Solar-Light-Responsive Nanomaterials for the Photoelectrocatalytic Degradation of Stubborn Pollutants
Abstract
:1. Introduction
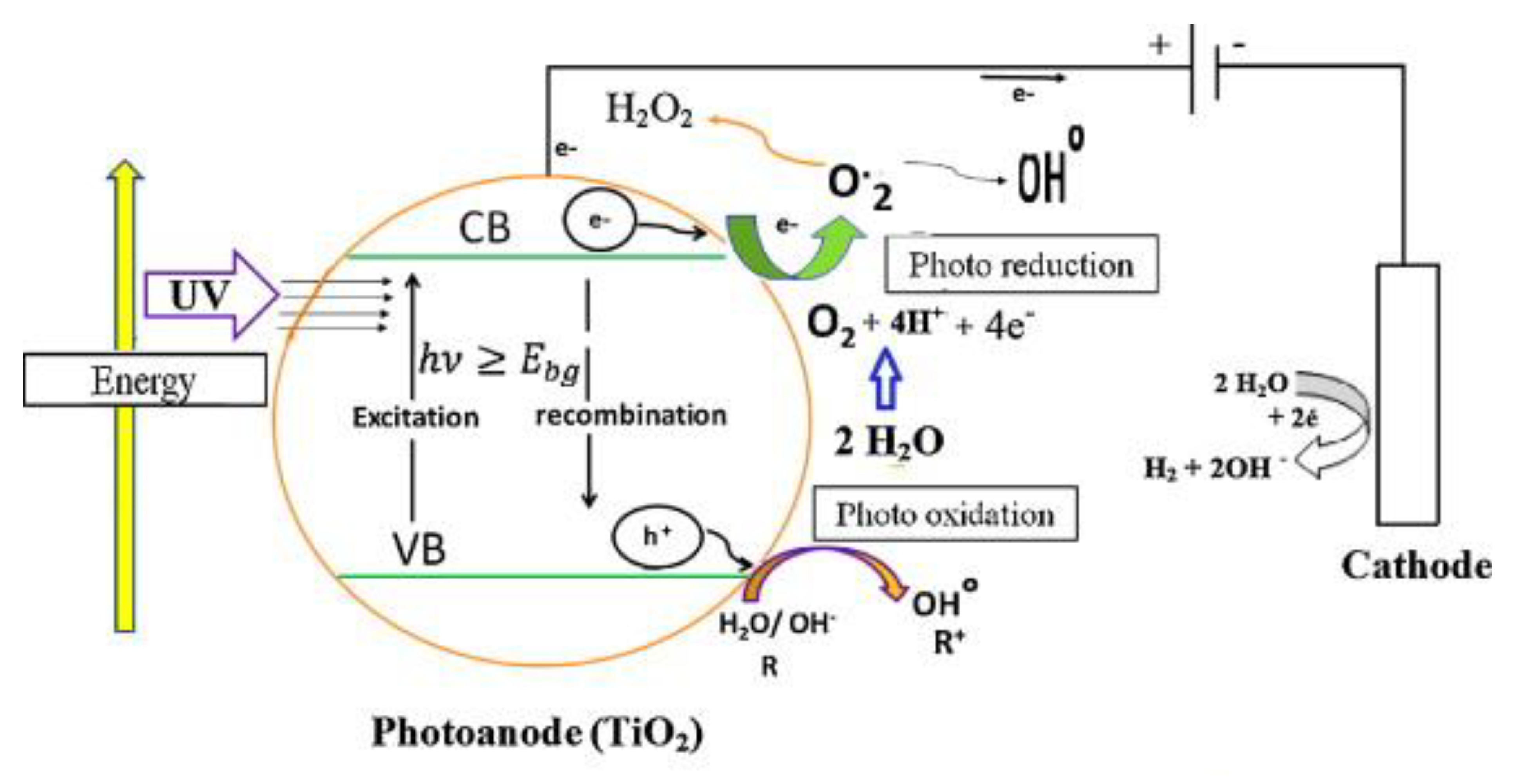
1.1. Fundamentals of Photoelectrocatalytic Degradation
1.2. Essential Characteristics of Photoelectrocatalyst Nanomaterials
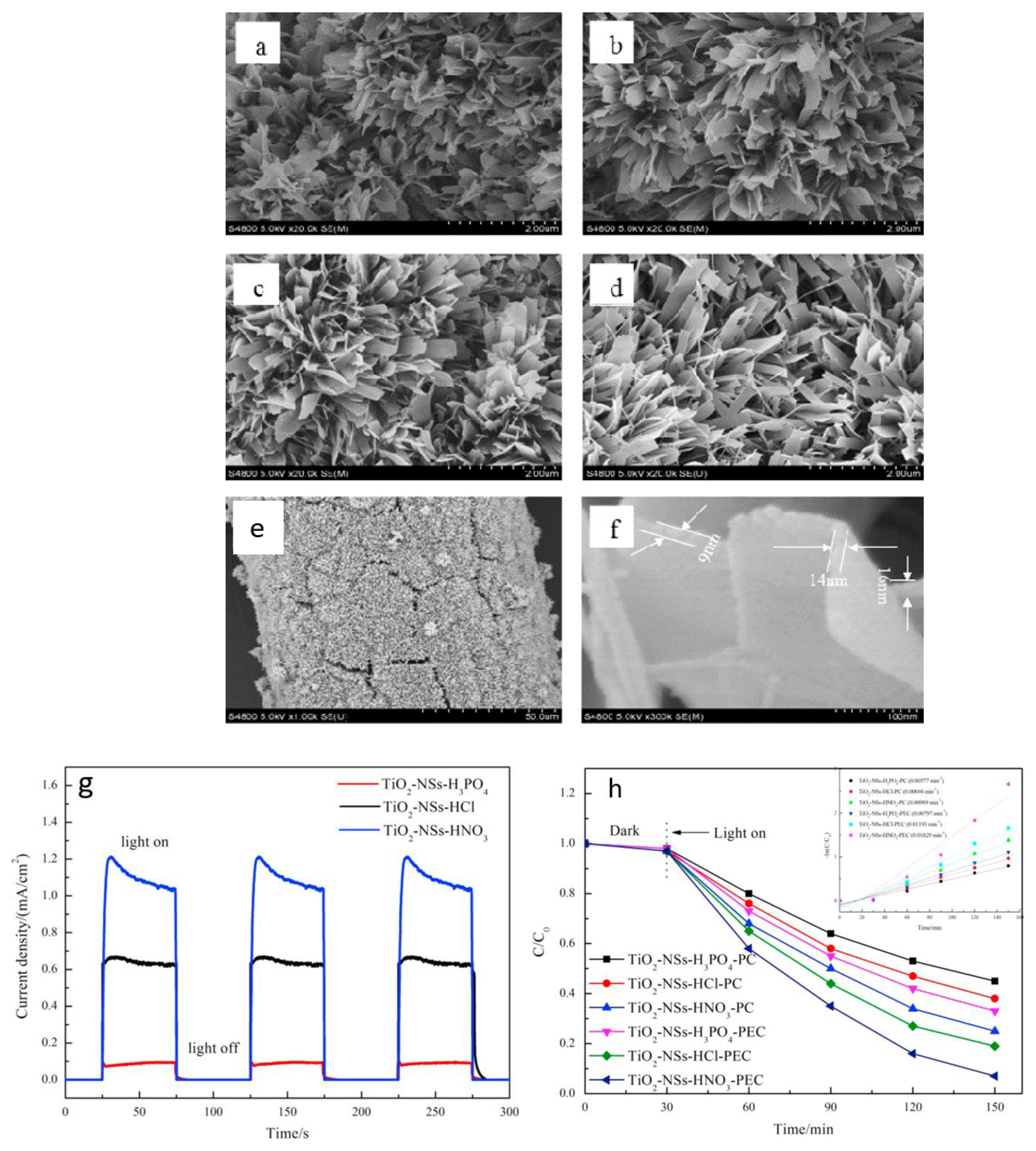
1.3. TiO2 Nanostructures in PEC Degradation of Organics
1.4. Zinc Oxides Nanomaterials as Photoanodes
1.5. Tungsten Trioxides Nanostructured Photoanodes
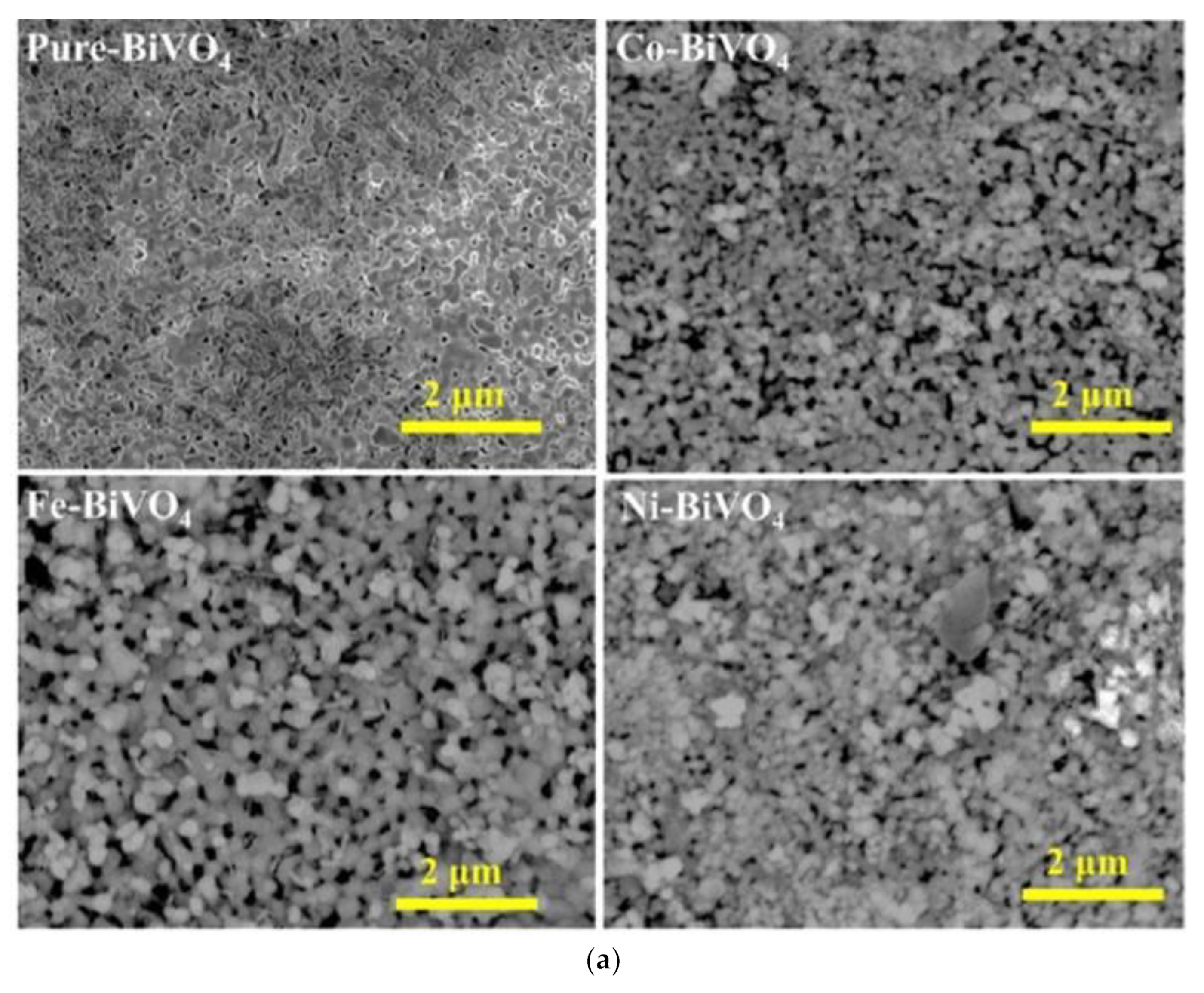
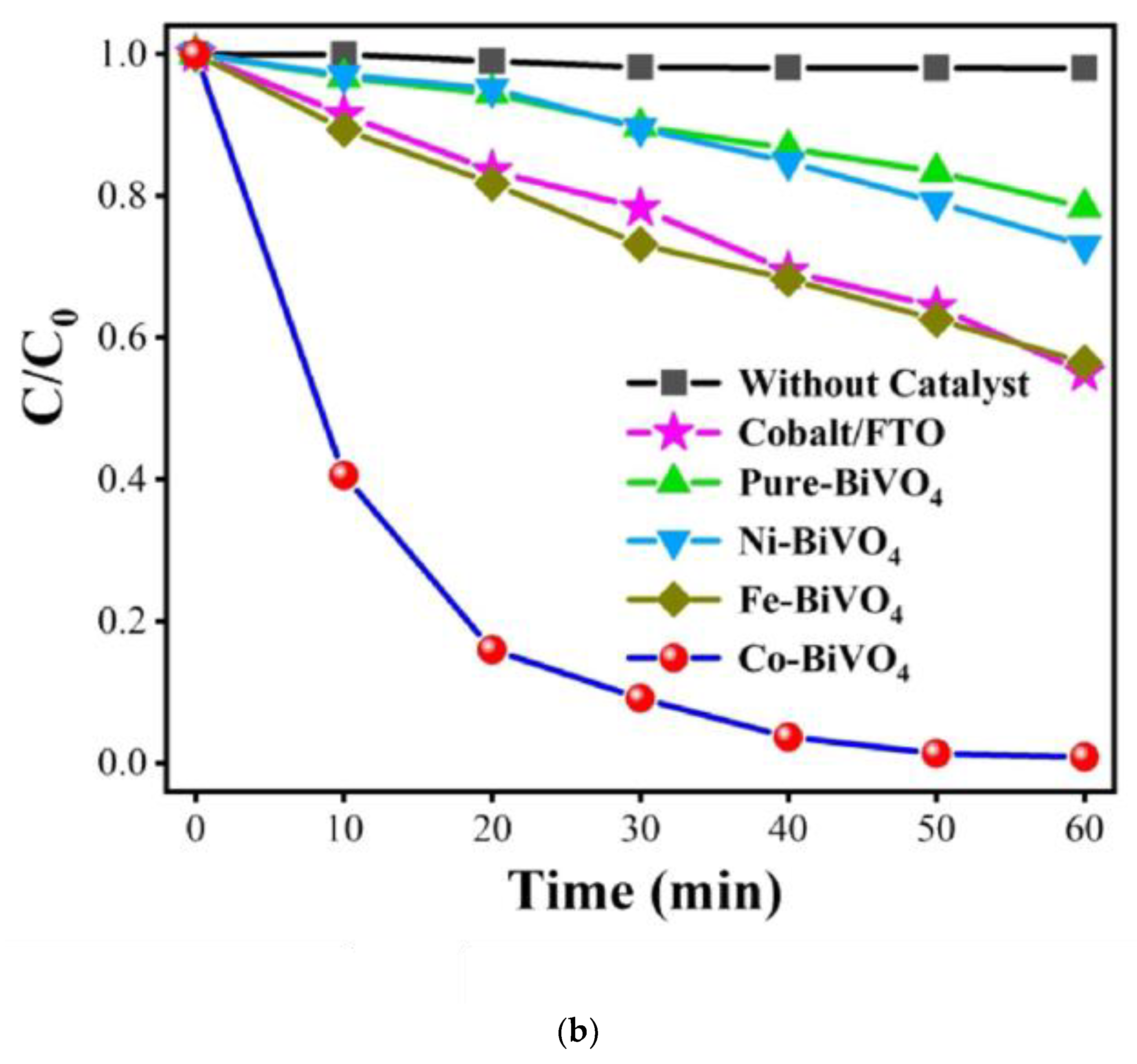
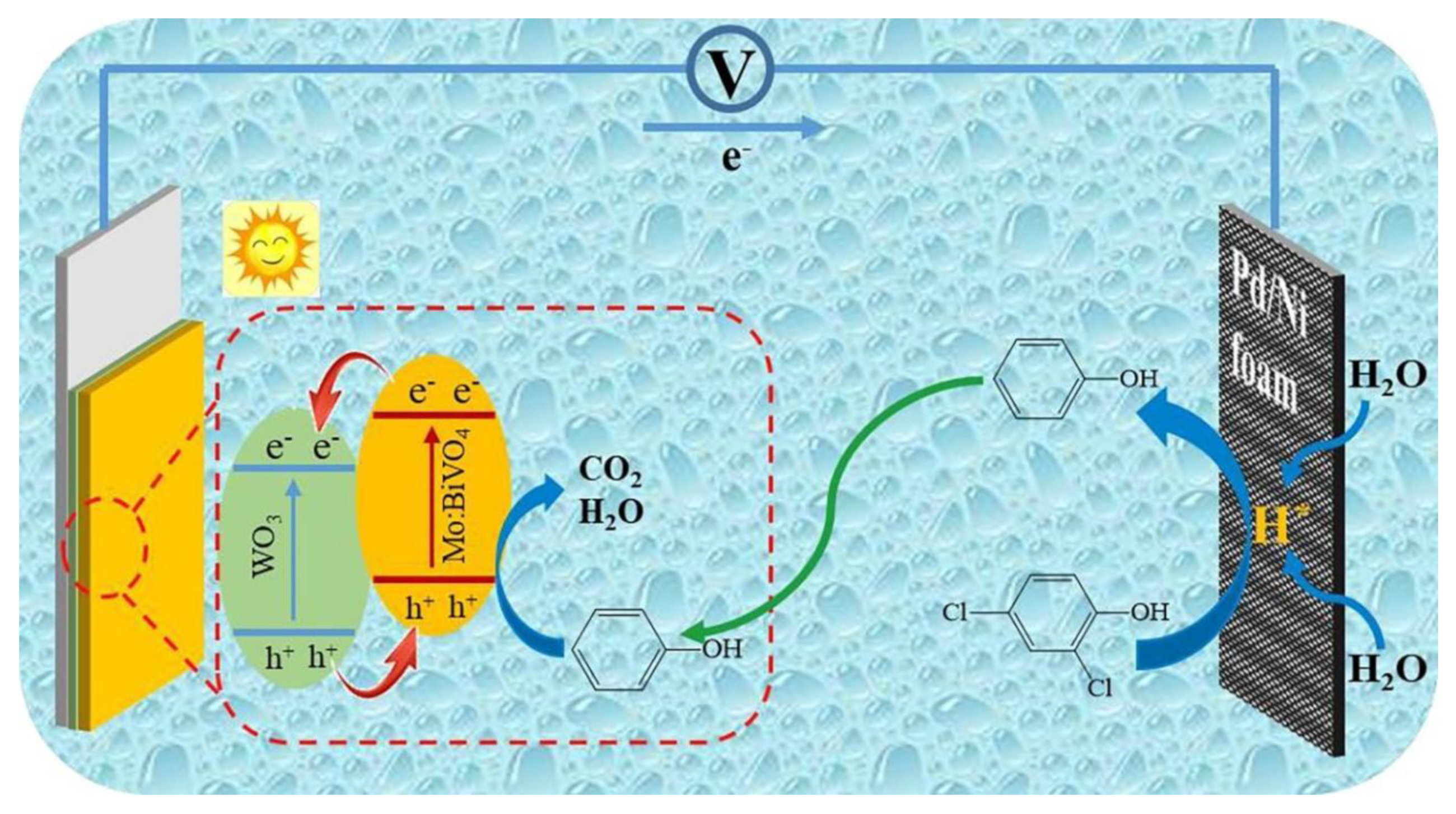
1.6. Bismuth Vanadate Nanostructures as Photoanodes
1.7. Nanostructured Semiconductors Heterojunctions
2. Conclusions and Future Perspective
- Most PEC degradation studies involving the use of nanostructured solar light-responsive photoanodes are often applied for the treatment of simulated wastewater on a laboratory scale. It is unfortunate that a large vacuum still exists in the literature concerning the real-life implementation of these photoanodes for the treatment of real wastewater effluents. Since the overall aim of developing the PEC degradation process is to remediate wastewater in the environment and make available cleaner water for use, it will be of more interest if future research focuses more on the treatment of real effluents either from industries or wastewater treatment plants. With this in view, researchers will endeavour to perform preliminary studies using a continuous flow process for PEC degradation rather than the batch process often use.
- Additionally, the experimental conditions (such as analyte concentration) used to access the performance of materials in PEC degradation should be as close as possible to the conditions attainable in real-life situations.
- It is also interesting to realise that the solar light responsiveness of the nanostructured photoanodes is often established through a series of experimental techniques in the laboratory whereas simulated solar light is commonly used as a light source for the degradation process. Future research should endeavor to use direct sunlight as a source of illumination to excite these photoanodes as this will establish, without a doubt, the solar light responsiveness of these photoanodes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amoatey, P.; Baawain, M.S. Effects of pollution on freshwater aquatic organisms. Water Environ. Res. 2019, 91, 1272–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Maurer, C.; Wang, Y.; Xue, S.; Davis, D.L. Water pollution and human health in China. Environ. Health Perspect. 1999, 107, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Vasilachi, I.; Asiminicesei, D.; Fertu, D.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-driven advanced oxidation processes in the disposal of emerging pharmaceutical contaminants in aqueous media: A brief review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Mondal, S.K.; Saha, A.K.; Sinha, A. Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization. J. Clean. Prod. 2018, 171, 1203–1214. [Google Scholar] [CrossRef]
- Välitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Zwane, B.N.; Orimolade, B.O.; Koiki, B.A.; Mabuba, N.; Gomri, C.; Petit, E.; Bonniol, V.; Lesage, G.; Rivallin, M.; Cretin, M.; et al. Combined Electro-Fenton and Anodic Oxidation Processes at a Sub-Stoichiometric Titanium Oxide (Ti4O7) Ceramic Electrode for the Degradation of Tetracycline in Water. Water 2021, 13, 2772. [Google Scholar] [CrossRef]
- Saafie, N.; Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Current Scenario of MXene-Based Nanomaterials for Wastewater Remediation: A Review. Chemistry 2022, 4, 1576–1608. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Ahmed, B.; Mehnaz, T.; Mehejabin, F.; Maliat, D.; Hoang, A.T.; Shafiullah, G.M. Nanomaterials as a sustainable choice for treating wastewater. Environ. Res. 2022, 214, 113807. [Google Scholar] [CrossRef] [PubMed]
- Palani, G.; Arputhalatha, A.; Kannan, K.; Lakkaboyana, S.K.; Hanafiah, M.M.; Kumar, V.; Marella, R.K. Current Trends in the Application of Nanomaterials for the Removal of Pollutants from Industrial Wastewater Treatment—A Review. Molecules 2021, 26, 2799. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Castro-Muñoz, R.; Lieder, M. New insights of nanomaterials usage toward superhydrophobic membranes for water desalination via membrane distillation: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2104–2149. [Google Scholar] [CrossRef]
- Daneshkhah, M.; Hossaini, H.; Malakootian, M. Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. J. Environ. Chem. Eng. 2017, 5, 3490–3499. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Deegan, A.M.; Shaik, B.; Nolan, K.; Urell, K.; Oelgemöller, M.; Tobin, J.; Morrissey, A. Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol. 2011, 8, 649–666. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Peleyeju, M.G.; Arotiba, O.A. Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1389–1411. [Google Scholar] [CrossRef]
- Zarei, E.; Ojani, R. Fundamentals and some applications of photoelectrocatalysis and effective factors on its efficiency: A review. J. Solid State Electrochem. 2017, 21, 305–336. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Umukoro, E.H.; Tshwenya, L.; Moutloali, R.; Babalola, J.O.; Arotiba, O.A. Photoelectrocatalytic water treatment systems: Degradation, kinetics and intermediate products studies of sulfamethoxazole on a TiO2-exfoliated graphite electrode. RSC Adv. 2017, 7, 40571–40580. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic technologies for environmental applications. J. Photochem. Photobiol. A Chem. 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Orimolade, B.O.; Masekela, D.; Mamba, B.; Mabuba, N. The application of photoelectrocatalysis in the degradation of rhodamine B in aqueous solutions: A review. RSC Adv. 2022, 12, 26176–26191. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, X.; Zhou, B.; Xiong, B.; Li, J.; Dong, C.; Bai, J.; Cai, W. Photoelectrocatalytic degradation of tetracycline by highly effective TiO2 nanopore arrays electrode. J. Hazard. Mater. 2009, 171, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, X.; Han, Y.; Zhang, X.; Shen, F.; Deng, S.; Xiao, H.; Yang, X.; Yang, G.; Peng, H. Photoelectrocatalytic degradation of recalcitrant organic pollutants using TiO2 film electrodes: An overview. Chemosphere 2012, 88, 145–154. [Google Scholar] [CrossRef]
- Martins, A.S.; Cordeiro-Junior, P.J.M.; Bessegato, G.G.; Carneiro, J.F.; Zanoni, M.V.B.; de Vasconcelos Lanza, M.R. Electrodeposition of WO3 on Ti substrate and the influence of interfacial oxide layer generated in situ: A photoelectrocatalytic degradation of propyl paraben. Appl. Surf. Sci. 2019, 464, 664–672. [Google Scholar] [CrossRef]
- Li, R.; Zhao, X.; Chen, X.; Jiang, T.; Leung, D.Y.C.; Pan, D.; Li, G.; Wang, W. g-C3N4 photoanode for photoelectrocatalytic synergistic pollutant degradation and hydrogen evolution. Appl. Surf. Sci. 2018, 467–468, 658–665. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, W.; Pan, S.; Okoth, O.K.; Yang, C.; Zhang, J. Photoelectrocatalytic activity of liquid phase deposited α-Fe2O3 films under visible light illumination. J. Alloys Compd. 2015, 648, 719–725. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, X.; Zhang, G.; Dong, W. Fabricating MoS 2 nanoflakes photoanode with unprecedented high photoelectrochemical performance and multi-pollutants degradation test for water treatment. Chem. Eng. J. 2019, 356, 1003–1013. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. An Exfoliated Graphite-Bismuth Vanadate Composite Photoanode for the Photoelectrochemical Degradation of Acid Orange 7 Dye. Electrocatalysis 2019, 10, 429–435. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Peroxymonosulfate assisted photoelectrocatalytic degradation of pharmaceuticals at a FTO-Bi2WO6 electrode: Mechanism and kinetics studies. Catal. Commun. 2022, 169, 106481. [Google Scholar] [CrossRef]
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic Materials for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Wan, J.; Yu, X. Hydrothermal synthesis of TiO2 nanosheets photoelectrocatalyst on Ti mesh for degradation of norfloxacin: Influence of pickling agents. Mater. Sci. Semicond. Process. 2016, 43, 47–54. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhou, J.; Xi, Q.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Fabricating I doped TiO2 photoelectrode for the degradation of diclofenac: Performance and mechanism study. Chem. Eng. J. 2019, 369, 968–978. [Google Scholar] [CrossRef]
- Xiang, G.; Yu, Z.; Hou, Y.; Chen, Y.; Peng, Z.; Sun, L.; Sun, L. Simulated solar-light induced photoelectrocatalytic degradation of bisphenol-A using Fe3+-doped TiO2 nanotube arrays as a photoanode with simultaneous aeration. Sep. Purif. Technol. 2016, 161, 144–151. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, R.; Zhang, M.; Gao, S. Solvothermal preparation of Fe-doped TiO2 nanotube arrays for enhancement in visible light induced photoelectrochemical performance. J. Alloys Compd. 2017, 690, 139–144. [Google Scholar] [CrossRef]
- Rajput, H.; Changotra, R.; Kumar, V.; Kumar, S.; Dhir, A. Chemosphere A facile synthesis of Cs loaded TiO2 nanotube photoelectrode for the removal of 4-chloroguaiacol. Chemosphere 2019, 218, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Deng, X.; Ma, Q.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q.; Li, B. Kinetics of photoelectrocatalytic degradation of diclofenac using N, S CO-doped TIO2 nano-crystallite decorated TIO2 nanotube arrays photoelectrode. Environ. Prot. Eng. 2018, 44, 117–130. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, J.; Wang, J.; Tian, R.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Enhanced visible light photoelectrocatalytic degradation of organic contaminants by F and Sn co-doped TiO2 photoelectrode. Chem. Eng. J. 2018, 344, 332–341. [Google Scholar] [CrossRef]
- Da Silva, T.F.; Cavalcante, R.P.; Guelfi, D.R.V.; de Oliveira, S.C.; Casagrande, G.A.; Caires, A.R.L.; de Oliveira, F.F.; Gubiani, J.R.; Cardoso, J.C.; Machulek, A. Photo-anodes based on B-doped TiO2 for photoelectrocatalytic degradation of propyphenazone: Identification of intermediates, and acute toxicity evaluation. J. Environ. Chem. Eng. 2022, 10, 107212. [Google Scholar] [CrossRef]
- Ghanbarnezhad, M.; Parvareh, A.; Keshavarz Moraveji, M.; Jorfi, S. La, S, N tri-doped TiO2/nickel foam as efficient photoelectrode for degradation of BTX solution under visible light irradiation. J. Photochem. Photobiol. A Chem. 2022, 431, 114044. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, E.; Li, H.; Wan, J.; Fan, J.; Hu, X.; Li, J.; Tang, C.; Pu, C. Fabrication of plasmonic Au/TiO2 nanotube arrays with enhanced photoelectrocatalytic activities. Ceram. Int. 2016, 42, 9387–9395. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Zhang, M.; Li, G.; Gao, S.; Li, M.; Zhang, Y. Influence of Ag-Au microstructure on the photoelectrocatalytic performance of TiO2 nanotube array photocatalysts. J. Colloid Interface Sci. 2016, 463, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, J.; Yang, X.; Zhao, Y.; Kang, J.; Yang, F.; Zhang, Y.; Cheng, M.; Wang, G.; Duanmu, Q. Porous Si/TiO2 nanowire photoanode for photoelectric catalysis under simulated solar light irradiation. Appl. Organomet. Chem. 2018, 32, 2–10. [Google Scholar] [CrossRef]
- Wang, H.; Han, B.; Lu, J.; Wu, P.; Cui, W. Excellent photoelectrocatalytic degradation and superior charge separation of polyaniline nanosheets wrapped TiO2 nanotube arrays. Mater. Lett. 2020, 260, 126906. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, Z.; Sun, Q.; Chen, X.; Zhu, Y.; Li, M.; Wang, J.; Qiu, H.; Li, X.; Li, Y.; et al. TiO2 nanotube array modified with polypyrrole for efficient photoelectrocatalytic decolorization of methylene blue. J. Alloys Compd. 2020, 820, 153128. [Google Scholar] [CrossRef]
- Sboui, M.; Niu, W.; Li, D.; Lu, G.; Zhou, N.; Zhang, K.; Pan, J.H. Fabrication of electrically conductive TiO2/PANI/PVDF composite membranes for simultaneous photoelectrocatalysis and microfiltration of azo dye from wastewater. Appl. Catal. A Gen. 2022, 644, 118837. [Google Scholar] [CrossRef]
- Fan, M.; Yang, C.; Pu, W.; Zhang, J. Liquid phase deposition of ZnO film for photoelectrocatalytic degradation of p-nitrophenol. Mater. Sci. Semicond. Process. 2014, 17, 104–109. [Google Scholar] [CrossRef]
- Lunkham, C.; Ngernchuklin, P.; Ponchio, C. Photoelectrocatalytic and ultrasonic-assisted effects for organic dye degradation using zinc oxide (ZnO) electrode. Key Eng. Mater. 2019, 798, 404–411. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A multifunctional ZnO thin film based devises for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sens. Actuators B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Feng, J.; Tian, Y.; Okoth, O.K.; Cheng, L.; Zhang, J. Liquid Phase Deposition of Nickel-Doped ZnO Film with Enhanced Visible Light Photoelectrocatalytic Activity. J. Electrochem. Soc. 2019, 166, H685–H690. [Google Scholar] [CrossRef]
- Hosseini, M.; Esrafili, A.; Farzadkia, M.; Kermani, M.; Gholami, M. Degradation of ciprofloxacin antibiotic using photo-electrocatalyst process of Ni-doped ZnO deposited by RF sputtering on FTO as an anode electrode from aquatic environments: Synthesis, kinetics, and ecotoxicity study. Microchem. J. 2020, 154, 104663. [Google Scholar] [CrossRef]
- Gholami, M.; Rasoulzadeh, H.; Ahmadi, T.; Hosseini, M. Synthesis, characterization of Nickel doped Zinc oxide by radio-frequency sputtering and application in photo-electrocatalysis degradation of Norfloxacin. Mater. Lett. 2020, 269, 127647. [Google Scholar] [CrossRef]
- Nada, A.A.; Orimolade, B.O.; El-maghrabi, H.H.; Koiki, B.A.; Rivallin, M.; Bekheet, M.F.; Viter, R.; Damberga, D.; Lesage, G.; Iatsunskyi, I.; et al. Photoelectrocatalysis of paracetamol on Pd–ZnO/N-doped carbon nanofibers electrode. Appl. Mater. Today 2021, 24, 101129. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In Situ Charge Transfer at the Ag@ZnO Photoelectrochemical Interface toward the High Photocatalytic Performance of H2 Evolution and RhB Degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–12206. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cheng, L.; Zhang, J. Fabrication of Co-doped ZnO photoanode by liquid phase deposition for photoelectrocatalytic degradation of ofloxacin under visible light. J. Electrochem. Soc. 2018, 165, H284–H290. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Enhanced photocatalytic performance of tungsten oxide through tuning exposed facets and introducing oxygen vacancies. J. Alloys Compd. 2017, 708, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Li, J.; Bai, J.; Li, X.; Xia, L.; Zhou, B. Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance. Appl. Catal. B Environ. 2017, 202, 388–396. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Mahadik, M.A.; Mathe, V.L.; Bhosale, C.H. A highly efficient visible-light responsive sprayed WO3/FTO photoanode for photoelectrocatalytic degradation of brilliant blue. J. Taiwan Inst. Chem. Eng. 2018, 85, 273–281. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Mahadik, M.A.; Mohite, V.S.; Kumbhar, S.S.; Deshpande, N.G.; Rajpure, K.Y.; Moholkar, A.V.; Patil, P.S.; Bhosale, C.H. Photoelectrocatalytic degradation of methyl blue using sprayed WO3 thin films. J. Mater. Sci. Mater. Electron. 2016, 27, 1629–1635. [Google Scholar] [CrossRef]
- Mohite, S.V.; Ganbavle, V.V.; Rajpure, K.Y. Solar photoelectrocatalytic activities of rhodamine-B using sprayed WO3 photoelectrode. J. Alloys Compd. 2016, 655, 106–113. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Muñoz-Portero, M.J.; Ramírez-Grau, R.; García-Antón, J. Visible-light photoelectrodegradation of diuron on WO3 nanostructures. J. Environ. Manag. 2018, 226, 249–255. [Google Scholar] [CrossRef]
- Roselló-Márquez, G.; Fernández-Domene, R.M.; Sánchez-Tovar, R.; García-Antón, J. Photoelectrocatalyzed degradation of organophosphorus pesticide fenamiphos using WO3 nanorods as photoanode. Chemosphere 2020, 246, 125677. [Google Scholar] [CrossRef]
- Roselló-Márquez, G.; Fernández-Domene, R.M.; García-Antón, J. Organophosphorus pesticides (chlorfenvinphos, phosmet and fenamiphos) photoelectrodegradation by using WO3 nanostructures as photoanode. J. Electroanal. Chem. 2021, 894, 115366. [Google Scholar] [CrossRef]
- Roselló-Márquez, G.; Fernández-Domene, R.M.; Sánchez-Tovar, R.; García-Carrión, S.; Lucas-Granados, B.; García-Antón, J. Photoelectrocatalyzed degradation of a pesticides mixture solution (chlorfenvinphos and bromacil) by WO3 nanosheets. Sci. Total Environ. 2019, 674, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mohite, S.V.; Ganbavle, V.V.; Rajpure, K.Y. Photoelectrochemical performance and photoelectrocatalytic degradation of organic compounds using Ga:WO3 thin films. J. Photochem. Photobiol. A Chem. 2017, 344, 56–63. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, W.; Han, S.; Liu, C. Photoelectrochemical properties and photocatalytic activity of nitrogen-doped nanoporous WO3 photoelectrodes under visible light. Appl. Surf. Sci. 2012, 258, 5038–5045. [Google Scholar] [CrossRef]
- Umukoro, E.H.; Peleyeju, M.G.; Ngila, J.C.; Arotiba, O.A. Towards wastewater treatment: Photo-assisted electrochemical degradation of 2-nitrophenol and orange II dye at a tungsten trioxide-exfoliated graphite composite electrode. Chem. Eng. J. 2017, 317, 290–301. [Google Scholar] [CrossRef]
- Luan, P.; Zhang, J. Stepping towards Solar Water Splitting: Recent Progress in Bismuth Vanadate Photoanodes. ChemElectroChem 2019, 6, 3227–3243. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Ahn, C.H.; Koli, R.R.; Jamadar, A.S.; Kim, D.S.; Kim, Y.B.; Jung, S.H.; Cho, H.K. Controlled nanostructured morphology of BiVO4 photoanodes for efficient on-demand catalysis in solar water-splitting and sustainable water-treatment. Appl. Surf. Sci. 2020, 514, 146075. [Google Scholar] [CrossRef]
- Xia, L.; Chen, F.; Li, J.; Chen, S.; Bai, J.; Zhou, T.; Li, L.; Xu, Q.; Zhou, B. Efficient organic pollutants conversion and electricity generation for carbonate-containing wastewater based on carbonate radical reactions initiated by BiVO4-Au/PVC system. J. Hazard. Mater. 2020, 389, 122140. [Google Scholar] [CrossRef]
- Bacha, A.U.R.; Cheng, H.; Han, J.; Nabi, I.; Li, K.; Wang, T.; Yang, Y.; Ajmal, S.; Liu, Y.; Zhang, L. Significantly accelerated PEC degradation of organic pollutant with addition of sulfite and mechanism study. Appl. Catal. B Environ. 2019, 248, 441–449. [Google Scholar] [CrossRef]
- Bacha, A.U.R.; Nabi, I.; Cheng, H.; Li, K.; Ajmal, S.; Wang, T.; Zhang, L. Photoelectrocatalytic degradation of endocrine-disruptor bisphenol—A with significantly activated peroxymonosulfate by Co-BiVO4 photoanode. Chem. Eng. J. 2020, 389, 124482. [Google Scholar] [CrossRef]
- Wang, S.; Yun, J.H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Xiao, M.; Wang, L. Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. J. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jaroniec, M. Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Arotiba, O.A.; Orimolade, B.O.; Koiki, B.A. Visible Light Driven Photoelectrocatalytic Semiconductor Heterojunction Anodes for Water Treatment Applications. Curr. Opin. Electrochem. 2020, 22, 25–34. [Google Scholar] [CrossRef]
- Qiu, L.; Cui, Y.; Tan, X.; Zheng, S.; Zhang, H.; Xu, J.; Wang, Q. Construction of Ag3PO4/Ag4P2O7 nanospheres sensitized hierarchical titanium dioxide nanotube mesh for photoelectrocatalytic degradation of methylene blue. Sep. Purif. Technol. 2019, 215, 619–624. [Google Scholar] [CrossRef]
- Fei, W.; Li, H.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Facile fabrication of ZnO/MoS2 p-n junctions on Ni foam for efficient degradation of organic pollutants through photoelectrocatalytic process. Sol. Energy 2020, 199, 164–172. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Koiki, B.A.; Zwane, B.N.; Peleyeju, G.M.; Mabuba, N.; Arotiba, O.A. Interrogating solar photoelectrocatalysis on an exfoliated graphite–BiVO4 /ZnO composite electrode towards water treatment. RSC Adv. 2019, 9, 16586–16595. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Lei, E.; Zhao, D.; Xin, Y.; Wu, X.; Meng, Y.; Liu, Z. The p-n heterojunction of BiVO4/Cu2O was decorated by plasma Ag NPs for efficient photoelectrochemical degradation of Rhodamine B. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127834. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Guo, R.; Li, B.; Zhang, X.; Cheng, X.; Xie, M.; Cheng, Q. Construction of CuS/TiO2 nano-tube arrays photoelectrode and its enhanced visible light photoelectrocatalytic decomposition and mechanism of penicillin G. Electrochim. Acta 2018, 283, 1154–1162. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Feng, J.; Cheng, L.; Zhang, J.; Okoth, O.K.; Chen, F. Preparation of BiVO4/ZnO composite film with enhanced visible-light photoelectrocatalytic activity. Ceram. Int. 2018, 44, 3672–3677. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Tang, Y.; Ng, Y.H.; Li, L.; Jiang, F.; Wang, J.; Chen, W.; Li, L. Understanding photoelectrocatalytic degradation of tetracycline over three-dimensional coral-like ZnO/BiVO4 nanocomposite. Mater. Chem. Phys. 2021, 271, 124871. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Enhanced visible light driven photoelectrochemical degradation of tetracycline hydrochloride using a BiOI photoanode modified with MnO2 films. Environ. Sci. Pollut. Res. 2022, 29, 92530. [Google Scholar] [CrossRef] [PubMed]
- Orimolade, B.O.; Zwane, B.N.; Koiki, B.A.; Tshwenya, L.; Peleyeju, G.M.; Mabuba, N.; Zhou, M.; Arotiba, O.A. Solar photoelectrocatalytic degradation of ciprofloxacin at a FTO/BiVO4/MnO2 anode: Kinetics, intermediate products and degradation pathway studies. J. Environ. Chem. Eng. 2020, 8, 103607. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Koiki, B.A.; Peleyeju, G.M.; Arotiba, O.A. Visible light driven photoelectrocatalysis on a FTO/BiVO4 /BiOI anode for water treatment involving emerging pharmaceutical pollutants. Electrochim. Acta 2019, 307, 285–292. [Google Scholar] [CrossRef]
- Parsaei-Khomami, A.; Mousavi, M.; Habibi, M.M.; Shirzad, K.; Ghasemi, J.B.; Wang, L.; Yu, J.; Yu, H.; Li, X. Highly efficient visible light photoelectrochemical degradation of ciprofloxacin and azo dyes by novel TiO2/AgBiS2 photoelectrocatalyst. Solid State Sci. 2022, 134, 107044. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Towards visible light driven photoelectrocatalysis for water treatment: Application of a FTO/BiVO4/Ag2S heterojunction anode for the removal of emerging pharmaceutical pollutants. Sci. Rep. 2020, 10, 5348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Wang, Y.; Qiao, M.; Zhao, X. Enhanced photoelectrocatalytic degradation of norfloxacin by an Ag3PO4/BiVO4 electrode with low bias. J. Catal. 2018, 360, 240–249. [Google Scholar] [CrossRef]
- Du, H.; Pu, W.; Wang, Y.; Yan, K.; Feng, J.; Zhang, J.; Yang, C.; Gong, J. Synthesis of BiVO4/WO3 composite film for highly efficient visible light induced photoelectrocatalytic oxidation of norfloxacin. J. Alloys Compd. 2019, 787, 284–294. [Google Scholar] [CrossRef]
- Li, K.; Yang, Y.; Bacha, A.U.R.; Feng, Y.; Ajmal, S.; Nabi, I.; Zhang, L. Efficiently complete degradation of 2,4-DCP using sustainable photoelectrochemical reduction and sequential oxidation method. Chem. Eng. J. 2019, 378, 122191. [Google Scholar] [CrossRef]
- Wei, W.; Zhu, Y.; Wang, J.; Chen, X.; Yao, W.; Gao, X.; Zong, R.; Yang, Z. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B Environ. 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Cui, Y.; Deng, X.; Guo, R.; Cheng, X.; Xie, M.; Cheng, Q. Fabrication of Cu2O/TiO2 nano-tube arrays photoelectrode and its enhanced photoelectrocatalytic performance for degradation of 2,4,6-trichlorophenol. J. Ind. Eng. Chem. 2018, 57, 181–187. [Google Scholar] [CrossRef]
- Wang, W.K.; Zhu, W.; Mao, L.; Zhang, J.; Zhou, Z.; Zhao, G. Two-dimensional TiO2-g-C3N4 with both Ti[sbnd]N and C[sbnd]O bridges with excellent conductivity for synergistic photoelectrocatalytic degradation of bisphenol A. J. Colloid Interface Sci. 2019, 557, 227–235. [Google Scholar] [CrossRef] [PubMed]

| Photoanodes | Experimental Conditions | Results | Ref. |
|---|---|---|---|
| TiO2 | Pollutant = norfloxacin; Co = 5 mg L−1; SE = 0.5 M Na2SO4; LS = 350 W Xe lamp | 93% removal after 3 h | [40] |
| I-TiO2 | Pollutant = diclofenac; Co = 0.1 M; SE = 0.1 M Na2SO4; Bias potential = 1.4 V; LS = 400 W halogen lamp | 99.4% removal after 2 h 60.2% TOC removal after 2 h | [41] |
| Fe3+-TiO2 | Pollutant = bisphenol A; Co = 10 mg L−1; SE = 0.1 M Na2SO4; Current density = 1.25 mA cm−2; LS = 150 W Xe lamp | 82.59% removal after 4 h 73.8% TOC removal after 4 h | [42] |
| Fe-TiO2 | Pollutant = methylene blue; Co = 10 mg L−1; SE = 0.1 M K2SO4; Potential = 1 V; LS = 500 W Xe lamp | 98.79% removal after 2 h | [43] |
| Cs-TiO2 | Pollutant = 4-chloroguaiacol; Co = 20 mg L−1; SE = 160 mg L−1 Na2SO4; Current: 0.03 A | 92% removal after 6 h | [44] |
| B-TiO2 | Pollutant = propyphenazone; Co = 30 mg L−1; SE = 0.05 M Na2SO4; Potential = 1 V | 94% removal after 2 h 18% TOC removal after 2 h | [47] |
| La, S, N—TiO2 | Pollutant = benzene, toluene, xylene (BTX) solution; Co = 50 mg L−1; SE = 0.1 M Na2SO4; Potential = 0.8 V; LS = 400 W Osram lamp | 83.7, 71.4, and 62.28% removal of benzene, toluene and xylene after 3 h | [48] |
| PPy—TiO2 | Pollutant = methylene blue; Co = 10 mg L−1; SE = 0.5 g L−1 NaCl; Potential = 15 V; LS = 250 W Hg lamp | 91.25% removal after 1 h | [53] |
| TiO2/PANI/PVDF | Pollutants = methylene blue and methyl orange; Co = 0.5 mg L−1; Potential = 1 V; LS = 100 W Xe lamp | 73.1% and 59.4% removal of methylene blue and methyl orange after 4 h | [54] |
| ZnO | Pollutants = methylene blue; Co = 5 mg L−1; Potential = 1 V; LS = 100 W Xe lamp | 71% removal after 1 h | [56] |
| Ni-ZnO | Pollutants = tetracycline; Co: 10 mg L−1; Potential = 0.8 V; SE = 0.1 M Na2SO4; LS = 300 Xe lamp | 87.5% removal after 3 h | [58] |
| Ni-ZnO | Pollutant = ciprofloxacin; Co = 5 mg L−1; Current density = 1.87 mA cm−2; SE = 0.75 g L−1 | 100% removal after 90 min 83.7% TOC removal after 90 min | [59] |
| Pd-ZnO | Pollutant = paracetamol; Co = 0.1 mM; Current density = 10 mA cm−2; SE = 0.05 M Na2SO4; LS = 150 W linear halogen lamp | 100% removal after 3 h 71% TOC removal after 4 h | [61] |
| Ag@ZnO | Pollutant = rhodamine B; Co = 10 mg L−1; Potential = 1.5 V; SE = 0.1 M Na2SO4; LS = 500 W Xe lamp | 82% removal after 2 h | [62] |
| WO3 | Pollutant = brilliant blue; Co: 0.5 mM; Potential = 1.5 V; SE = 0.1 M Na2SO4; LS = 100 W Xe lamp | 85% COD removal after 4 h | [66] |
| WO3 | Pollutant = fenamiphos; Co = 20 mg L−1; Potential = 1 V; SE = 0.1 M Na2SO4; LS = 100 W Xe lamp | 100% removal after 2 h | [70] |
| Co-BiVO4 | Pollutant = bisphenol A; Co = 20 mg L−1; Potential = 1.2 V; SE = 2 mM peroxymonosulphate; LS = 300 W Xe lamp | 99.16% removal after 1 h | [80] |
| TiO2/Ag3PO4/Ag4P2O7 | Pollutant = methylene blue; Co = 10 mg L−1; Potential = 0.5 V; SE = 0.2 M Na2SO4; LS = 100 W Xe lamp | 99.39% removal after 40 min | [86] |
| ZnO/MoS2 | Pollutant = acid red 1; Co: 20 mg L−1; Potential = 0.4 V; SE = 0.1 Na2SO4; LS = 300 W Xe lamp | 100% removal after 6 h | [87] |
| ZnO/BiVO4 | Pollutant = tetracycline; Co = 20 mg L−1; Potential = 1.2 V; SE = 0.1 M Na2SO4; LS = 300 W Xe lamp | 84.5% removal in 1 h | [93] |
| BiVO4/MnO2 | Pollutant = ciprofloxacin; Co = 10 mg L−1; Potential = 1.5 V; SE = 0.1 M Na2SO4; LS = 100 W Xe lamp | 76% removal in 2 h | [95] |
| BiVO4/Ag2S | Pollutant = sulphamethoxazole; Co = 10 mg L−1; Potential = 1.2 V; SE = 0.1 M Na2SO4; LS = 100 W Xe lamp | 86% removal in 2 h | [98] |
| TiO2/g-C3N4 | Pollutant = bisphenol A; Co = 10 mg L−1; SE = 0.1 M Na2SO4; Potential = 1.3 V; LS = 300 W Xe lamp | 99.7% removal in 4 h | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orimolade, B.O.; Idris, A.O.; Akanji, S.P.; Adekola, F.A.; Azizi, S.; Maaza, M.; Mamba, B. Solar-Light-Responsive Nanomaterials for the Photoelectrocatalytic Degradation of Stubborn Pollutants. Coatings 2023, 13, 159. https://doi.org/10.3390/coatings13010159
Orimolade BO, Idris AO, Akanji SP, Adekola FA, Azizi S, Maaza M, Mamba B. Solar-Light-Responsive Nanomaterials for the Photoelectrocatalytic Degradation of Stubborn Pollutants. Coatings. 2023; 13(1):159. https://doi.org/10.3390/coatings13010159
Chicago/Turabian StyleOrimolade, Benjamin O., Azeez O. Idris, Seyi Philemon Akanji, Folahan A. Adekola, Shohreh Azizi, Malik Maaza, and Bhekie Mamba. 2023. "Solar-Light-Responsive Nanomaterials for the Photoelectrocatalytic Degradation of Stubborn Pollutants" Coatings 13, no. 1: 159. https://doi.org/10.3390/coatings13010159
APA StyleOrimolade, B. O., Idris, A. O., Akanji, S. P., Adekola, F. A., Azizi, S., Maaza, M., & Mamba, B. (2023). Solar-Light-Responsive Nanomaterials for the Photoelectrocatalytic Degradation of Stubborn Pollutants. Coatings, 13(1), 159. https://doi.org/10.3390/coatings13010159






