A Comparative Study of Organic Dye-Sensitized Solar Cells Based on Anatase TiO2 and Amorphous Free Mixed Phase’s Anatase/Rutile P25 TiO2 Photoanodes
Abstract
:1. Introduction
2. Experiment
2.1. Materials Synthesis
2.2. DSC Devices Assembly
2.3. Material and Device Characterizations
3. Results and Discussions
3.1. Material Structure Analysis
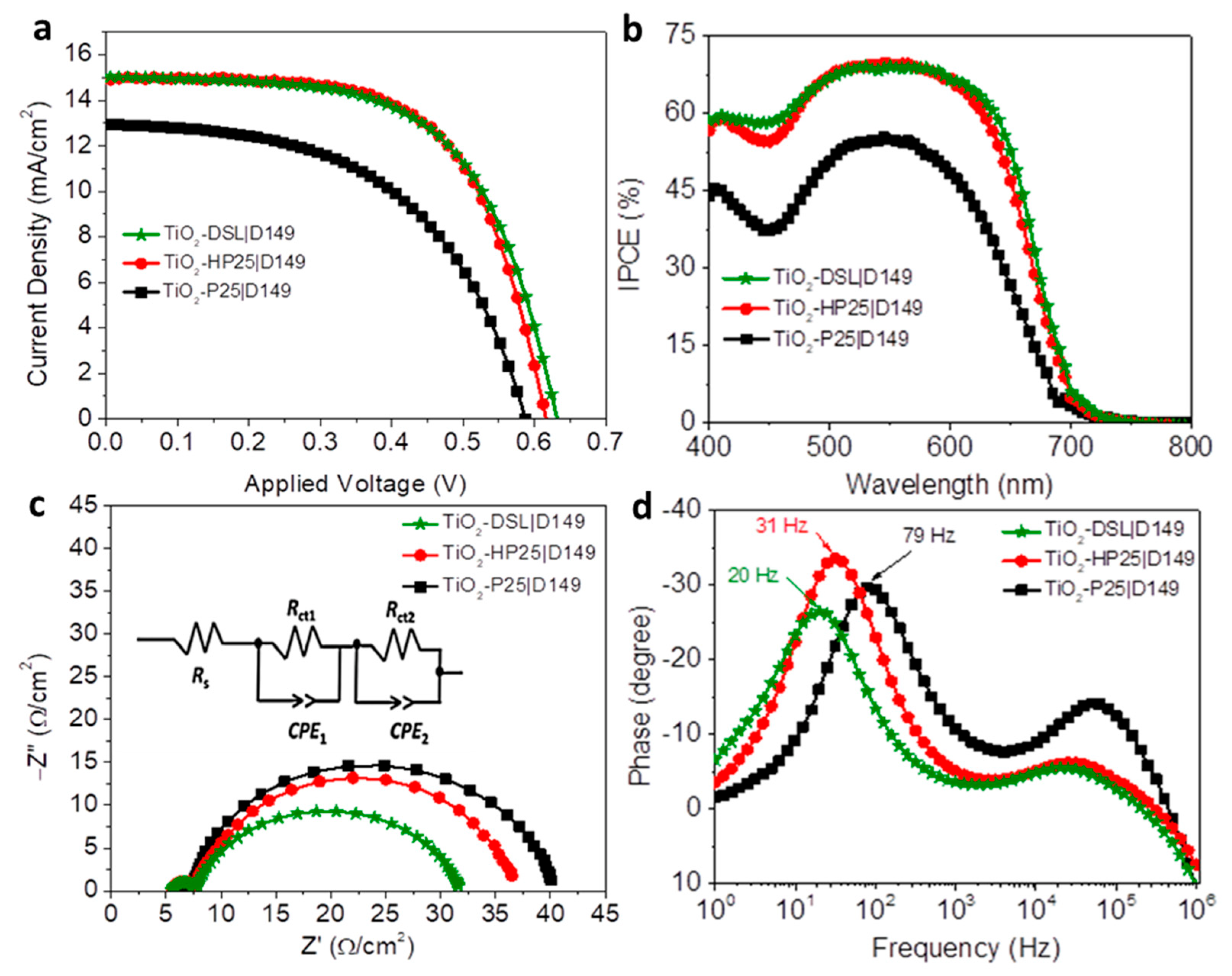
3.2. Photovoltaic Performance of DSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Krebs, F.C.; Tromholt, T.; Jørgensen, M. Upscaling of polymer solar cell fabrication using full roll-to-roll processing. Nanoscale 2010, 2, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Rasheduzzaman, M.; Pillai, P.B.; Mendoza, A.N.C.; De Souza, M.M. A study of the performance of solar cells for indoor autonomous wireless sensors. In Proceedings of the 2016 10th International Symposium on Communication Systems, Networks and Digital Signal Processing (CSNDSP), Prague, Czech Republic, 20–22 July 2016; pp. 1–6. [Google Scholar]
- Yoon, S.; Tak, S.; Kim, J.; Jun, Y.; Kang, K.; Park, J. Application of transparent dye-sensitized solar cells to building integrated photovoltaic systems. Build. Environ. 2011, 46, 1899–1904. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photon. 2017, 11, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Al-Attafi, K.; Nattestad, A.; Qutaish, H.; Park, M.S.; Shrestha, L.K.; Ariga, K.; Dou, S.X.; Kim, J.H. Solvothermally synthesized anatase TiO2 nanoparticles for photoanodes in dye-sensitized solar cells. Sci. Technol. Adv. Mater. 2021, 22, 100–112. [Google Scholar] [CrossRef]
- Fan, K.; Yu, J.; Ho, W. Improving photoanodes to obtain highly efficient dye-sensitized solar cells: A brief review. Mater. Horiz. 2017, 4, 319–344. [Google Scholar] [CrossRef]
- Park, N.-G.; van de Lagemaat, J.; Frank, A.J. Comparison of Dye-Sensitized Rutile- and Anatase-Based TiO2 Solar Cells. J. Phys. Chem. B 2000, 104, 8989–8994. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Heo, Y.-U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D Hierarchical Rutile TiO2 and Metal-free Organic Sensitizer Producing Dye-sensitized Solar Cells 8.6% Conversion Efficiency. Sci. Rep. 2014, 4, 5769. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ikeuchi, Y.; Xu, L.; Sewvandi, G.A.; Kusunose, T.; Tanaka, Y.; Nakanishi, S.; Wen, P.; Feng, Q. Synthesis of [111]- and {010}-faceted anatase TiO2 nanocrystals from tri-titanate nanosheets and their photocatalytic and DSSC performances. Nanoscale 2015, 7, 7980–7991. [Google Scholar] [CrossRef]
- Li, C.; Koenigsmann, C.; Ding, W.; Rudshteyn, B.; Yang, K.R.; Regan, K.P.; Konezny, S.J.; Batista, V.S.; Brudvig, G.W.; Schmuttenmaer, C.A.; et al. Facet-Dependent Photoelectrochemical Performance of TiO2 Nanostructures: An Experimental and Computational Study. J. Am. Chem. Soc. 2015, 137, 1520–1529. [Google Scholar] [CrossRef]
- Fang, W.Q.; Yang, X.H.; Zhu, H.; Li, Z.; Zhao, H.; Yao, X.; Yang, H.G. Yolk@ shell anatase TiO2 hierarchical microspheres with exposed {001} facets for high-performance dye sensitized solar cells. J. Mater. Chem. 2012, 22, 22082–22089. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Sewvandi, G.A.; Kusunose, T.; Tanaka, Y.; Nakanishi, S.; Feng, Q. Synthesis of {010}-faceted anatase TiO2 nanoparticles from layered titanate for dye-sensitized solar cells. CrystEngComm 2014, 16, 8885–8895. [Google Scholar] [CrossRef]
- Ide, Y.; Inami, N.; Hattori, H.; Saito, K.; Sohmiya, M.; Tsunoji, N.; Komaguchi, K.; Sano, T.; Bando, Y.; Golberg, D.; et al. Remarkable Charge Separation and Photocatalytic Efficiency Enhancement through Interconnection of TiO2 Nanoparticles by Hydrothermal Treatment. Angew. Chem. Int. Ed. 2016, 55, 3600–3605. [Google Scholar] [CrossRef]
- Al-Attafi, K.; Nattestad, A.; Wu, Q.; Ide, Y.; Yamauchi, Y.; Dou, S.X.; Kim, J.H. The effect of amorphous TiO2 in P25 on dye-sensitized solar cell performance. Chem. Commun. 2018, 54, 381–384. [Google Scholar] [CrossRef]
- Kurian, S.; Sudhagar, P.; Lee, J.; Song, D.; Cho, W.; Lee, S.; Kang, Y.S.; Jeon, H. Formation of a crystalline nanotube–nanoparticle hybrid by post water-treatment of a thin amorphous TiO2 layer on a TiO2 nanotube array as an efficient photoanode in dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 4370–4375. [Google Scholar] [CrossRef]
- Mohammadi, M.; Louca, R.; Fray, D.; Welland, M. Dye-sensitized solar cells based on a single layer deposition of TiO2 from a new formulation paste and their photovoltaic performance. Sol. Energy 2012, 86, 2654–2664. [Google Scholar] [CrossRef]
- Ito, S.; Murakami, T.N.; Comte, P.; Liska, P.; Grätzel, C.; Nazeeruddin, M.K.; Grätzel, M. Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%. Thin Solid Film. 2008, 516, 4613–4619. [Google Scholar] [CrossRef]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; Takata, M.; Miura, H.; et al. High-Efficiency Organic-Dye- Sensitized Solar Cells Controlled by Nanocrystalline-TiO2 Electrode Thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Lee, C.-P.; Lin, R.Y.-Y.; Lin, L.-Y.; Li, C.-T.; Chu, T.-C.; Sun, S.-S.; Lin, J.T.; Ho, K.-C. Recent progress in organic sensitizers for dye-sensitized solar cells. RSC Adv. 2015, 5, 23810–23825. [Google Scholar] [CrossRef]
- Lin, J.; Nattestad, A.; Yu, H.; Bai, Y.; Wang, L.; Dou, S.X.; Kim, J.H. Highly connected hierarchical textured TiO2 spheres as photoanodes for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 8902–8909. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Cole, D.R. Characterization and Analysis of Porosity and Pore Structures. Rev. Miner. Geochem. 2015, 80, 61–164. [Google Scholar] [CrossRef] [Green Version]
- Wayment-Steele, H.K.; Johnson, L.E.; Tian, F.; Dixon, M.C.; Benz, L.; Johal, M.S. Monitoring N3 Dye Adsorption and Desorption on TiO2 Surfaces: A Combined QCM-D and XPS Study. ACS Appl. Mater. Interfaces 2014, 6, 9093–9099. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J. Theory of the impedance of charge transfer via surface states in dye-sensitized solar cells. J. Electroanal. Chem. 2010, 646, 43–51. [Google Scholar] [CrossRef]
- Kern, R.; Sastrawan, R.; Ferber, J.; Stangl, R.; Luther, J. Modeling and interpretation of electrical impedance spectra of dye solar cells operated under open-circuit conditions. Electrochim. Acta 2002, 47, 4213–4225. [Google Scholar] [CrossRef]
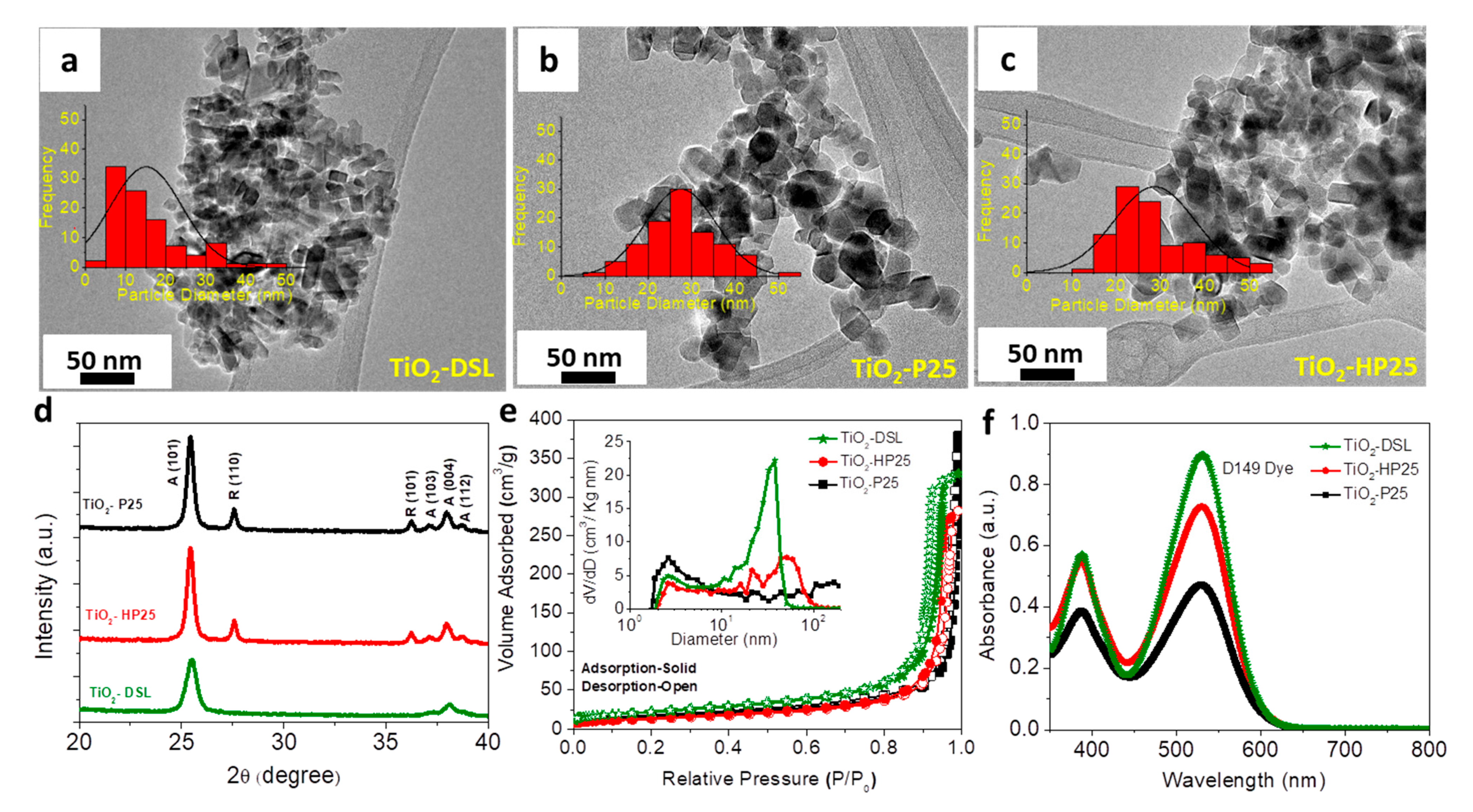
| Material | SA (m2·g−1) | DP (nm) | Dye Loading (µmol·cm−3) | Dye Loading (nmol·cm−2) |
|---|---|---|---|---|
| TiO2-DSL | 79 | 26 | 99 ± 1 | 64 ± 1 |
| TiO2-HP25 | 47 | 37 | 42 ± 2 | 50 ± 1 |
| TiO2-P25 | 57 | 41 | 27 ± 1 | 33 ± 1 |
| Devices | Jsc (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| TiO2-DSL|D149 | 15.3 ± 0.9 | 0.608 ± 0.01 | 62 ± 2 | 5.81 ± 0.4 |
| TiO2-HP25|D149 | 15.4 ± 0.4 | 0.620 ± 0.02 | 60 ± 1 | 5.78 ± 0.2 |
| TiO2-P25|D149 | 13.3 ± 1.3 | 0.600 ± 0.02 | 49 ± 4 | 3.89 ± 0.4 |
| Devices | RS (Ω·cm−2) | Rct1 (Ω·cm−2) | Rct2 (Ω·cm−2) | Cµ (µF·m−1) |
|---|---|---|---|---|
| TiO2-DSL|D149 | 5.5 ± 0.1 | 2.2 ± 0.2 | 24.1 ± 0.3 | 1450 ± 100 |
| TiO2-HP25|D149 | 5.0 ± 0.1 | 2.5 ± 0.1 | 30.5 ± 0.3 | 540 ± 40 |
| TiO2-P25|D149 | 5.2 ± 0.1 | 2.5 ± 0.1 | 33.5 ± 0.5 | 210 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Attafi, K.; Dwech, M.H.; Mezher, H.A.; Nattestad, A.; Kim, J.H. A Comparative Study of Organic Dye-Sensitized Solar Cells Based on Anatase TiO2 and Amorphous Free Mixed Phase’s Anatase/Rutile P25 TiO2 Photoanodes. Coatings 2023, 13, 121. https://doi.org/10.3390/coatings13010121
Al-Attafi K, Dwech MH, Mezher HA, Nattestad A, Kim JH. A Comparative Study of Organic Dye-Sensitized Solar Cells Based on Anatase TiO2 and Amorphous Free Mixed Phase’s Anatase/Rutile P25 TiO2 Photoanodes. Coatings. 2023; 13(1):121. https://doi.org/10.3390/coatings13010121
Chicago/Turabian StyleAl-Attafi, Kadhim, Majed H. Dwech, Hamza A. Mezher, Andrew Nattestad, and Jung Ho Kim. 2023. "A Comparative Study of Organic Dye-Sensitized Solar Cells Based on Anatase TiO2 and Amorphous Free Mixed Phase’s Anatase/Rutile P25 TiO2 Photoanodes" Coatings 13, no. 1: 121. https://doi.org/10.3390/coatings13010121
APA StyleAl-Attafi, K., Dwech, M. H., Mezher, H. A., Nattestad, A., & Kim, J. H. (2023). A Comparative Study of Organic Dye-Sensitized Solar Cells Based on Anatase TiO2 and Amorphous Free Mixed Phase’s Anatase/Rutile P25 TiO2 Photoanodes. Coatings, 13(1), 121. https://doi.org/10.3390/coatings13010121








