Bioactive Surface of Zirconia Implant Prepared by Nano-Hydroxyapatite and Type I Collagen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Characterization
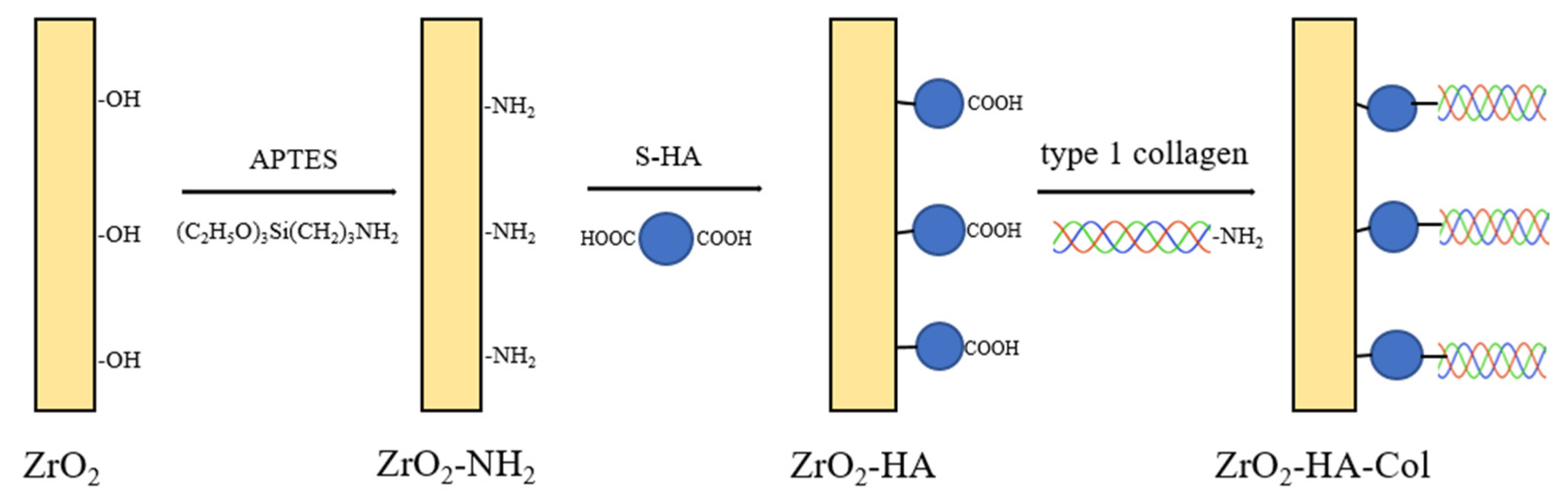
2.3. Chemical Bonding of Succinic Acid to the Surface of nHA
2.4. Chemical Bonding of nHA Particles to the Surface of Zirconia Implant
2.5. Chemical Bonding of Type I Collagen to the Surface of ZrO2-HA
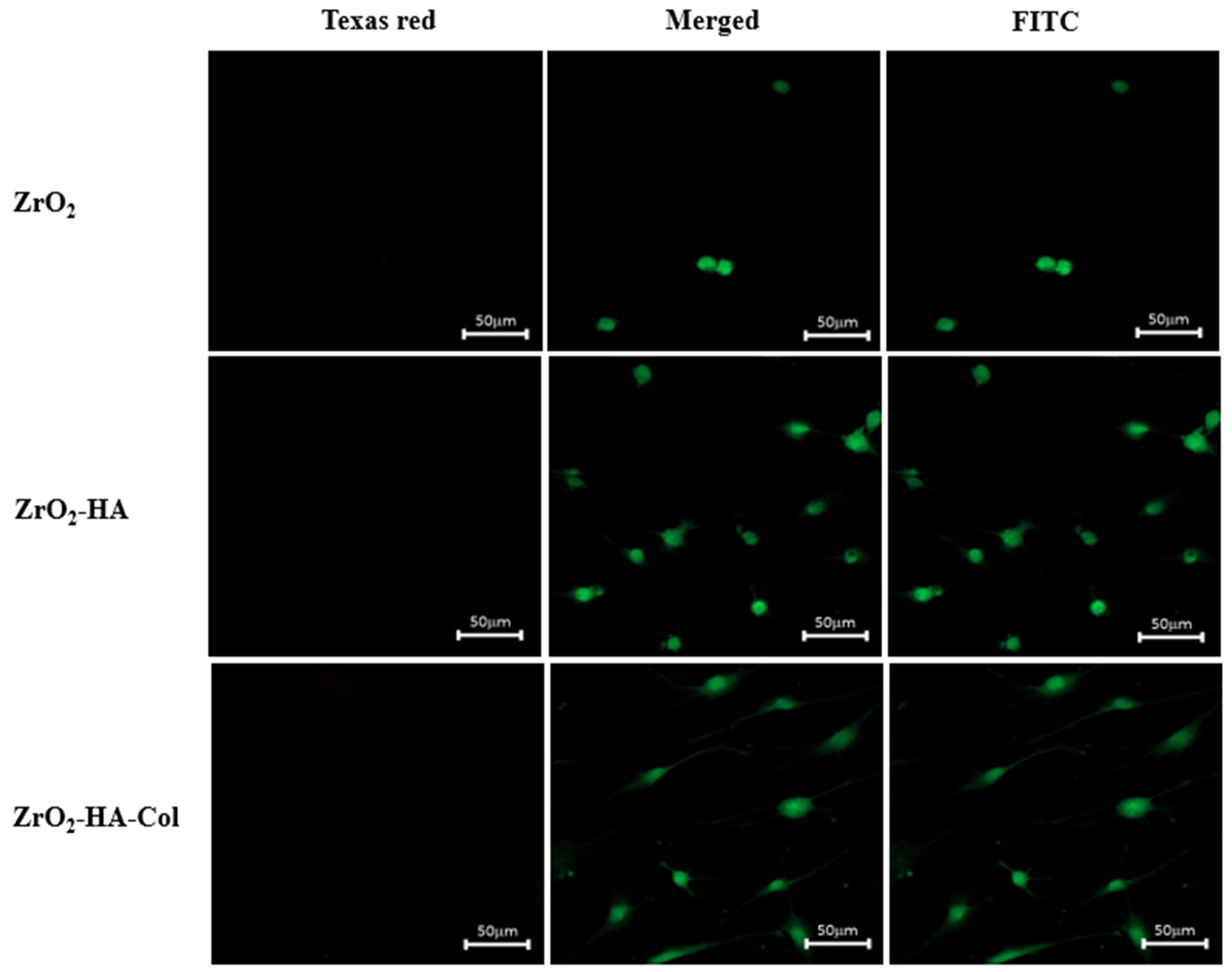
2.6. Cell Viability of ZrO2-HA and ZrO2-HA-Col
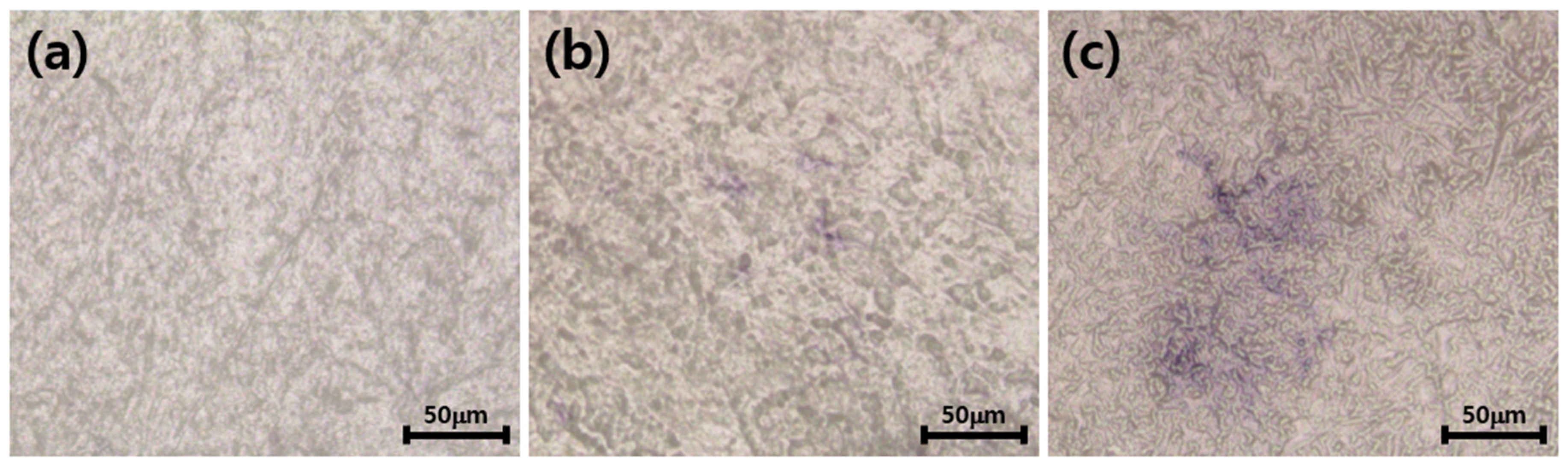
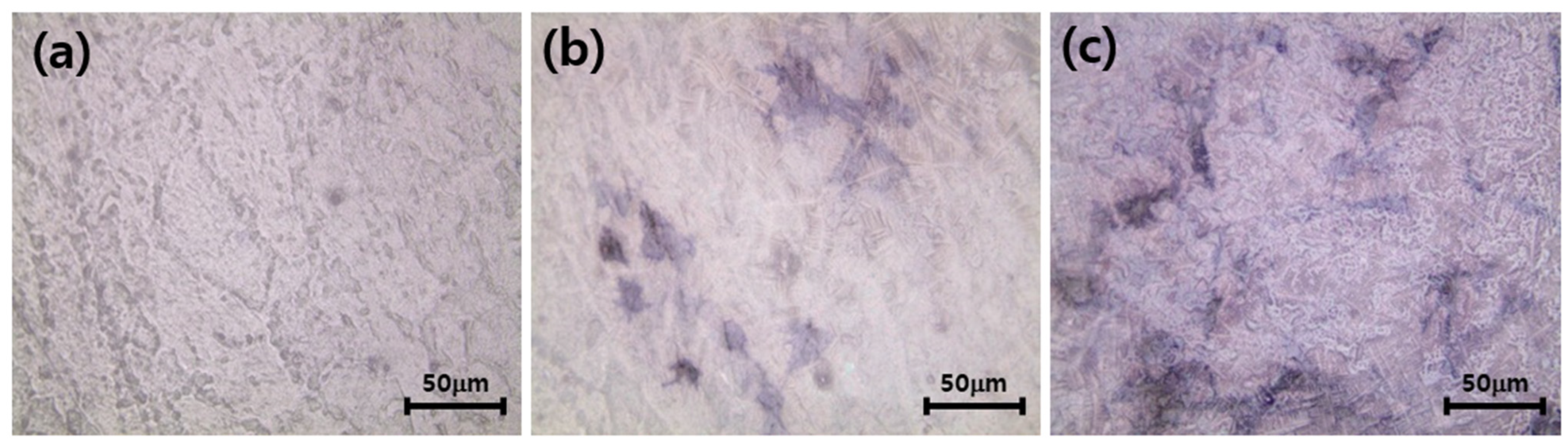
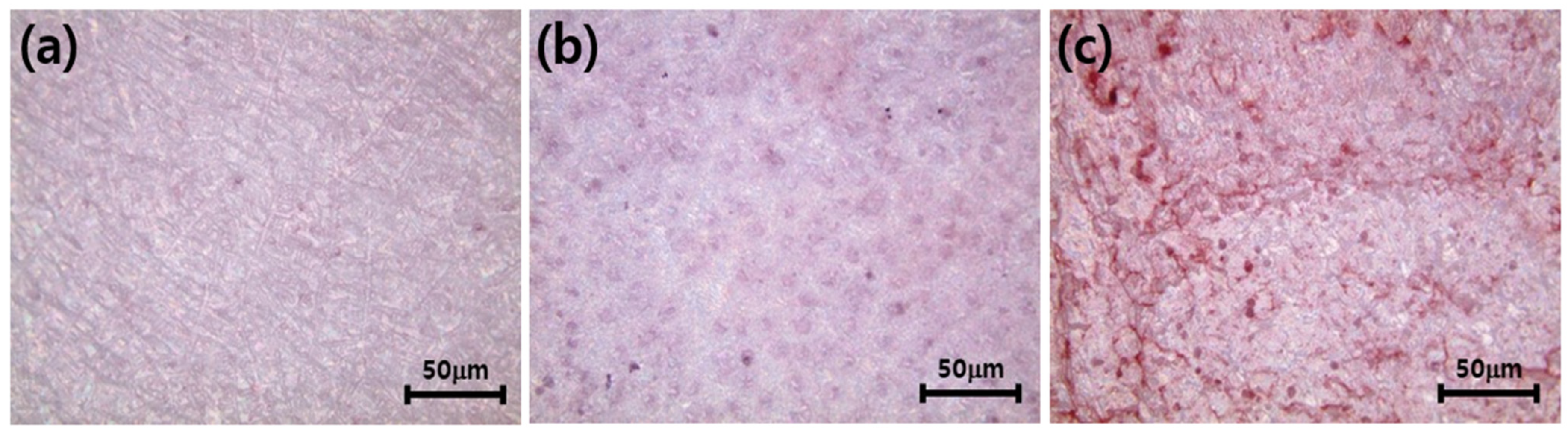
2.7. Differentiation of Osteoblasts Cultured on the Surface-Modified Zirconia
3. Results
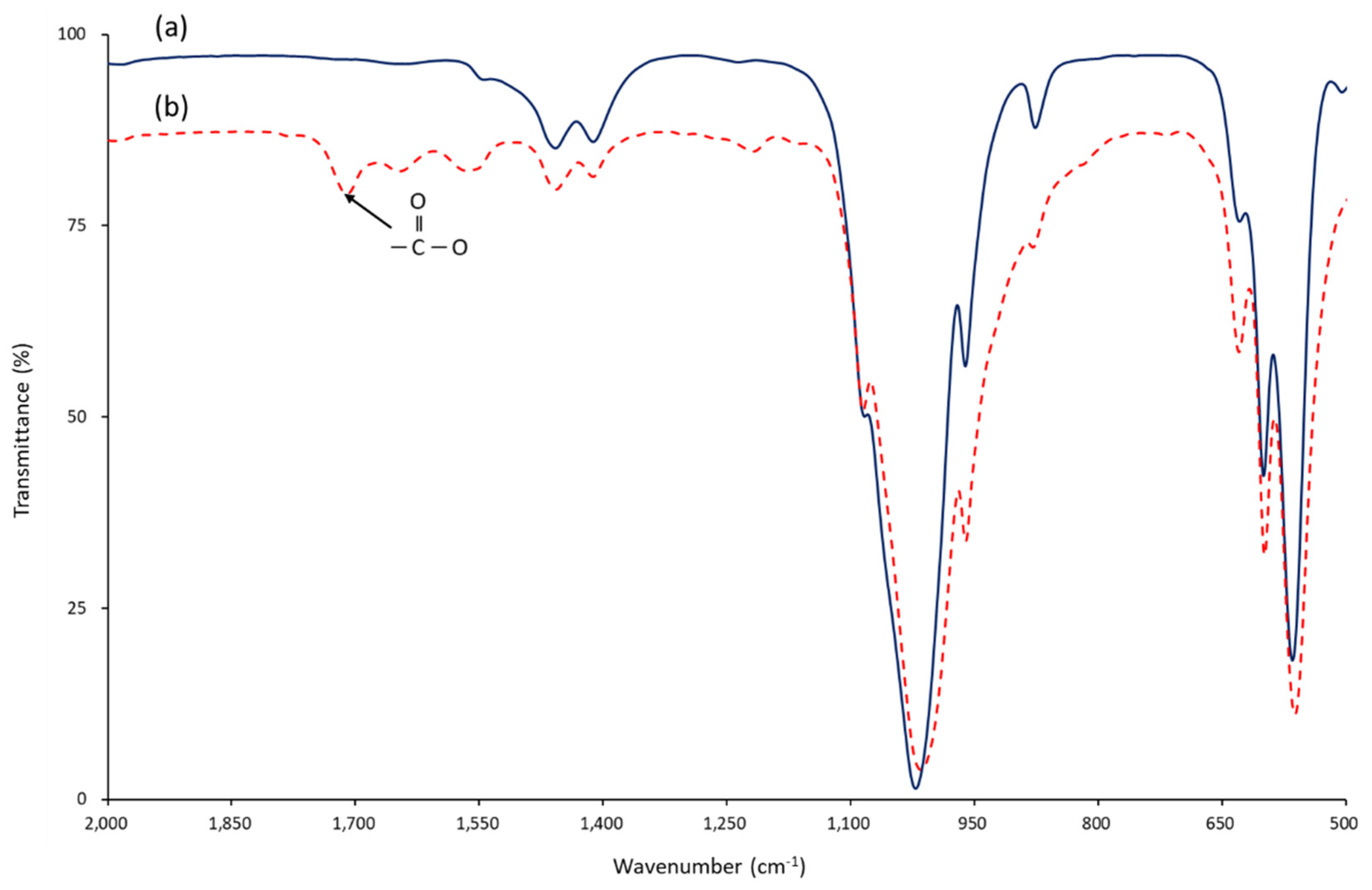
3.1. Chemical Bonding of Succinic Acid to the Surface of nHA
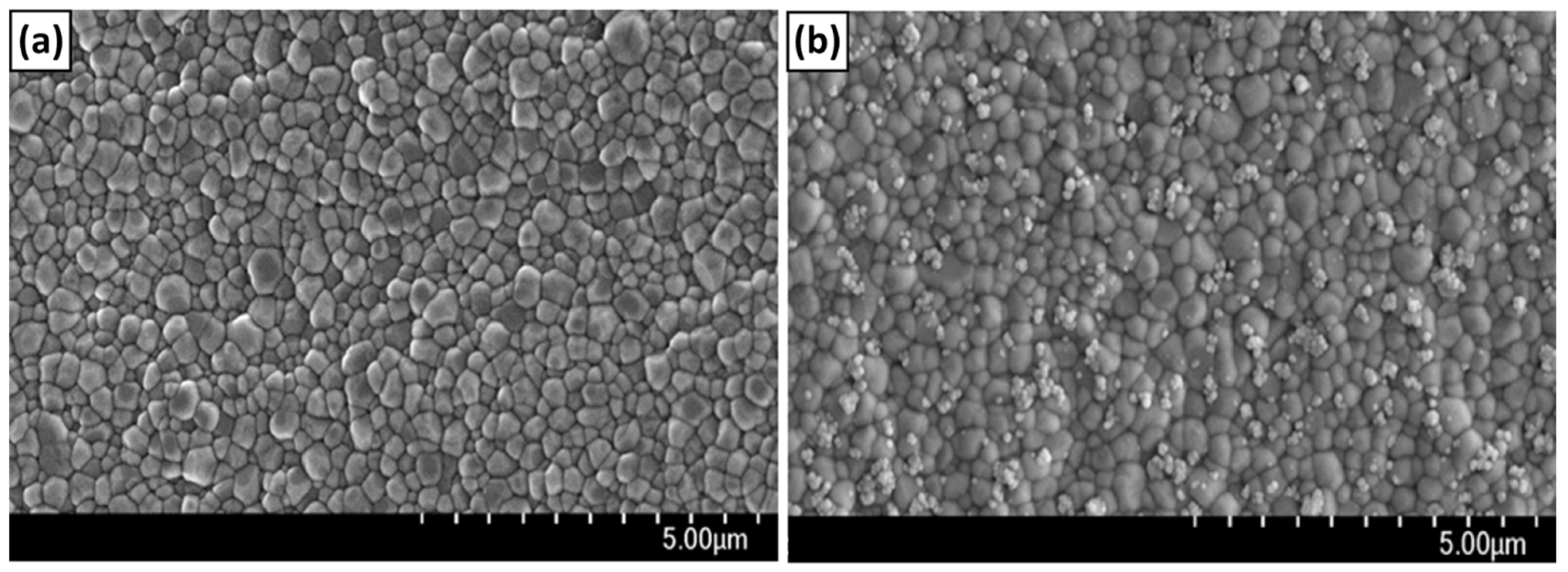
3.2. Chemical Bonding of nHA to the Surface of ZrO2 Implant
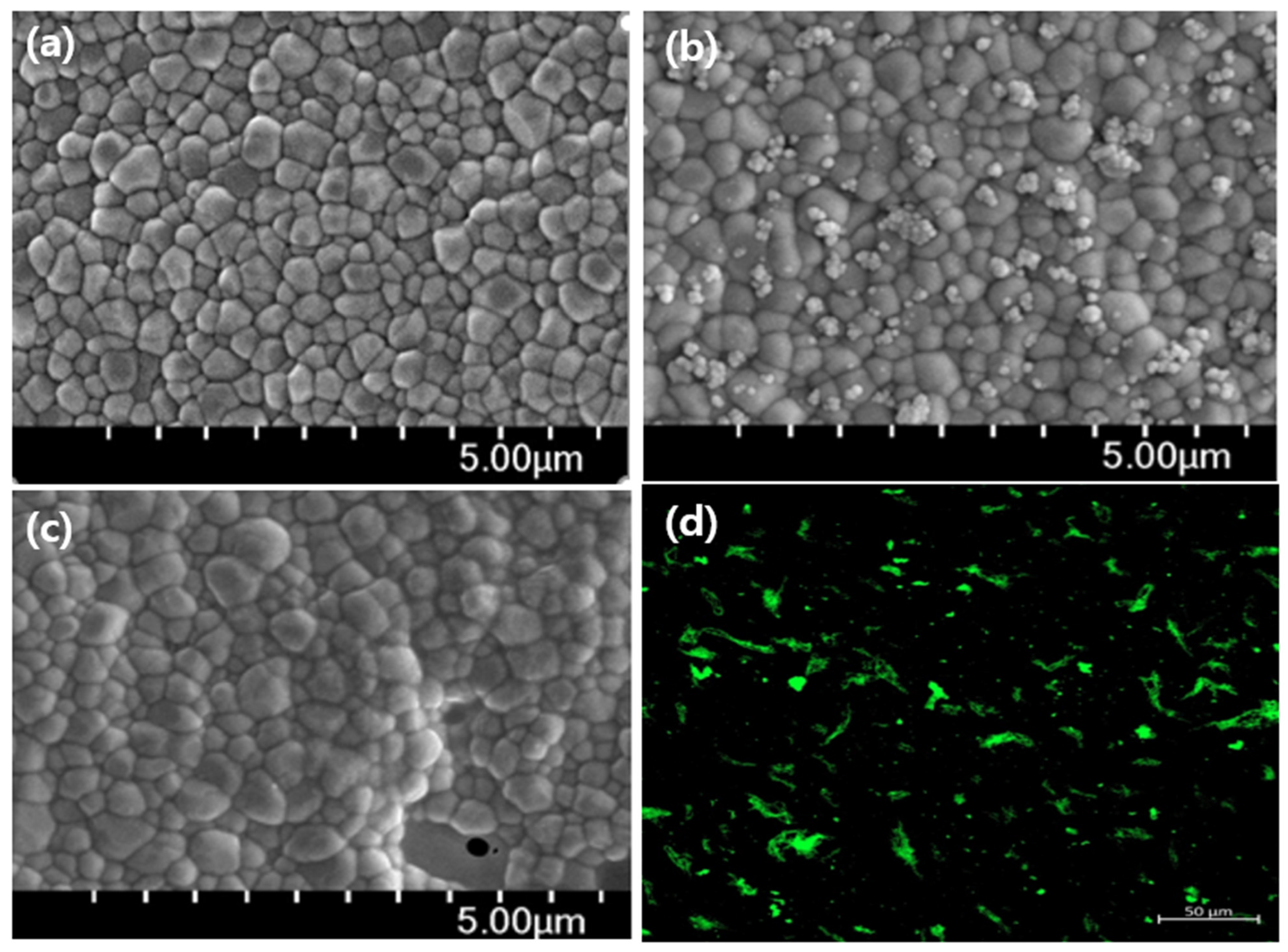
3.3. Chemical Bonding of Type I Collagen to the Surface of ZrO2 Implant
3.4. Cytotoxicity of Osteoblasts Cultured on the Zirconia Implants Immobilized with nHA and Type I Collagen
3.5. Differentiation of Osteoblasts Cultured on the Zirconia Implants Immobilized with nHA and Type I Collagen
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohal, R.J.; Weng, D.; Bachle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. Relat. Res. 2005, 7, S13–S20. [Google Scholar] [CrossRef]
- Hoffmann, O.; Angelov, N.; Gallez, F.; Jung, R.E.; Weber, F.E. The zirconia implant-bone interface: A preliminary histologic evaluation in rabbits. Int. J. Oral Maxillofac. Implant. 2008, 23, 369–374. [Google Scholar]
- Ahmad, I. Yttrium-partially stabilized zirconium dioxide posts: An approach to restoring coronally compromised nonvital teeth. Int. J. Periodon. Restor. Dent. 1998, 18, 455–465. [Google Scholar]
- Jackson, M.C. Restoration of posterior implants using a new ceramic material. J. Dent. Technol. 1999, 16, 19–22. [Google Scholar]
- Scarano, A.; DiCarlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Kohal, R.J.; Schwindling, F.S.; Bächle, M.; Spies, B.C. Peri-implant bone response to retrieved human zirconia oral implants after a 4-year loading period: A histologic and histomorphometric evaluation of 22 cases. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1622–1631. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Lauer, H.-C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef]
- Dubruille, J.H.; Viguier, E.; Le Naour, G.; Dubruille, M.T.; Auriol, M.; Charpentier, Y.L. Evaluation of combinations of titanium, zirconia, and alumina implants with 2 bone fillers in the dog. Int. J. Oral Maxillofac. Implant. 1999, 14, 271–277. [Google Scholar]
- Karoussis, I.K.; Salvi, G.E.; Heitz-Mayfield, L.J.A.; Bragger, U.; Hämmerle, C.H.F.; Lang, N.P. Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI dental implant system. Clin. Oral Implant. Res. 2003, 14, 329–339. [Google Scholar] [CrossRef]
- Mareci, D.; Trincă, L.C.; Căilean, D.; Souto, R.M. Corrosion resistance of Zr/Ti alloys with hydroxyapatite-zirconia-silver layer in simulated physiological solution containing proteins for biomaterial applications. Appl. Surf. Sci. 2016, 389, 1069–1075. [Google Scholar] [CrossRef]
- Hsu, C.M.; Sun, Y.S.; Huang, H.H. Enhanced cell response to zirconia surface immobilized with type I collagen. J. Dent. Res. 2019, 98, 556–563. [Google Scholar] [CrossRef]
- Zhou, B.; Niu, L.N.; Shi, W.; Zhang, W.; Arola, D.D.; Breschi, L.; Mao, J.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Synthesis of hierarchical mesoporous zirconia fiber by using collagen fiber as a template. J. Mater. Res. 2008, 23, 3263–3268. [Google Scholar]
- Kim, J.; Kang, I.G.; Cheon, K.H.; Lee, S.; Park, S.; Kim, H.E.; Han, C.M. Stable sol–gel hydroxyapatite coating on zirconia dental implant for improved osseointegration. J. Mater. Sci. Mater. Med. 2021, 32, 81. [Google Scholar] [CrossRef]
- Lee, J.; Eum, S.; Kim, J.; Jang, W.Y. Fabrication of hydroxyapatite-coated zirconia by room temperature spray process and microstructural change by heat-treatment. J. Korean Soc. Heat Treat. 2015, 28, 17–23. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, J.; Ryoo, H.; Kim, D.; Park, J.; Han, J. Osteogenic responses to zirconia with hydroxyapatite coating by aerosol deposition. J. Dent. Res. 2015, 94, 491–499. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive ceramics: Physical chemistry. In Comprehensive Biomaterials; Ducheyne, P., Healy, K., Hutmacher, D., Grainger, D.E., Kirkpatrick, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 187–221. [Google Scholar]
- Anwar, A.; Kanwal, Q.; Akbar, S.; Munawar, A.; Durrani, A.; Farooq, M.H. Synthesis and characterization of pure and nano-sized hydroxyapatite bioceramics. Nanotechnol. Rev. 2017, 6, 149–157. [Google Scholar] [CrossRef]
- Haider, A.; Gupta, K.C.; Kang, I.K. PLGA/nHA hybrid nanofiber scaffold as a nano-cargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res. Lett. 2014, 9, 314. [Google Scholar] [CrossRef]
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2013, 26, 209–220. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, H.; Gupta, K.C.; Kang, I.-K. Enhanced Tissue Compatibility of Polyetheretherketone Disks by Dopamine-Mediated Protein Immobilization. Macromol. Res. 2018, 25, 128–138. [Google Scholar] [CrossRef]
- You, C.K.; Kim, S.M.; Ahn, M.W.; Kim, S.Y. Evaluation of hydroxyl groups on hydroxyapatite and calcium metaphosphate by grafting TEOS and APTES. Key Eng. Mater. 2007, 342–343, 677–680. [Google Scholar] [CrossRef]
- Uskokovic, V.; Uskokovi, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. 2011, 96, 152–191. [Google Scholar] [CrossRef]
- Kandori, K.; Oda, S.; Fukusumi, M.; Morisada, Y. Synthesis of positively charged calcium hydroxyapatite nano-crystals and their adsorption behavior of proteins. Coll. Surf. 2009, 73, 140–145. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, W.M. Helmholtz–Smoluchowski velocity for viscoelastic electroosmotic flows. J. Colloid Interface Sci. 2008, 317, 631–636. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Eftekhari, M.; Schwarzenberger, K.; Javadi, A.; Eckert, K. The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: Electrostatic repulsion or the effect of ionic strength? Phys. Chem. Chem. Phys. 2020, 22, 2238–2248. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H.; Kim, N.K.; Kang, I.-K. Immobilization of collagen on the surface of a PEEK implant with monolayer nanopores. Polymers 2022, 14, 1633. [Google Scholar] [CrossRef]
- Titus, J.A.; Haugland, R.; Sharrow, S.O.; Segal, D.M. Texas red, a hydrophilic, red-emitting flourophore for use with flourescein in dual parameter flow microfluorometric and fluorescence microscopic studies. J. Immunol. Methods 1982, 50, 193–204. [Google Scholar] [CrossRef]
- Kohal, R.J.; Knauf, M.; Larsson, B.; Sahlin, H.; Butz, F. One-piece zirconia oral implants: One-year results from a prospective cohort study. Single tooth replacement. J. Clin. Periodontol. 2012, 39, 590–597. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Flückiger, L.B.; Attin, T.; Schmidlin, P.R. Effect of titanium and zirconium oxide microparticles on pro-Inflammatory response in human macrophages under induced sterile inflammation: An in vitro study. Materials 2021, 14, 4166. [Google Scholar] [CrossRef]
- Smielak, B.; Klimek, L. Effect of hydrofluoric acid concentration and etching duration on select surface roughness parameters for zirconia. J. Prosth. Dent. 2015, 113, 596–602. [Google Scholar] [CrossRef]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2022, 13, 161–178. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Spies, B.C.; Bauer, A.; Butz, F. One-piece zirconia oral implants for single-tooth replacement: Three-year results from a long-term prospective cohort study. J. Clin. Periodon. 2017, 45, 114–124. [Google Scholar] [CrossRef]
- Naleway, S.E.; Fickas, K.C.; Maker, Y.N.; Meyers, M.A.; McKittrick, J. Reproducibility of ZrO2-based freeze casting for biomaterials. Mater. Sci. Eng. C 2016, 61, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Aboushelib, M.N.; Shawky, R. Osteogenesis ability of CAD/CAM porous zirconia scaffolds enriched with nanohydroxyapatite particles. Int. J. Implant Dent. 2017, 3, 21. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, K.C.; Kim, N.-H.; Moon, H.S. Evaluation of zirconia sueface after strong acid etching and its effects on the shear bond strength of dental resin cement. Int. J. Dent. 2019, 2019, 3564275. [Google Scholar] [CrossRef]
- Turp, V.; Akgungor, G.; Sen, D.; Tuncelli, B. Evaluation of surface topography of zirconia ceramic after Er:YAG laser etching. Photomed. Laser. Surg. 2014, 32, 533–539. [Google Scholar] [CrossRef]
- An, S.-H.; Matsumoto, T.; Miyajima, H.; Nakahira, A.; Kim, K.-H.; Imazato, S. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 2012, 28, 1221–1231. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.S.; Gupta, K.C.; Lee, D.Y.; Kang, I.-K. Hydroxyapatite nanorod-modified sand blasted titanium disk for endosseous dental implant applications. Tissue Eng. Regen. Med. 2018, 15, 601–614. [Google Scholar] [CrossRef]
- Liu, G.; Wu, C.; Zhang, X.; Liu, Y.; Meng, H.; Xu, J.; Xu, X.; Xu, Y. Surface functionalization of zirconium dioxide nano-adsorbents with 3-aminopropyltriethoxysilane and promoted adsorption activity for bovine serum albumin. Mater. Chem. Phys. 2016, 176, 129–135. [Google Scholar] [CrossRef]




| Substrates | Atomic Percent of Elements (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Y | Zr | C | N | O | S | Si | Ca | P | |
| ZrO2 | 1.3 | 18.0 | 15.6 | 1.3 | 55.1 | 1.0 | 1.5 | - | 0.4 |
| ZrO2-A | 1.1 | 10.1 | 36.6 | 4.0 | 37.0 | 0.3 | 10.7 | - | 0.3 |
| ZrO2-HA | 0.7 | 8.9 | 16.5 | 1.6 | 55.9 | 0.3 | 2.5 | 7.4 | 6.5 |
| ZrO2-HA-Col. | 0.9 | 11.7 | 25.8 | 5.0 | 49.7 | 0.1 | 5.4 | - | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lee, Y.-H.; Kim, N.-K.; Kang, I.-K. Bioactive Surface of Zirconia Implant Prepared by Nano-Hydroxyapatite and Type I Collagen. Coatings 2022, 12, 1335. https://doi.org/10.3390/coatings12091335
Kim H, Lee Y-H, Kim N-K, Kang I-K. Bioactive Surface of Zirconia Implant Prepared by Nano-Hydroxyapatite and Type I Collagen. Coatings. 2022; 12(9):1335. https://doi.org/10.3390/coatings12091335
Chicago/Turabian StyleKim, Hun, Yang-Ho Lee, Nam-Kwon Kim, and Inn-Kyu Kang. 2022. "Bioactive Surface of Zirconia Implant Prepared by Nano-Hydroxyapatite and Type I Collagen" Coatings 12, no. 9: 1335. https://doi.org/10.3390/coatings12091335
APA StyleKim, H., Lee, Y.-H., Kim, N.-K., & Kang, I.-K. (2022). Bioactive Surface of Zirconia Implant Prepared by Nano-Hydroxyapatite and Type I Collagen. Coatings, 12(9), 1335. https://doi.org/10.3390/coatings12091335







