The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review
Abstract
:1. Introduction
2. Geopolymer Aluminosilicate Materials
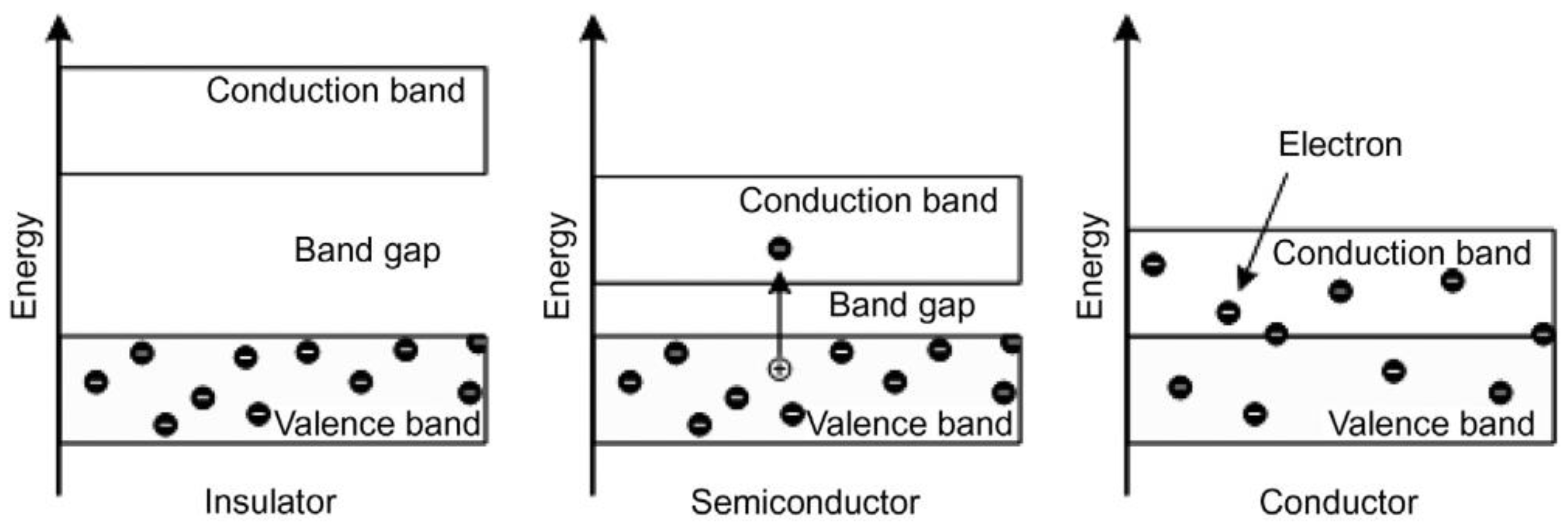
3. Photocatalyst
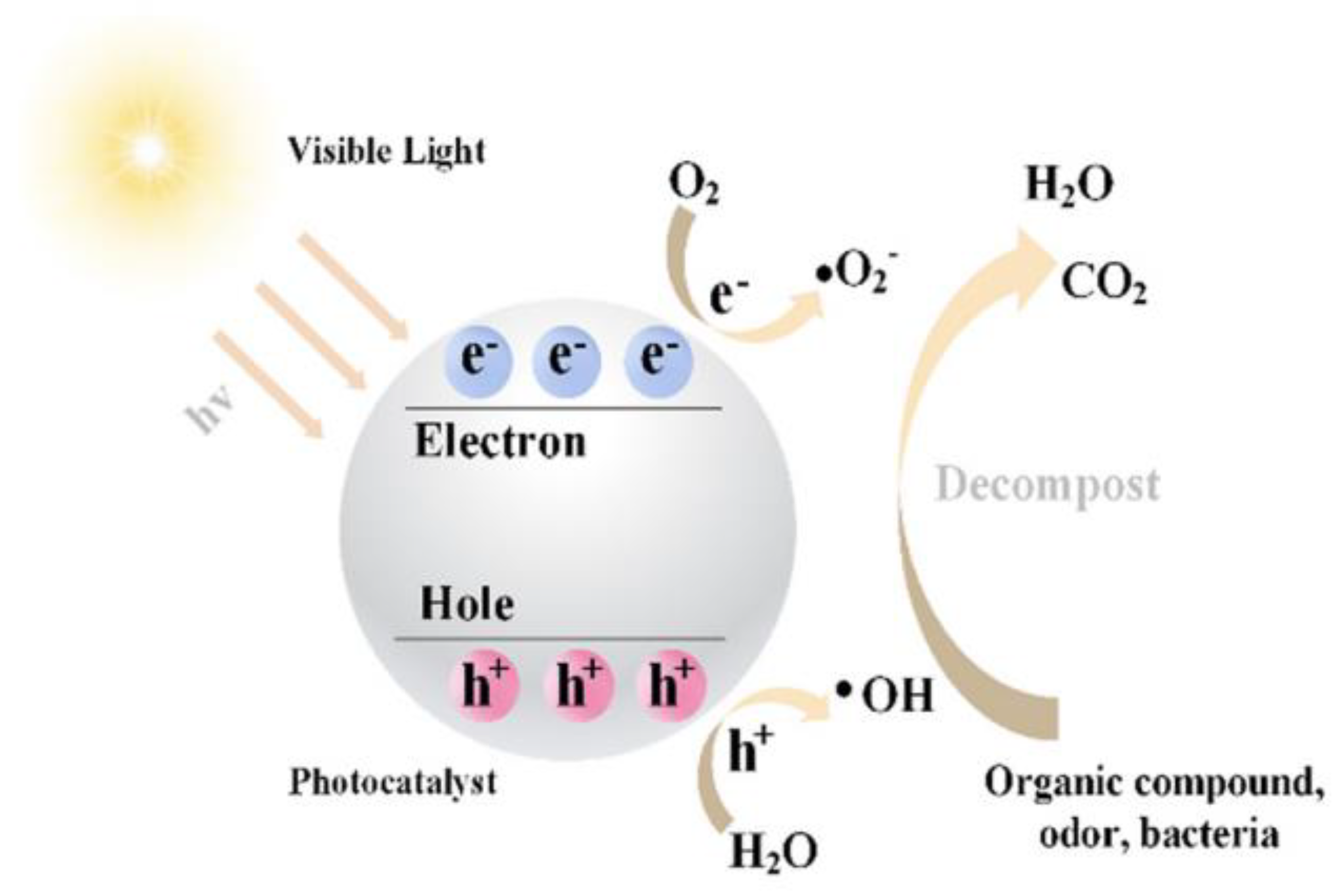
3.1. Photocatalyst Mechanism
3.2. Photocatalyst Precursor Materials
3.2.1. Titanium Dioxide Nanoparticles Precursor Material
3.2.2. Zinc Oxide Nanoparticles Precursor Material
4. Coating
4.1. Conventional Coating
4.2. Geopolymer Coating
5. Factor Affecting Geopolymer Paste
6. Photocatalyst Degradation Evaluation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillay, D.L.; Olalusi, O.B.; Awoyera, P.O.; Rondon, C.; Echeverría, A.M.; Kolawole, J.T. A Review of the Engineering Properties of Metakaolin Based Concrete: Towards Combatting Chloride Attack in Coastal/Marine Structures. Adv. Civ. Eng. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Petroche, D.M.; Ramirez, A.D. The Environmental Profile of Clinker, Cement, and Concrete: A Life Cycle Perspective Study Based on Ecuadorian Data. Buildings 2022, 12, 311. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Mechanism of Photocatalysis. In Photocatalysis. Lecture Notes in Chemistry; Springer: Singapore, 2018; Volume 100. [Google Scholar]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Halin, D.S.C.; Sandu, A.V.; Vizureanu, P.; Yahya, Z. Potential Applications of Geopolymer Cement-Based Composite as Self-Cleaning Coating: A Review. Coatings 2022, 12, 133. [Google Scholar] [CrossRef]
- Burghardt, T.E.; Pashkevich, A.; Zakowska, L. Influence of Volatile Organic Compounds Emissions from Road Marking Paints on Ground-Level Ozone Formation: Case Study of Kraków, Poland. Transp. Res. Procedia 2016, 14, 714–723. [Google Scholar] [CrossRef]
- Jiménez-López, A.M.; Hincapié-Llanos, G.A. Identification of Factors Affecting the Reduction of VOC Emissions in the Paint Industry: Systematic Literature Review-SLR. Prog. Org. Coat. 2022, 170, 106945. [Google Scholar] [CrossRef]
- Zhu, A. Feasibility Study on Novel Fire-Resistant Coating Materials. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2020. [Google Scholar]
- Warid Wazien, A.Z.; Mustafa, M.; Abdullah, A.B.; Razak, R.A.; Rozainy, M.M.A.Z.R.; Faheem, M.; Tahir, M.; Faris, M.A.; Hamzah, H.N. Review on Potential of Geopolymer for Concrete Repair and Rehabilitation. MATEC Web Conf. 2016, 78, 01065. [Google Scholar] [CrossRef]
- Falah, M.; Mackenzie, K.J.D. Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts 2020, 10, 1158. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation—A Review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Mashuri, S.I.S.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Asikin-Mijan, N.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for Organic Wastewater Treatment: From the Basis to Current Challenges for Society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied Photoelectrocatalysis on the Degradation of Organic Pollutants in Wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Dziechciarz, A. Self-Cleaning Coatings and Surfaces of Modern Building Materials for the Removal of Some Air Pollutants. Materials 2021, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Gururani, P.; Gairola, S.P. Metal Oxide Nanoparticles and Their Nanocomposite-Based Materials as Photocatalysts in the Degradation of Dyes. Biointerface Res. Appl. Chem. 2022, 12, 6557–6579. [Google Scholar]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO₂)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent Progress on Doped ZnO Nanostructures for Visible-Light Photocatalysis. Thin Solid Films. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Sharifi, T.; Crmaric, D.; Kovacic, M.; Popovic, M.; Rokovic, M.K.; Kusic, H.; Jozić, D.; Ambrožić, G.; Kralj, D.; Kontrec, J.; et al. Tailored BiVO4 for Enhanced Visible-Light Photocatalytic Performance. J. Environ. Chem. Eng. 2021, 9, 106025. [Google Scholar] [CrossRef]
- Shan, L.; Lu, C.; Dong, L.; Suriyaprakash, J. Efficient Facet Regulation of BiVO4 and Its Photocatalytic Motivation. J. Alloys Compd. 2019, 804, 385–391. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Shafique, S.; Rashid, R.; Akhter, P.; Ahmed, A.; Jeon, J.-K.; Park, Y.-K. Oxidative Desulfurization of Refinery Diesel Pool Fractions Using LaVO4 Photocatalyst. J. Ind. Eng. Chem. 2021, 98, 283–288. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Chen, M. Novel Z-Scheme LaVO4/Bi3O4Cl Heterojunctions for Highly Efficient Degradation of Ofloxacin under Visible Light Irradiation. J. Alloys Compd. 2022, 925, 166653. [Google Scholar] [CrossRef]
- Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5-WO3/TiO2 Catalyst Synthesized from Waste Catalyst on Selective Catalytic Reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Ardelean, I.; Sandu, A.V.; Corbu, O.C.; Matei, E. Revealing the Influence of Microparticles on Geopolymers’ Synthesis and Porosity. Materials 2020, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Meor Ahmad Tajudin, M.A.F.; Abdullah, M.M.A.B.; Sandu, A.V.; Nizar, K.; Moga, L.; Neculai, O.; Muniandy, R. Assessment of Alkali Activated Geopolymer Binders as an Alternative of Portland Cement. Mater. Plastice. 2017, 54, 145–154. [Google Scholar]
- Vizureanu, P.; Samoila, C.; Cotfas, D. Materials Processing using Solar Energy. Environ. Eng. Manag. J. 2009, 8, 301–306. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Corbu, O. Synthesis and Characteristics of Local Fly Ash Based Geopolymers Mixed with Natural Aggregates. Rev. De Chim. 2019, 70, 1262–1267. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A Review of the Multi-Component Utilisation of Coal Fly Ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A Review on Geopolymers as Emerging Materials for the Adsorption of Heavy Metals and Dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Saloma; Hanafiah; Elysandi, D.O.; Meykan, D.G. Effect of Na2SiO3/NaOH on Mechanical Properties and Microstructure of Geopolymer Mortar Using Fly Ash and Rice Husk Ash as Precursor. AIP Conf. Proc. 2017, 1903, 050013. [Google Scholar]
- Al Bakri, A.M.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Mastura, W. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement—A Review. J. Asian Sci. Res. 2011, 1, 247–253. [Google Scholar]
- Castillo, H.; Collado, H.; Droguett, T.; Vesely, M.; Garrido, P.; Palma, S. State of the Art of Geopolymers: A Review. E-Polymer 2022, 22, 108–124. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Recent Advances in Fly-Ash-Based Geopolymers: Potential on the Utilization for Sustainable Environmental Remediation. ACS Omega 2021, 6, 15532–15542. [Google Scholar] [CrossRef] [PubMed]
- Zidi, Z.; Ltifi, M.; ben Ayadi, Z.; Mir, L.E.L.; Nóvoa, X.R. Effect of Nano-ZnO on Mechanical and Thermal Properties of Geopolymer. J. Asian Ceram. Soc. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Cao, R.; Fang, Z.; Jin, M.; Shang, Y. Study on the Activity of Metakaolin Produced by Traditional Rotary Kiln in China. Minerals 2022, 12, 365. [Google Scholar] [CrossRef]
- Rocha, J.; Klinowski, J. Physics and Chemistry of Minerals, 295i and 27A1 Magic-Angle-Spinning NMR Studies of the Transformation of Kaolinite; University of Cambridge: Cambridge, UK, 1990; Volume 17. [Google Scholar]
- De Rossi, A.; Simão, L.; Ribeiro, M.J.; Novais, R.M.; Labrincha, J.A.; Hotza, D.; Moreira, R.F.P.M. In-Situ Synthesis of Zeolites by Geopolymerization of Biomass Fly Ash and Metakaolin. Mater. Lett. 2019, 236, 644–648. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly Ash-Based Geopolymer: Clean Production, Properties and Applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Kalombe, R.M.; Ojumu, V.T.; Eze, C.P.; Nyale, S.M.; Kevern, J.; Petrik, L.F. Fly Ash-Based Geopolymer Building Materials for Green and Sustainable Development. Materials 2020, 13, 5699. [Google Scholar] [CrossRef]
- Prochon, P.; Zhao, Z.; Courard, L.; Piotrowski, T.; Michel, F.; Garbacz, A. Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars. Materials 2020, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Humad, A.M.; Kothari, A.; Provis, J.L.; Cwirzen, A. The Effect of Blast Furnace Slag/Fly Ash Ratio on Setting, Strength, and Shrinkage of Alkali-Activated Pastes and Concretes. Front. Mater. 2019, 6, 9. [Google Scholar] [CrossRef]
- Abbas, R.; Khereby, M.A.; Ghorab, H.Y.; Elkhoshkhany, N. Preparation of Geopolymer Concrete Using Egyptian Kaolin Clay and the Study of Its Environmental Effects and Economic Cost. Clean Technol. Environ. Policy 2020, 22, 669–687. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of Metakaolin-Based Geopolymer Concrete for Different Mix Design Parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Ionescu, B.A.; Lăzărescu, A.-V.; Hegyi, A. The Possibility of Using Slag for the Production of Geopolymer Materials and Its Influence on Mechanical Performances—A Review. Proceedings 2020, 63, 30. [Google Scholar]
- Vignesh, T.; Sumathi, A.; Saravana, K.; Mohan, R. Study on Self-Cleaning Concrete Using Nano-Liquid TiO2. Int. J. Eng. Technol. 2018, 7, 860–863. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 135–175. [Google Scholar]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-Light Photocatalysts and Their Perspectives for Building Photocatalytic Membrane Reactors for Various Liquid Phase Chemical Conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Ma, W.; Shi, W.; Zhu, Y.; Choi, W. Photocatalytic Activity Enhanced via Surface Hybridization. Carbon Energy. 2020, 2, 308–349. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding Mechanism of Photocatalytic Microbial Decontamination of Environmental Wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Zhang, W.; Jin, B.; Feng, Q.; Huang, J.; Jiao, Z. Advances in Z-Scheme Semiconductor Photocatalysts for the Photoelectrochemical Applications: A Review. Carbon Energy 2022, 4, 294–331. [Google Scholar] [CrossRef]
- Anandan, S.; Ohashi, N.; Miyauchi, M. ZnO-Based Visible-Light Photocatalyst: Band-Gap Engineering and Multi-Electron Reduction by Co-Catalyst. Appl. Catal. B Environ. 2010, 100, 502–509. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, K.; Yu, H.; Zhao, L.; Zhu, X.; Zhang, J. Laboratory Experiment on the Nano-TiO2 Photocatalytic Degradation Effect of Road Surface Oil Pollution. Nanotechnol. Revis. 2020, 9, 922–933. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Gómez-Avilés, A.; Peñas-Garzón, M.; Rodriguez, J.J. Semiconductor Photocatalysis for Water Purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–651. [Google Scholar]
- Rana, A.; Sudhaik, A.; Raizada, P.; Khan, A.A.P.; van Le, Q.; Singh, A.; Selvasembian, R.; Nadda, A.; Singh, P. An Overview on Cellulose-Supported Semiconductor Photocatalysts for Water Purification. Nanotechnol. Environ. Eng. 2021, 6, 40. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. In-Depth Study of Heavy Metal Removal by an Etidronic Acid-Functionalized Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2022, 14, 7450–7463. [Google Scholar] [CrossRef]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.B. Photocatalytic Behaviour of TiO2-Geopolymer Paste under Sunlight. IOP Conf. Ser. Mater. Sci. Eng. 2020, 957, 012006. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable Concrete: Building a Greener Future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Peng, R.; Wang, H. Geopolymer-TiO2 Nanocomposites for Photocsatalysis: Synthesis by One-Step Adding Treatment versus Two-Step Acidification Calcination. Minerals 2019, 9, 658. [Google Scholar] [CrossRef]
- Jdm, K.; Yusoff, M.M.; Aqilah, N.S. Degradation of Methylene Blue via Geopolymer Composite Photocatalyst. Solid State Sci. Technol. 2013, 21, 23–30. [Google Scholar]
- Maniarasan, S.K.; Santhosh Kumar, V.; Chandrasekaran, P. Index Terms: Application of Titania in Geopolymer Concrete. Int. J. Sci. Technol. Res. 2020, 9, 806–813. [Google Scholar]
- Mondragón-Figueroa, M.; Guzmán-Carrillo, H.R.; Rico, M.Á.; Reyez-Araiza, J.L.; Pineda-Piñón, J.; López-Naranjo, E.J.; Columba-Palomares, M.C.; López-Romero, J.M.; Gasca-Tirado, J.R. Development of a Construction Material for Indoor and Outdoor, Metakaolinite-Based Geopolymer, with Environmental Properties. J. Mater. Sci. Eng. A 2019, 9, 131–142. [Google Scholar] [CrossRef]
- Isabel Bravo, P.; Shimizu, E.; Alvin Malenab, R.; Anne Tigue, A.; Mae Dela Cerna, K.; Isagani Janairo, J.; Angelo Promentilla, M.; Ethelbhert Yu, D. Nanocrystalline Titania Coated Metakaolin and Rice Hull Ash Based Geopolymer Spheres for Photocatalytic Degradation of Dyes in Wastewater. Orient. J. Chem. 2019, 35, 167–172. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase—A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ding, S.; Yu, X.; Han, B.; Ou, J. Multifunctional Cementitious Composites Modified with Nano Titanium Dioxide: A Review. Compos. Part A: Appl. Sci. Manuf. 2018, 111, 115–137. [Google Scholar] [CrossRef]
- Ningthoujam, R.; Singh, Y.D.; Babu, P.J.; Tirkey, A.; Pradhan, S.; Sarma, M. Nanocatalyst in Remediating Environmental Pollutants. Chem. Phys. Impact 2022, 4. [Google Scholar] [CrossRef]
- Hamidi, F.; Aslani, F. TiO2-Based Photocatalytic Cementitious Composites: Materials, Properties, Influential Parameters, and Assessment Techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Ketan, M.; Jibhenkar, B.; Vaidya, P.V.D.; Waghmare, M.S.S.; Singh, D.P. Eco-Sustainable Pervious Concrete Using Titanium Dioxide. Int. J. Sci. Res.Dev. 2015, 3, 391–392. [Google Scholar]
- Liu, J.; Li, Q.; Xu, S. Influence of Nanoparticles on Fluidity and Mechanical Properties of Cement Mortar. Constr. Build. Mater. 2015, 101, 892–901. [Google Scholar] [CrossRef]
- Jamaludin, L.; Razak, R.A.; al Bakri Abdullah, M.M.; Kusbiantoro, A.; Yahya, Z.; Abdullah, A.; Sandu, A.V. Geopolymer Coating Paste on Concrete for Photocatalytic Performance. AIP Conf. Proc. 2021, 2339, 020187. [Google Scholar]
- Cha, B.J.; Saqlain, S.; Seo, H.O.; Kim, Y.D. Hydrophilic Surface Modification of TiO2 to Produce a Highly Sustainable Photocatalyst for Outdoor Air Purification. Appl. Surf. Sci. 2019, 479, 31–38. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 Nanoparticles by Chemical and Green Synthesis Methods and Their Multifaceted Properties. SN Appl. Sci. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Nabi, G.; Raza, W.; Tahir, M.B. Green Synthesis of TiO2 Nanoparticle Using Cinnamon Powder Extract and the Study of Optical Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1425–1429. [Google Scholar] [CrossRef]
- Honarmand, M.M.; Mehr, M.E.; Yarahmadi, M.; Siadati, M.H. Effects of Different Surfactants on Morphology of TiO2 and Zr-Doped TiO2 Nanoparticles and Their Applications in MB Dye Photocatalytic Degradation. SN Appl. Sci. 2019, 1, 505. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Šubrt, J.; Štengl, V.; Dianez, M.J.; Sayagues, M.J. Photoactivity of Anatase–Rutile TiO2 Nanocrystalline Mixtures Obtained by Heat Treatment of Homogeneously Precipitated Anatase. Appl. Catal. B Environ. 2005, 58, 193–202. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Niaei, A.; Rasoulifard, M.H.; Khataee, A.R. Immobilization of TiO2 Nanopowder on Glass Beads for the Photocatalytic Decolorization of an Azo Dye C.I. Direct Red 23. J. Environ. Sci. Health Part A 2005, 40, 1605–1617. [Google Scholar] [CrossRef]
- Khan, M.I.; Bhatti, K.A.; Qindeel, R.; Althobaiti, H.S.; Alonizan, N. Structural, Electrical and Optical Properties of Multilayer TiO2 Thin Films Deposited by Sol–Gel Spin Coating. Results Phys. 2017, 7, 1437–1439. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Photocatalytic Activities of Titanium Dioxide Incorporated Architectural Mortars: Effects of Weathering and Activation Light. Build. Environ. 2015, 94, 395–402. [Google Scholar] [CrossRef]
- Loh, K.; Gaylarde, C.C.; Shirakawa, M.A. Photocatalytic Activity of ZnO and TiO2 ‘Nanoparticles’ for Use in Cement Mixes. Constr. Build. Mater. 2018, 167, 853–859. [Google Scholar] [CrossRef]
- Wu, X. Applications of Titanium Dioxide Materials. In Titanium Dioxide—Advances and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Zulkifly, K.; Heah, C.Y.; Liew, Y.M.; Abdullah, M.M.A.B.; Abdullah, S.F.A. The Synergetic Compressive Strength and Microstructure of Fly Ash and Metakaolin Blend Geopolymer Pastes. AIP Conf. Proc. 2018, 2045, 020100. [Google Scholar]
- Ratan, J.K.; Saini, A. Enhancement of Photocatalytic Activity of Self-Cleaning Cement. Mater. Lett. 2019, 244, 178–181. [Google Scholar] [CrossRef]
- Pathak, S.S.; Vesmawala, G.R. Effect of Nano TiO2 on Mechanical Properties and Microstructure of Concrete. Mater. Today Proc. 2022, 65, 1915–1921. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Fariza, M.; Rashid, A.; Ainuddin, A.R. Zinc Oxide Nanoparticle Synthesize by Green Approach. Res. Prog. Mech. Manuf. Eng. 2021, 2, 390–398. [Google Scholar]
- Garg, N.; White, C.E. Mechanism of Zinc Oxide Retardation in Alkali-Activated Materials: An in-Situ X-Ray Pair Distribution Function Investigation. J. Mater. Chem. A 2017, 5, 11794–11804. [Google Scholar] [CrossRef]
- Nochaiya, T.; Sekine, Y.; Choopun, S.; Chaipanich, A. Microstructure, Characterizations, Functionality and Compressive Strength of Cement-Based Materials Using Zinc Oxide Nanoparticles as an Additive. J. Alloys Compd. 2015, 630, 1–10. [Google Scholar] [CrossRef]
- McKeen, L.W. The Components of Paint. In Fluorinated Coatings and Finishes Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 51–58. [Google Scholar]
- Goncharenko, D.; Aleinikova, A.; Kabus, O.; Kolomiiets, Y. Study of the Efficiency of Epoxy Coating Protection of Concrete Surfaces from Sulfuric Acid Corrosion. IOP Conf. Ser. Mater. Sci. Eng. 2019, 708, 012081. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, Y.; Wang, Y.; Shi, J. TA/Fe (III) Anti-Chloride Coating to Protect Concrete. J. Clean. Prod. 2020, 259, 120922. [Google Scholar] [CrossRef]
- Papakonstantinou, C.G.; Balaguru, P.N. Geopolymer protective coatings for concrete. In Proceedings of the SAMPE ‘07: M and P—From Coast to Coast and Around the World, Conference Proceedings, International SAMPE Symposium and Exhibition, Baltimore, MD, USA, 3–7 June 2007. [Google Scholar]
- Pradhan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Insight on the Chemistry of Epoxy and Its Curing for Coating Applications: A Detailed Investigation and Future Perspectives. Polym. Plast. Technol. Eng. 2016, 55, 862–877. [Google Scholar] [CrossRef]
- Kozak, A. Application of Acrylic-Based Coatings for Concrete Protection. MATEC Web Conf. 2018, 163, 05011. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, A.; Bao, X.; Chen, Z.; Ni, T.; Wang, Z. Protective Geopolymer Coatings Containing Multi-Componential Precursors: Preparation and Basic Properties Characterization. Materials 2020, 13, 3448. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Biasetto, L.; Colombo, P. Metakaolin-Based Geopolymer Coatings on Metals by Airbrush Spray Deposition. J. Coat. Technol. Res. 2020, 17, 991–1002. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; Robayo-Salazar, R.A.; de Gutiérrez, R.M. A Novel Geopolymer Application: Coatings to Protect Reinforced Concrete against Corrosion. Appl. Clay Sci. 2017, 135, 437–446. [Google Scholar] [CrossRef]
- Sarumathi, M.; Ramaswamy, S.N. Performance and Effectiveness of Concrete Coatings-A State of Art Review. Int. J. Sci. Res. 2016, 5, 294–298. [Google Scholar]
- Rosales, A.; Esquivel, K. SiO2@TiO2 Composite Synthesis, and Its Hydrophobic Applications: A Review. Catalysts 2020, 10, 171. [Google Scholar] [CrossRef]
- Guzmán-Aponte, L.A.; de Gutiérrez, R.M.; Maury-Ramírez, A. Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics. Coatings 2017, 7, 233. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; el Achouri, M.; Taibi, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The Role of Inorganic Polymer Technology in the Development of ‘Green Concrete. ’ Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Jaya, N.A.; Abdullah, M.M.A.B.; Li, L.-Y.; Sandu, A.V.; Hussin, K.; Ming, L.Y. Durability of Metakaolin Geopolymers with Various Sodium Silicate/Sodium Hydroxide Ratios against Seawater Exposure. AIP Conf. Proc. 2017, 1887, 020063. [Google Scholar]
- Guzmán-Carrillo, H.R.; Manzano-Ramírez, A.; Garcia Lodeiro, I.; Fernández-Jiménez, A. ZnO Nanoparticles for Photocatalytic Application in Alkali-Activated Materials. Molecules 2020, 25, 5519. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, S.M.; Rabat, N.E.; Sufian, S. Effects of Na: Al and Water: Solid Ratios on the Mechanical Properties of Fly Ash Based Geopolymer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012011. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C.W. The Role of Zinc in Metakaolin-Based Geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO2-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef]
- Saufi, H.; el Alouani, M.; Alehyen, S.; el Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Kaya-Özkiper, K.; Uzun, A.; Soyer-Uzun, S. Red Mud- and Metakaolin-Based Geopolymers for Adsorption and Photocatalytic Degradation of Methylene Blue: Towards Self-Cleaning Construction Materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L. Fly Ash-Based Geopolymer as a Novel Photocatalyst for Degradation of Dye from Wastewater. Particuology 2013, 11, 353–358. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, K.; Liu, Y. Geopolymer-supported photocatalytic TiO2 film: Preparation and characterization. Constr. Build. Mater. 2017, 151, 63–70. [Google Scholar] [CrossRef]



| Author | Aluminosilicate Material | Finding |
|---|---|---|
| Yusuf G. Adewuyi [37] | Class F Fly Ash | Elimination of trace noxious heavy metals in aqueous environment. The geopolymer adsorbent as a substance is recyclable since it can be synthesized by leveraging abundant waste materials. |
| Rafik Abbas et al. [46] | Kaolin | Used to produce geopolymer concrete as it does not require energy for pretreatment and contains high alumina silicate. |
| Abdulrahman et al. [47] | Metakaolin | Metakaolin geopolymer with different mix design for producing geopolymer concrete. |
| Ionescu et al. [48] | Slag | Steel slag or blast furnace slag in the production of geopolymer for construction building materials |
| Author | Photocatalyst Precursor | Application | Finding |
|---|---|---|---|
| Jdm et al. 2013 [67] | TiO2 anatase Titanyl sulfate |
| Titanyl sulphate results in high photocatalyst activity. |
| Maniasaran et al. 2020 [68] | TiO2 |
| Geopolymer concrete add TiO2 is superior in self-cleaning. |
| M. Mondragon-Figueroa et al. 2019 [69] | TiO2 |
| Metakaolin geopolymer add TiO2 improved antimicrobial testing. |
| Isabel Bravo et al. 2019 [70] | Titania |
| Metakaolin and rice husk geopolymer deposited titania degrade 90% of dye pollutants in wastewater. |
| Anandan et al. 2010 [58] | Zinc oxide |
| Efficient ZnO based visible-light photocatalysts, consisting of band-engineering by formation of a solid solution and surface modification of co-catalysts. |
| Shayegan et al. 2018 [71] | Titanium Dioxide |
| Photocatalyst can eliminate indoor air contaminants effectively at room temperature and contaminants to carbon dioxide and water. |
| Authors | Field of Study | Finding Descriptions | Research Gap |
|---|---|---|---|
| Jiang et al. 2022 [104] | Fly ash combined with ground granulated blast-furnace slag, metakaolin and ordinary Portland cement added superplasticizer for geopolymer coating concrete. | The adhesive strength of the recommended GPC mixes varied from 1.5 to 3.4 MPa and fully met the surface protection criteria. | The study shows the effect of geopolymer coating for surface protection in building construction and several gaps can be filled regarding the function of superplasticizer. Their properties for enhancing adhesion strength are not fully discussed. |
| Mao et al. 2020 [105] | Metakaolin-based geopolymer coatings on metal by air brush deposition. | Applied metakaolin-based geopolymer spraying on hot aluminium and steel metal surfaces (40–150 °C) at sealed and unsealed conditions for thermal protection applications. | The study focuses on the effects of curing conditions but gives less explanation regarding the effect of curing temperature on the surface deterioration of metal substrates. |
| Rosales et al. 2020 [108] | Development of a SiO2@TiO2 coating applicable to cement-based materials for hydrophobic applications. | The photocatalytic activity of the SiO2@TiO2 coating showed a removal of RhB establishing itself as a photocatalytic material. | Hydrophobic effect relevant for self-cleaning application on coating but this research focus on conventional coating with different synthesis method such as sol gel and hydrothermal. No research study on the effect of hydrophobic towards geopolymer coating. |
| Falah et al. 2020 [9] | The effective activation and utilization of metakaolin as an alkali activated geopolymer precursor and its use surface protection. | Geopolymer nanocomposites capable of eliminating hazardous pollutants from wastewater or the atmosphere. | This review of the utilization of metakaolin geopolymer for surface protection did not cover the strength of coating in term of adhesion, corrosion resistance, or abrasion. |
| Alouani et al. 2019 [110] | The ability of geopolymer powder produced from metakaolin and alkaline activators to react as an adsorbent to remove methylene blue. | Geopolymer has high selectivity and considered an economical adsorbent for the elimination of methylene blue. | The adsorption of MB in geopolymer explained by pseudo-second-order kinetic model, but there is less literature on the testing to eliminate dyes. |
| Loh et al. 2018 [88] | Titanium and zinc nanoparticle suspensions use in degradation of pollutants and protection of built concrete structures. | TiO2 and ZnO nanoparticles protected calciferous materials from fungal fouling and light exposure was not necessary for antifungal activity. | The focusing method are incorporated nanoparticles into OPC for concrete building. No relevant information on strength and surface characteristics after addition of nanoparticles. |
| Aguirre-Guerrero et al. 2017 [106] | Geopolymer mortars containing fly ash and metakaolin as coatings for reinforced concrete against chloride-induced corrosion. | The findings led to the conclusion that the MK based geopolymer coating performed the best, reducing the corrosion rate compared to concrete without coating. | The corrosion reinforcing steel exposed only to the chloride environment and less investigates on the surface deterioration after concrete exposed to chloride attack. |
| Guzman-Aponte et al. 2017 [109] | TiO2 addition into the physical and mechanical characteristics of a geopolymer process derived on metakaolin. | Based on the findings, the addition of TiO2 particles at up to 10 wt.% had no effect on the development of the KASH gel. | The research mentions the physical and mechanical characterization but says less about the strength behaviour of coating after the addition of TiO2 into the geopolymer. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaludin, L.; Razak, R.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Bras, A.; Imjai, T.; Sandu, A.V.; Abd Rahim, S.Z.; Yong, H.C. The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings 2022, 12, 1348. https://doi.org/10.3390/coatings12091348
Jamaludin L, Razak RA, Abdullah MMAB, Vizureanu P, Bras A, Imjai T, Sandu AV, Abd Rahim SZ, Yong HC. The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings. 2022; 12(9):1348. https://doi.org/10.3390/coatings12091348
Chicago/Turabian StyleJamaludin, Liyana, Rafiza Abd Razak, Mohd Mustafa Al Bakri Abdullah, Petrica Vizureanu, Ana Bras, Thanongsak Imjai, Andrei Victor Sandu, Shayfull Zamree Abd Rahim, and Heah Cheng Yong. 2022. "The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review" Coatings 12, no. 9: 1348. https://doi.org/10.3390/coatings12091348
APA StyleJamaludin, L., Razak, R. A., Abdullah, M. M. A. B., Vizureanu, P., Bras, A., Imjai, T., Sandu, A. V., Abd Rahim, S. Z., & Yong, H. C. (2022). The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings, 12(9), 1348. https://doi.org/10.3390/coatings12091348











