.C.; validation, H.-C.K. and F.-C.C.; writing—original draft preparation, Y.-A.L.; writing—review and editing, C.-C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author.
Acknowledgments
The authors acknowledge the reliability evaluation support of the IST group.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suntola, T.; Antson, J. Method for Producing Compound Thin Films. U.S. Patent 4,058,430, 15 November 1977. [Google Scholar]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A review of conductive metal nanomaterials as conductive, transparent, and flexible coatings, thin films, and conductive fillers: Different deposition methods and applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef]
- Profijt, H.; Potts, S.; Van de Sanden, M.; Kessels, W. Plasma-assisted atomic layer deposition: Basics opportunities, and challenges. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 050801. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Chang, C.Y.; Pan, F.M.; Lin, J.S.; Yu, T.Y.; Li, Y.M.; Chen, C.Y. Lateral amorphous selenium metal-insulator-semiconductor-insulator-metal photodetectors using ultrathin dielectric blocking layers for dark current suppression. J. Appl. Phys. 2016, 120, 234501. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Hu, H.; Chen, F.; Kong, M.; Zhang, Q.; Wan, L. Atomic layer deposition of TiO2 for a high-efficiency hole-blocking layer in hole-conductor-free perovskite solar cells processed in ambient air. ACS Appl. Mater. Interfaces 2016, 8, 17999–18007. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Weng, Y.; Sun, F.; Zhou, X.; Wu, C.; Yan, Q.; Guo, T.; Zhang, Y. Low-temperature atomic layer deposition of Al2O3/alucone nanolaminates for OLED encapsulation. RSC Adv. 2019, 9, 20884. [Google Scholar] [CrossRef]
- Weaver, M.; Michalski, L.; Rajan, K.; Rothman, M.; Silvernail, J.; Brown, J.J.; Burrows, P.E.; Graff, G.L.; Gross, M.E.; Martin, P.M. Organic light-emitting devices with extended operating lifetimes on plastic substrates. Appl. Phys. Lett. 2002, 81, 2929–2931. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Low temperature atomic layer deposition of ZnO: Applications in photocatalysis. Appl. Catal. B Environ. 2016, 196, 68–76. [Google Scholar] [CrossRef]
- Vayrynen, K.; Mizohata, K.; Ra isa nen, J.; Peeters, D.; Devi, A.; Ritala, M.; Leskela, M. Low-temperature atomic layer deposition of low-resistivity copper thin films using Cu(dmap)2 and tertiary butyl hydrazine. Chem. Mater. 2017, 29, 6502–6510. [Google Scholar] [CrossRef]
- Vangelista, S.; Mantovan, R.; Lamperti, A.; Tallarida, G.; Kutrzeba-Kotowska, B.; Spiga, S.; Fanciulli, M. Low-temperature atomic layer deposition of MgO thin films on Si. J. Phys. D Appl. Phys. 2013, 46, 485304. [Google Scholar] [CrossRef]
- Jurca, T.; Moody, M.J.; Henning, A.; Emery, J.D.; Wang, B.; Tan, J.M.; Lohr, T.L.; Lauhon, L.J.; Marks, T.J. Low-temperature atomic layer deposition of MoS2 films. Angew. Chem. Int. Ed. 2017, 56, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, J.; Ramachandran, R.K.; Devloo-Casier, K.; Rampelberg, G.; Filez, M.; Poelman, H.; Marin, G.B.; Fonda, E.; Detavernier, C. Low-temperature atomic layer deposition of platinum using (methylcyclopentadienyl) trimethylplatinum and ozone. J. Phys. Chem. C 2013, 117, 20557–20561. [Google Scholar] [CrossRef]
- Putkonen, M.; Sippola, P.; Svärd, L.; Sajavaara, T.; Vartiainen, J.; Buchanan, I.; Forsström, U.; Simell, P.; Tammelin, T. Low-temperature atomic layer deposition of SiO2/Al2O3 multilayer structures constructed on self-standing films of cellulose nanofibrils. Philos. Trans. R. Soc. A 2018, 376, 20170037. [Google Scholar] [CrossRef]
- Nam, T.; Kim, J.-M.; Kim, M.-K.; Kim, H.; Kim, W.-H. Low-temperature atomic layer deposition of TiO2, Al2O3, and ZnO thin films. J. Korean Phys. Soc. 2011, 59, 452–457. [Google Scholar] [CrossRef]
- Kwak, I.; Park, J.H.; Grissom, L.; Fruhberger, B.; Kummel, A. Mechanism of low temperature ALD of Al2O3 on graphene terraces. ECS Trans. 2016, 75, 143. [Google Scholar] [CrossRef]
- Vandalon, V.; Kessels, W. What is limiting low-temperature atomic layer deposition of Al2O3? A vibrational sum-frequency generation study. Appl. Phys. Lett. 2016, 108, 011607. [Google Scholar] [CrossRef]
- Klaus, J.W.; Sneh, O.; George, S.M. Growth of SiO2 at room temperature with the use of catalyzed sequential half-reactions. Science 1997, 278, 1934–1936. [Google Scholar] [CrossRef]
- Klaus, J.; George, S. Atomic layer deposition of SiO2 at room temperature using NH3-catalyzed sequential surface reactions. Surf. Sci. 2000, 447, 81–90. [Google Scholar] [CrossRef]
- Knez, M.; Nielsch, K.; Niinistö, L. Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 2007, 19, 3425–3438. [Google Scholar] [CrossRef]
- Biercuk, M.; Monsma, D.; Marcus, C.; Becker, J.; Gordon, R. Low-temperature atomic-layer-deposition lift-off method for microelectronic and nanoelectronic applications. Appl. Phys. Lett. 2003, 83, 2405–2407. [Google Scholar] [CrossRef]
- Temperature and Humidity Test—iST-Integrated Service Technology. Available online: https://www.istgroup.com/en/service/temperature-humidity/ (accessed on 1 June 2021).
- Chiu, Y.T.; Chiang, T.H.; Yang, P.F.; Huang, L.; Hung, C.P.; Uegaki, S.; Lin, K.L. The corrosion behavior of Ag alloy wire bond on Al pad in molding compounds of various chlorine contents under biased-HAST. In Proceedings of the 2016 International Conference on Electronics Packaging (ICEP), Hokkaido, Japan, 20–22 April 2016; pp. 497–501. [Google Scholar]
- Fu, S.W.; Lee, C.C. A corrosion study of Ag–Al intermetallic compounds in chlorine-containing epoxy molding compounds. J. Mater. Sci. Mater. Electron. 2017, 28, 15739–15747. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Wang, C.T.; Hsu, C.H. The effect of epoxy molding compound on thermal/residual deformations and stresses in IC packages during manufacturing process. IEEE Trans. Compon. Packag. Manuf. Technol. 2006, 29, 625–635. [Google Scholar] [CrossRef]
- Lancaster, A.; Keswani, M. Integrated circuit packaging review with an emphasis on 3D packaging. Integration 2018, 60, 204–212. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thangaraj, M.; Moiduddin, K.; Al-Ahmari, A.M. Fabrication and performance analysis of 3D inkjet flexible printed touch sensor based on AgNP electrode for infotainment display. Coatings 2022, 12, 416. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).