1. Introduction
To improve the energy efficiency of vehicle engines, aluminum alloys replacing steel components has been developed widely for weight reduction during the last few decades. However, surface protection has to be introduced to some aluminum alloy components, such as cylinder liners and piston crowns, due to the relatively low melting point and poor wear resistance of aluminum alloys compared to steels [
1]. Technological enhancements in engine thermal efficiency require engine components to have ability to operate under higher temperatures and peak loads. The use of TBCs can lead to a temperature reduction of as much as 200 °C at the piston crown of an engine, thus improving the durability of engine components as well as enhancing the fuel efficiency [
2].
From other side, internal combustion (IC) engines are significant sources of environmental pollution. The strengthening of environmental legislation had stimulated the improvement of existing methods for emission reduction and a search for new approaches. The main regulated pollutants in engine exhausts are nitrogen oxides (NOx), carbon monoxide (CO), unburned hydrocarbons (HC) and soot. In diesel engines the most significant emissions are NOx and soot, and new technologies being developed are expected to lower their emission levels. These new technologies can be classified into two different categories, according to their emission-control techniques. The first prevents emission formation in the engine cylinder using improved combustion technologies, such as high-pressure injection, low-compression-ratio bowls and exhaust gas recirculation (EGR). The second uses after-treatment systems, such as diesel particulate filters (DPFs), selective catalytic reduction (SCR) and NOx converters. However, these systems are rather expensive, and their performance deteriorates over time [
3]. Therefore, the integration of both in-cylinder and after-treatment purification technologies looks more efficient. Now, there is growing interest in the development of in-cylinder emission-control methods, especially because upcoming regulations place more emphasis on the reduction of NOx emissions [
4]. For example, the piston crown catalytic coatings are being applied [
5]. However, the application of combined thermal barrier–catalytic coatings is not being developed because of a lack of deposition technologies and methods of controlling the coating structure and properties. So, the goal this paper is to develop a design of combined thermal barrier–catalytic coatings and cold-spraying technology for their deposition on piston crowns.
Thermal-spraying technologies allow for TBCs to be obtained to alleviate mentioned drawbacks [
6,
7]. TBCs made by thermal-spraying methods have attracted much attention because of the combination of thermal and wear resistance properties [
4]. However, the application of low-pressure cold-spraying (LPCS) technology for TBC deposition has not been developed to a high extent. Some benefits of TBCs made by LPCS could also be obtained due to the thermal barrier effect of decreasing of the piston crown surface temperature, which could diminish the temperature difference between the coated crown surface and gas, thereby enhancing heat energy conversion and power efficiency [
8,
9]. Many ceramic-based materials have been well-established as proper TBC materials on account of their low thermal conductivity, for instance, yttria-stabilized zirconia (YSZ). Nevertheless, the inherent brittle character significantly restricts the reliability of ceramic-based TBCs under mechanical loading or thermal shock [
10,
11].
Intermetallic compounds are of considerable interest because of their high-temperature strength, low density and high creep resistance. However, the application of intermetallics has been limited due to their highly brittle behavior at ambient temperature. Whereas intermetallics are complemented with a ductile metallic phase, together they offer a good combination of strength and toughness. Based on this idea, a new class of the multilayer structural material known as metal–intermetallic laminate (MIL) composites was developed by D. Harach et al. [
12]. It includes MIL composites, based on the combination of Fe-Al, Ti–Al
3Ti and Ni–Al
3Ni, which have been attained recently by means of reactive foil sintering [
13]. However, there is a lack of reports on MILs’ application as TBCs yet. In this case, the one-layer TBCs with thicknesses of about 100–500 μm might be replaced by MILs with thermal barrier intermetallic layers, which would have a total thickness similar to that of one-layer TBCs. That is why developing novel metal matrix composite TBCs with high mechanical properties is attracting the increasing attention of researchers and related industries due to the MMC’s great potential in this field. Metals such as the MMC matrix are well-known to be good thermal conductors compared to ceramic reinforcement particles. In order to reduce the thermal conductivity of metals, MMCs with a low-conductive ceramic phase are believed to be designed. As shown in [
14], the application of the layered structure of MMC TBCs will enhance the thermal barrier properties. However, the high thermal conductivity of Al layers reduces the effect of low-conductive Fe-Al intermetallic layers formed during reaction sintering of the multilayer structure. That is why it seems to be possible to diminish the thermal conductivity of Al layers by its reinforcement with a ceramic phase such as YSZ with low thermal conductivity. Therefore, the layered structure of TBC composites is believed to consist of intermetallic layers of low thermal conductivity and Al-YSZ layers, allowing for high mechanical properties to be achieved and the negative effect of Al on the integral thermal barrier properties of the composite to be diminished. Thus, the terminology “three-phase multilayer structure” looks reasonable.
It is of great importance to increase the fracture toughness of the engine thermal barrier coating working in both harsh mechanical loading and thermal conditions. A multiscale structural design was innovatively adopted in [
15] to increase the toughness of monolithic HfB2 ceramics. In this case, the strategy of ceramic reinforcement is usually associated with design of ceramic composites that can enable the action of the different mechanisms of fracture energy absorption and/or dissipation. In the three-phase composite materials, even more complex layered microstructural architectures have to be developed. Different structure elements such as metal, ceramic, intermetallic particles and their layers are combined to tailor properties in accordance with different service requirements. TBCs are complicated systems that need to be characterized by a multitude of interdependent parameters such as mechanical (hardness, fracture toughness and other parameters), thermal (thermal conductivity) and exploitation properties, resulting in improved engine thermal efficiency, which leads to measured gains in fuel economy and emissions reduction. The experimental study of mechanical behavior is of great importance to provide information related to the failure of such a composite material. The strength of a ceramic matrix three-phase layered composite depends on the properties of the separate layers and the process of composite failure on the system of layers. However, a lack of these data hinders the development and application of such a composite. Therefore, analysis of composite materials for possible use as TBCs reveals about two main directions of the development: (i) metal matrix composites and (ii) multilayer composite materials with high thermal barrier properties and fracture toughness. The main approach of designing composite materials being made by CS is by using two types of CS deposition technology (spraying of a powder mixtures and layer-by-layer spraying) associated with subsequent sintering.
Many automobile companies use TBCs of about 300 μm thickness. However, just TBCs cannot improve performance and emissions of engines [
16]. The thermophysical, mechanical and catalytic properties of TBC materials need to be optimized. For example, authors [
17] used mullite as their TBC material on the turbocharged engine piston crown and cylinder head, as well as on the valves. A 12% increase in exhaust gas temperature and engine efficiency were observed. It is shown that whilst CO is reduced by 28%, NOx emission is increased by 21% due to the increase in in-cylinder temperature. The similar effects of NOx emissions increasing with TBCs’ application were observed in [
16]. That is why the presence of the catalytic layer on the TBC is believed to facilitate a reduction in NOx emission. In oxygen-lean zones of the combustion chamber, near to the piston crown coated by the catalyst, nitrogen oxides can be reduced by hydrogen and carbon monoxide by the reactions:
The hydrogen results from the of the fuel hydrocarbons.
The dehydrogenation reaction can be promoted by the appropriate catalysts, such as Cu-Zn/Ni-zeolite [
18] and molybdenum compounds such as MoSi
2/Mo
2C and chromium oxides Cr
2O
3 [
19]. The temperatures in the cylinder during combustion are higher than those in the exhaust, so an increased catalyst activity might be anticipated. However, the development of TBCs with additional catalytic layers made by cold spraying is not described in literature.
As shown above, TBCs are complex, multilayered and multimaterial systems with various options related to composition, processing and microstructure. These coatings, typically 100–200 mm thick, are applied using a variety of deposition processes such as atmospheric plasma spray (APS), high-velocity oxygen fuel (HVOF), low-pressure plasma spray (LPPS), cathodic arc/ion plasma deposition and others [
20]. In the case of intermetallic-based coatings such as MCrAlY bond coats, the deposition technologies allow for a great control of the coating composition, which is dictated essentially by the coating source. The structure of the MCrAlY coating is dictated by thermodynamic and kinetic constraints of the intermetallic phase formation [
21]. The authors of [
21,
22] showed that using nanocrystalline MCrAlY coatings deposited by the HVOF process greatly influences the coating oxidation reactions. The effect is considerably important for the development of catalytic coatings [
18,
19]. From the other side, the post-spraying sintering steps of TBCs allows for careful control of the diffusion processes of structure formation, as is shown in [
23]. That is why the coating synthesis route to be developed will consist of cold spraying for particle consolidation and sintering steps for final targeted structure formation.
Thus, the main tasks of the combined thermal barrier–catalytic coating development in this work are as follows: (i) to develop the design of a three-phase multilayer structure of TBCs and a study of their thermophysical properties; (ii) to study the mechanical properties of TBCs; and (iii) to develop TBCs with cold-sprayed catalytic layers.
2. Materials and Methods
2.1. Powder Materials, Cold Spraying and Sintering
The Fe-Al-YSZ-Al TBCs synthesis requires the two main powder technologies: (i) cold spraying and (ii) subsequent sintering. Based on the results shown in paper [
14], Fe-Al intermetallic phase was synthesized during reaction sintering of stainless steel and Al particles in the powder aggregate previously obtained by cold-spraying layered structure. A double-nozzle low-pressure cold-spraying gun was used to deposit two layers (stainless steel and Al-YSZ) per one track.
The following powder materials were chosen for study: (i) AISI 304 powder (composition Fe-0.07%C, 18.0%Cr, 9.5%Ni, 2%Mn, 0.75%Si) supplied by Atlantic Equipment Engineers Ltd., Upper Saddle River, NJ, USA; (ii) SHS-717 (composition Fe-25%Cr-8%Mo-10%W-5%Mn-5%B-2%C-2%Si) stainless steel powder from Nanosteel Company, Upper Saddle River, NJ, USA; (iii) YSZ ZrO2 supplied by Atlantic Equipment Engineers Ltd., Upper Saddle River, NJ, USA; and (iv) aluminum powder, supplied by Atlantic Equipment Engineers Ltd., USA, which served bifold purposes as a bond coat for metal powder layers as well as a base reagent to synthesize intermetallics. The multimaterial catalytic layer is formed with Cu-Zn-Graphene powder mixture. Cu and Zn powder was supplied by Atlantic Equipment Engineers Ltd., Upper Saddle River, NJ, USA, and graphene powder was supplied by Cheap Tubes Inc., Grafton, VT, Canada. The particle size distribution was measured by laser diffraction particle size analyzer Mastersizer 3000 (Malvern, UK). The measurement of powder particle size distribution of the powders indicated their average size (i.e., particle diameter for 80% content) is in the range of 45 µm. Graphene particles were 8–15 nm thick across the layers and larger than 2 μm in the layer plane, according to supplier data sheet. The cold-sprayed multilayer thermal barrier and catalytic coatings and their characterization are shown in
Table 1.
The spray experiments were carried out using robotic spraying system equipped with dual-flow low-pressure cold-spray machine (Tessonics Inc., Windsor, ON, Canada). The powders were supplied by a presize powder hopper and were injected into the divergent portion of the nozzle near the throat area by means of vacuum developed by an accelerated stream of compressed air passing through the nozzle. The injected particles were accelerated in the high-velocity air stream and projected onto flat A356 substrate of blocks of d = 25.4mm. Additionally, A356 alloy and steel piston prototypes were deposited. To increase the air velocity—and ultimately, the particle velocity—the compressed air was preheated to 350 °C for spraying of Al-YSZ and to 600 °C for spraying of stainless steel powders. The pressure and temperature of the compressed air were monitored by a pressure gauge and a thermocouple positioned inside the gun. The gun was installed on a gantry robot to scan the air-powder jet over the substrate surface with a step size 0.5–4mm (
Table 1). The compressed air pressure varied in the range of 0.6–0.8 MPa. The gun transverse speed was about 30 mm/s. The powder feeding rate was varied in the range of 0.5~1.0 g/s, while the stand-off distance, measured from the exit of the nozzle to the substrate, was held constant at 10 mm. Up to five layers of Al-YSZ powder mixture with thicknesses of 100~150 µm were interlayed with stainless steel particles embedded into them, and the average thickness of stainless steel layers was about 50 µm. The total thickness of final TBC was set to 1 mm, similar to [
14], and the TBC deposited by CS contained 12 layers in total: 5 stainless steel layers and 6 aluminum interlayers (
Figure 1).
The second stage of the coating formation process was heat treatment, which allowed for reaction sintering of aluminum and stainless steel particles and the formation of intermetallic phases. TBC sintering was performed by heating the blocks in the furnace (Carbolite Gero Ltd., Newtown, PA, USA) in nitrogen atmosphere at temperatures of 550–600 °C over a period of 3 h.
Two types of stainless-steel- and two types of Al-based layers were studied in the multilayer TBCs: SHS 717 high chromium steel and AISI 304 chromium-nickel steel were the precursors for intermetallic layer formation during sintering. ZrO
2-Al mixtures were deposited on Al-13Si alloy substrate to obtain the two-phase homogenous Al-ZrO
2 layer. (
Figure 1a). The deposition efficiencies of the two powders differ, and the fraction of ZrO
2 in the sprayed coatings after the CS is about 50–70%, as measured by analysis of the optical microscopy images (
Figure 1b). To obtain a TBC layered structure, ZrO
2-Al layers described above and stainless steel layers were deposited alternately (
Figure 1c). Catalytic coating made of Cu-10%Zn-2%Graphene powder mixture was deposited separately (
Figure 1d).
2.2. Thermal Conductivity Measurements
The methods for measuring thermal conductivity are usually classified as either steady-state or transient methods [
24,
25,
26]. Each method has advantages and shortcomings. The transient methods, including hot wire, hot strip and especially laser flash, are used when absolute values of thermal conductivity are needed. The laser flash method is used extensively for measurements on thin layers and is fast and accurate at high temperatures. As for negative aspects, the technique is quite expensive, requires relatively complex computations and is not recommended for insulating materials. The uncertainty of the method is in the range 3–5% [
25,
26]. The steady-state method is based on a simple mathematical treatment and is used for low-conductivity materials in bulk or thin layers. The construction is relatively simple and provides high accuracy if the experimental setup is well-designed [
26,
27]. There are several variants of the steady-state method, including guarded hot plate, comparative, heat-flow meter and cylinder [
26]. The experiment may take a long time (required to achieve the steady state of the thermal flux) and uncertainty in the estimate of the thermal flux may be reflected in the accuracy of the results. The reported values of uncertainty vary in the range of 2–5% for guarded hot plate and around 10% for comparative methods [
27].
For our specific purpose, which is characterization of the thermal properties of the thermal barrier layers, the main goal is to estimate the increase in the thermal insulation properties of the coating relative to the uncoated substrate. For this reason, a comparative method will satisfy the goal. For a substrate made of materials with well-known thermal properties, as is the case for our samples, we can estimate the actual (absolute) values of the thermal conductivity by using the values of the thermal conductivity of the substrate from literature.
The thermal conductivity of the coatings was measured by a steady-state method and using a variation of the comparative technique described in [
24]. In our case, the aluminum substrate of the coating was used as the reference standard. To ensure the uniformity of the heat flux across the surface of the coating, we used two samples with identical coating stacked on top of the other, with the two coatings in contact (see
Figure 2). In this way, the region of interest is in the middle of the heat flux path rather than at the edge, and we can expect a more uniform and axial heat flux through the coating.
The steady-state methods have the advantage that they avoid the necessity of measuring the heat flux through the sample. Using the material of the piston or other engine part to be coated as standard in the comparative technique, we can directly measure the relative increase in thermal resistivity of the coating.
In order to ensure the axial symmetry of the heat flux as much as possible, we enclosed the ensemble of the samples in a thick insulating chamber built from refractory brick (Anorthite brick–Morgan Thermal Ceramics). The small space between the sample stack and the walls of the chamber were filled with grains of expanded perlite to reduce to a minimum the vertical convection from the hot plate to the top of the sample stack and to further reduce the radial component of the heat flux, which is one of the main sources of error in the steady-state methods.
The thermal conductivity of the insulating brick used is less than 0.2 W/(K·m) and the minimum thickness of the oven insulation (between the lateral surface of the sample stack and environment) is 100 mm. The outside temperature of the oven wall is about 60 °C when the temperature inside is in the range of 200–300 °C. With these values, the radial thermal flux can be estimated to be of the order of (2–4) × 10−2 W/cm2, whereas the axial thermal flux through our samples has values of the order of 4 W/cm2. With the radial flux of the order of 1% of the axial flux, we see that the assumption of axial thermal flux at steady state is a good approximation. Other sources of error are the distortion in the temperature field induced by the thermocouples and the extrapolation of the temperatures on the two sides of the coating from the temperatures measured by thermocouples, about 1 mm away from the surfaces.
The main advantage of the steady-state method for our specific investigation is that it better simulates the conditions of the actual working of the thermal barrier coating on the engine piston. The heating of the piston surface during the ignition is mainly due to conduction and not radiation and is relatively uniform over the surface of the piston (rather than spot-like). We used the same method (hot plate) to measure the temperature drop across the thermal barrier coating on an actual piston model.
In the steady-state method, the thermal flux q is assumed to be the same through the unknown sample and the known reference. The thermal flux is
where ϕ is the thermal flux, K is the thermal conductivity, ΔT is the temperature difference and h is the distance between the two temperature sensors.
Assuming the same flux through both samples, we have
where “1” refers to the unknown sample and “2” refers to the reference sample.
Knowing K
2 and measuring the temperature differences and the thicknesses, the value of K
1 can be calculated. For our modified version of the method, we have two references, the two substrates of the coatings, stacked as in
Figure 3 (insert). The temperatures measured with thermocouples embedded in the substrates (
Figure 2b) are used to calculate the heat flux on each side of the coating and use the average of the two values to find the average flux:
with the notations in
Figure 3, and Ks is the thermal conductivity of the substrate.
Using this average value of the flux, we can calculate the thermal conductivity of the coatings as
where T’
2 and T’
3 are the temperatures at the interfaces between the substrate and the coatings. These values were extrapolated from the values T
2 and respective T
3 measured directly with the thermocouples and assuming a linear gradient of temperature along the substrate (
Figure 3).
Using the expression for the average flux, the ratio between the thermal conductivity of the coating and that of the substrate can then be written as
Precautions need to be taken to eliminate the effect of the contact thermal resistance at the interface between the two coatings as much as possible. We addressed this problem along two directions: first, we measured the thermal resistance between two samples with coatings of pure aluminum. As our coatings are all based on aluminum powder as the major component, this thermal resistance is a reasonable estimate of the thermal resistance between the two coatings. The calculation of the Kcoating was corrected by subtracting the contact thermal resistance from the thermal resistance of the coatings. The values of contact thermal resistance measured for the samples on 1-inch substrate were of the order of 15 K·mm2/W, whereas the total thermal resistance of the two coatings is in the range of 200–300 K·mm2/W. The effect of the contact thermal resistance can then be estimated to be less than 10%. To further reduce the effect of the thermal resistance, for some measurements we attached the two coatings through a thin layer of silver paint.
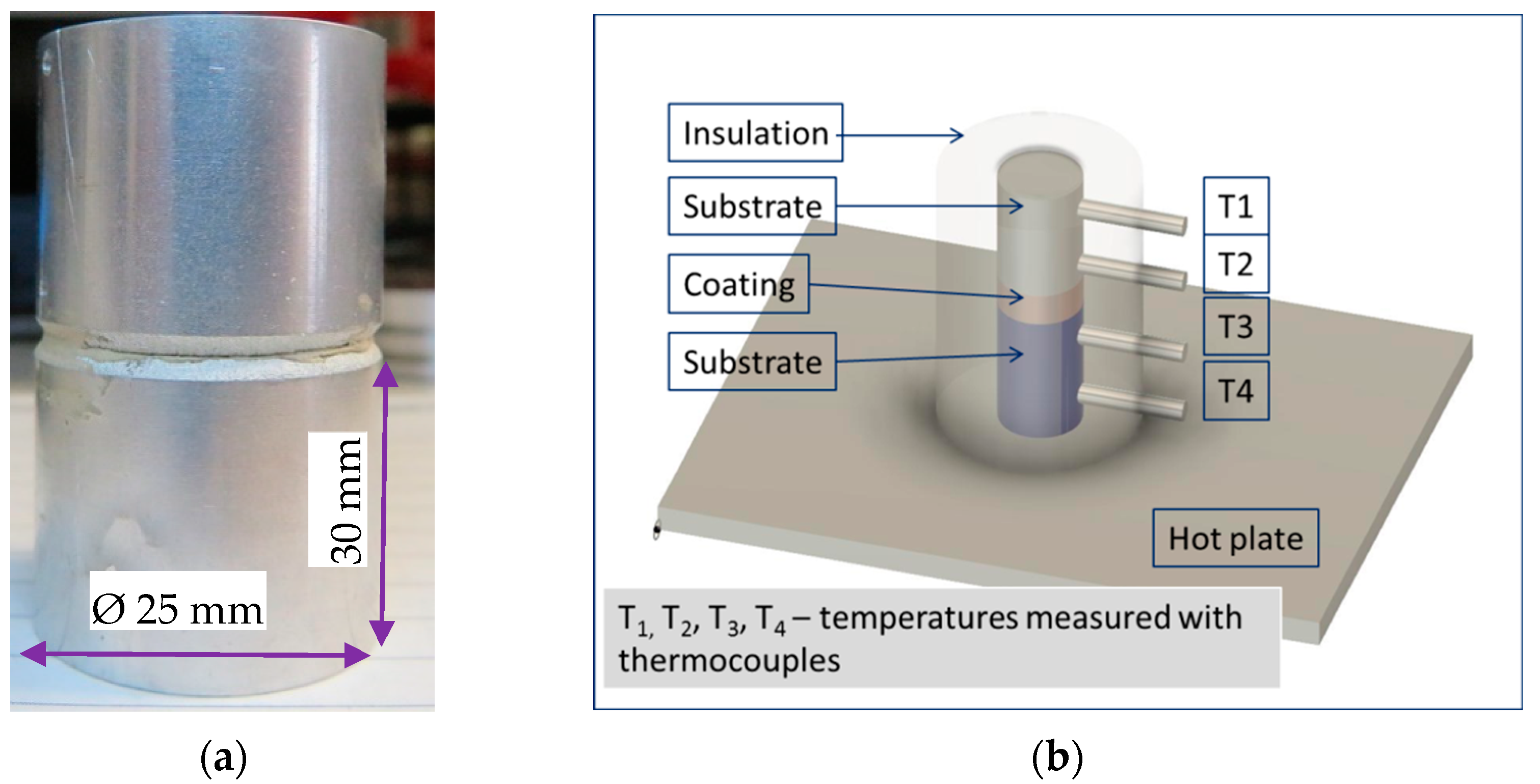
Thermal conductivity examination of piston models (
Figure 4a,b) was made, with setup shown in
Figure 4c. Models of flat piston heads were coated with the multilayer TBV and tested for determination of the temperature difference between the top of the coating and the substrate. The piston model was enclosed in an insulating chamber. One thermocouple was attached to the top of the coating (central area) with silver paint. The second thermocouple was inserted into a thin channel drilled about 1 mm below the substrate’s surface, along a radial direction.
2.3. Mechanical Properties and Structure Examination Procedures
As shown in the Introduction, the improved thermal efficiency results in measured gains in fuel economy and emissions reduction. To achieve these effects, ceramic-based TBCs are traditionally used to insulate combustion chambers due to the significantly reduced thermal conductivity as compared to engine block, head, and pistons metallic material. However, due to a mismatch of thermophysical properties between ceramics and the metals, these coatings are susceptible to failure far below the intended service lifetime of the engine [
28,
29]. Considering that the CTE of the examined intermetallic-based coatings matches the Al alloy substrate well, high values of their thermal shock resistance are foreseen. Nevertheless, a high brittleness of intermetallic compounds and the presence of some pores and cracks created at the synthesis process may be the reason for further accumulation of defects in intermetallic- and probably Al-based composite layers. Generally, a certain amount of porosity or microcracks seems to be favorable in achieving a high level of thermal shock resistance, because pores and microcracks accommodate deformation [
29]. In addition, various gradient and layered structures are known to improve the performance of coatings [
29]. In such systems, thermal stresses may be released, and the mechanical and thermal properties can be improved significantly. Normally, the thicker TBCs provide a greater temperature drop across the coatings. In addition, the increased thickness and number of layers of the coating will increase the total elastic strain energy stored, and hence the energy release rate for a crack [
30]. Thus, the failure mechanisms that cause spallation of multilayered TBCs are expected to be different in some degree from those of the traditional TBCs.
It is known [
18,
19,
24] that the failure of plasma-sprayed TBCs occurs in most cases by interface delaminating due to different thermomechanical properties of the coating and substrate and oxidation of the bond coat. In the case of multilayer intermetallic-based TBCs, the bonding strength between Al and intermetallic compounds is high due to gradual diffusion of Al to stainless steel particles and prevention the crack initiation at the interface. However, thermal shock and thermal fatigue behavior of multilayer coating itself have not been studied yet. Therefore, the aim of this experiment is to study the thermal shock behavior of the multilayer intermetallic-Al TBCs associated with different features of the microstructure.
The thermal shock behavior of TBCs was examined with resistance spot weld heat flux tests being performed with resistance spot welding gun, as shown on
Figure 5.
In this new method of testing, TBC specimens were subjected to discrete temperature exposure; the developed experimental technique uses resistance spot weld heating to create a heat flux and to model the thermal shock conditions of TBC action. It allows for modeling of the thermal cycling of the samples with various temperatures due to permanent cooling of one specimen edge. Thermal cycling of the TBCs results in crack accumulation in the intermetallic and ZrO
2 particles and particle–Al matrix interface (
Figure 1c). This process depends on the temperature regime and number of cycles. The real temperature of the sample surface near to heat source is being registered permanently by infrared pyrometer at the surface area with axis AA. An example of temperature–time diagram is shown in
Figure 6a. The thermal fatigue test at 500 and more cycles was made for Al plates coated with the multilayer TBC (
Figure 5b). To estimate the crack accumulation dependence on thermal cycles, measurements of electrical resistance of the samples shown in
Figure 5b were made by using a 4-probe (Kelvin) method, at a DC current of 3A.
The linear temperature distribution (
Figure 5a) may be approved for calculation of the temperatures of the cross sections BB and CC based on modeling results shown in [
21]. This conclusion allows one to define the procedure of layer-by-layer microhardness determination of the samples after thermal fatigue (TMF) test. The samples after TMF tests were cut and polished at the plane AA, in accordance with scheme shown in
Figure 5. After microhardness measurements, the samples were ground and polished at the plane BB. Similar procedure was made to measure the microhardness in the plane CC.
After sintering, the samples were mounted in epoxy resin, sectioned perpendicularly to the surface of the coating, polished using standard metallographic technique and examined by optical microscopy and SEM with EDS. Optical light microscopy Leica DMI5000 M with a digital camera DFS 320 R2 was used for metallographic characterization. SEM and EDS examinations were made by microscope FEI, including EDS X-ray micro-analyzer manufactured by EDAX. The thicknesses of intermetallic layers as well as the layers’ morphology were studied for different sintering conditions. The microhardness testing with depth-sensing indentation was used to record the indentation load–depth diagram. Mechanical characterization of the TBCs was performed using a computer-controlled dynamic ultra-microhardness tester (Shimadzu DUH-W201S, Kyoto, Japan). The microhardness measurements were performed on mounted samples with a Vickers diamond indenter to evaluate the coating and substrate hardness at various indentation loads. Five hardness experiments were performed for each sample.
4. Discussion
One of the main advantages of the LPCS system is the ability to spray the various powder mixtures. LPCS technology parameters chosen on the basis of experimental data shown in
Table 1 demonstrate that the LPCS method allows for both complex thermal barrier and catalytic coatings, and their combination, to be obtained. The coating structure and properties are well-controlled by propellant gas pressure and temperature, powder mixture composition and powder feeding rate. The main drawback of LPCS method is its low deposition efficiency in the range of 15–35%, which may be overcome up to 80% by the increase in gas pressure to 35 bar and using a middle-pressure cold-spraying system [
40].
The experimental results demonstrate that LPCS technology allows for both complex powder mixtures and multilayer coatings to be deposited. Two types of powder mixtures are successfully deposited: Al-ZrO
2 and Cu-Zn-Graphene. Some differences of coating structure formation mechanisms can be highlighted by comparison of the microstructures shown on
Figure 1,
Figure 9 and
Figure 12. Deposition of Al-ZrO
2 powder mixtures results in the breaking of the brittle ZrO
2 particles due to impingement with the substrate and formation of a relatively homogeneous structure with a ZrO
2 particle size of 3–10 μm (
Figure 12a,c). Additionally, the Al-ZrO
2 coating exhibits a much more pronounced flattened-splat microstructure (
Figure 12c). The further liquid-phase sintering of cold-sprayed coating leads to Al and Al + ZrO
2 composite layer formation (
Figure 1a,b) due to the flow of the molten Al phase.
The as-sprayed Al-ZrO
2 TBCs demonstrate the minimal effective thermal conductivity in the range of 8.0–10.0 W/mK, whilst the sintered intermetallic-based layered TBCs show K
eff ≈ 5.0–8.0 W/mK (
Figure 11). Intermetallic formation due to Al diffusion into steel particles leads to a considerable fall in thermal conductivity in the case of using Al bond layers. The intermetallics are formed due to reactive diffusion in alternatively stacked layers of two different metals. The intermetallic layers on the steel particles–Al matrix interface grow as a result of the annealing with the predetermined temperature and time. Therefore, the kinetics of the diffusion process is the key feature in controlling the properties of the synthesizing TBCs [
14]. The intermetallic layer thickness on the steel particles (
Figure 19) is proportional to growth kinetics, and the average thickness of the aluminide layer around the embedded particles may be used to calculate the kinetic parameters of the reaction diffusion. The further study and calculation of diffusion parameters will be made in future research.
The application of Al-ZrO
2 composite bond layers results in the diminishing of the effect of intermetallics on thermal conductivity. The main possible reason of such an effect is the small thickness of intermetallic layers formed on the AISI304 steel particles during heat treatment because of the lack of Al diffusion from Al-ZrO
2 and the Kirkendall pore effect (
Figure 14). It is worth mentioning that the SHS717-Al TBCs demonstrate low thermal conductivity because the intermetallics occupy about 70–80% of the particle volume. Only a small portion of the particle volume in the core belongs to austenite (
Figure 19,
Table 5).
Multilayer TBCs with Al/Al-ZrO
2 layers demonstrate high ductility due to the deformation of the Al matrix (
Figure 19b). It is of great importance because the TBCs work in harsh loading and thermal shock conditions. The microhardness measurement data (
Table 6) clearly show that microhardness of the Al layer falls after sintering at 500–575 °C due to the softening processes and increases after sintering at 625 °C due to the diffusion of Fe, Cr and other elements into the Al phase. In both cases, Al/Al-ZrO
2 layers have high ductility and are able to stop crack generation and movement (
Figure 13c). The results of thermal cycling’s influence on the electrical contact resistance (
Figure 18) reveal that contact resistance is controlled by multilayer TBC structure formation processes such as intermetallic synthesis and pore formation. It is possible to share these effects based on the Arrhenius model, which will be made in future work.
Comparison of the effective thermal conductivity of cold-sprayed multilayer coatings with that of plasma-sprayed Yttria stabilized zirconia with 7–8 wt% (7–8% YSZ) commonly used as TBC material [
16,
41] demonstrates the similar values of K
eff, a high thermal expansion coefficient similar to Al and the high ductility of the SHS717-Al composite due to the superior plastic properties of the Al matrix.
The main specific features of the catalytic coating structure (
Figure 8 and
Figure 9,
Table 3) clearly demonstrate the opportunities for DFCS technology to create the layers of heterogeneous catalysts. At the present time, heterogeneous single-atom catalysts (SACs) with atomically dispersed metal atoms facilitate the maximized atom utilization efficiency to reduce the catalyst cost and achieve their use for many applications [
42]. The experimental results of graphene NPs’ incorporation to the porous catalyst structure reveal the presence of Cu in the pores’ vicinity (spot 7
Table 3), which ensures high catalytic activity for a variety of hydrogenation and dehydrogenation reactions [
43]. The Cu-Graphene catalytic layer is believed to reduce NOx in a combustion chamber by about 30–40%, similar to [
44]. The real catalytic effectiveness of the Cu-Graphene coatings will be defined in future work.