A Review on the Transport-Chemo-Mechanical Behavior in Concrete under External Sulfate Attack
Abstract
:1. Introduction
2. Chemical Reaction Products of Sulfate Attack
3. Formation Mechanism of Reaction Products
4. Failure Forms Caused by Chemical Reaction Attack
4.1. Degradation Cause of Concrete
4.2. Degradation Mechanism of Concrete
5. Model for Chemical Sulfate Attack
5.1. Diffusion-Reaction Model of Sulfate
5.2. Volume Expansion or Crystallization Pressure
5.2.1. Free Volume Expansion of Concrete Caused by Sulfate Products
| Chemical Reactions | |
|---|---|
| 0.48 | |
| 0.51 | |
| 1.26 |
5.2.2. Equivalent Expansive Force Generated by Crystallization Pressure
5.3. Chemo-Mechanical Model of Sulfate Attack
5.4. Damage Characterization of Concrete
5.4.1. Chemical Damage
5.4.2. Mechanical Damage
5.4.3. LOAD DAMAGE
| Author | Basic Mechanical Equation | Number | |
|---|---|---|---|
| Chemical damage dc | |||
| Saetta et al. [110,111] | (19) | ||
| Cefis and Comi [92] | (20) | ||
| Sun et al. [73] Zhang et al. [115] | (21) | ||
| is related to the diffuse time or hydrated time. is the ratio of ion concentration in the concrete to that in the external solution. | |||
| Mechanical damage dm | |||
| I | Tixier and Mobasher [78] | (22) | |
| is a threshold strain for the initiation of microcracks or damage. | |||
| Wang et al. [116] | (23) | ||
| is the initial elastic modulus. is the ultimate tensile strain corresponding to the ultimate tensile stress at the peak of the stress–strain curve. | |||
| II | Bary et al. [84,87] | (24) | |
| is the equivalent strain. | |||
| Yin et al. [96,102] | (25) | ||
| are the positive and negative spectral decomposition parts of the effective stress tensors . is the compressive or tensile stress-induced mechanical damage. | |||
| Load damage dl | |||
| Grassl et al. [112] | (26) | ||
| is the parameter that controls the slope of the softening curve. | |||
| Wu et al. [114] | (27) | ||
| are the tensile damage and shear damage corresponding to positive and negative stress components. | |||
| Mazars et al. [109] Zheng et al. [117] | (28) | ||
| and represent the stiffness recovery effects of tension and compression. | |||
6. Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Wu, L.; Bindiganavile, V.; Yi, C.F. Coupled models to describe the combined diffusion-reaction behaviour of chloride and sulphate ions in cement-based systems. Constr. Build. Mater. 2020, 243, 118232. [Google Scholar] [CrossRef]
- Martins, M.C.; Langaro, E.A.; Macioski, G.; Medeiros, M.H.F. External ammonium sulfate attack in concrete: Analysis of the current methodology. Constr. Build. Mater. 2021, 277, 122252. [Google Scholar] [CrossRef]
- Salt Lake: Accelerating Chinese civilization and creating magnificent scenery. Chin. Natl. Geogr. 2011, 605, 13.
- Sun, W. Durability and service life of structure concrete under load and environment coupling effects. J. Southeast Univ. (Nat. Sci. Ed.) 2006, 36 (Suppl. SII), 7–14. [Google Scholar]
- Liu, Z.Y. Study on Methods of Accelerated Testing of Marine Concrete Durability Based on Simulating Environment and Service Life Prediction; Southeast University: Nanjing, China, 2006. [Google Scholar]
- Zhou, G.; Li, S.R.; Wang, Z.J.; Wang, C.F. Investigation and analysis on corrosion situation of concrete in saline soil region. J. Archit. Civ. Eng. 2011, 4, 121–126. [Google Scholar]
- Mehta, P.K. Sulfate attack on concrete: Separating myths from reality. Concr. Int. 2000, 22, 57–61. [Google Scholar]
- Hime, W.G.; Mather, B. “Sulfate attack,” or is it. Cem. Concr. Res. 1999, 29, 789–791. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Neville, A. Consideration of durability of concrete structure: Past, present, and future. Mater. Struct. 2001, 34, 114–118. [Google Scholar] [CrossRef]
- Bensted, J. Thaumasite-direct, woodfordite and other possible formation routes. Cem. Concr. Compos. 2003, 25, 873–877. [Google Scholar] [CrossRef]
- Harrison, W.H.; Cooke, R.W. Sulfate resistance of buried concrete. Proc. Inst. Civ. Eng. 1981, 70, 871–874. [Google Scholar]
- Santhanam, M.; Cohen, M.D.; Olek, J. Effects of gypsum formation on the performance of cement mortars during external sulfate attack. Cem. Concr. Res. 2003, 33, 325–332. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Lee, H.; Cody, R.D.; Cody, A.M.; Spry, P.G. The formation and role of ettringite in Iowa highway concrete deterioration. Cem. Concr. Res. 2005, 35, 332–343. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Z.; Shen, X.; Liu, W.Q. Stability and decomposition mechanism of ettringite in presence of ammonium sulfate solution. Constr. Build. Mater. 2016, 124, 786–793. [Google Scholar] [CrossRef]
- Pavoine, A.; Brunetaud, X.; Divet, L. The impact of cement parameters on delayed ettringite formation. Cem. Concr. Compos. 2012, 34, 521–528. [Google Scholar] [CrossRef]
- Abualgasem, J.M.; Cripps, J.C.; Lynsdale, C.J. Effects of wetting and drying cycles on thaumasite formation in cement mortars. J. Mater. Civ. Eng. 2015, 27, 1–6. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bassuoni, M.T. Thaumasite sulfate attack on concrete: Mechanisms, influential factors and mitigation. Constr. Build. Mater. 2014, 73, 652–662. [Google Scholar] [CrossRef]
- Bellmann, F.; Stark, J. Prevention of thaumasite formation in concrete exposed to sulphate attack. Cem. Concr. Res. 2007, 37, 1215–1222. [Google Scholar] [CrossRef]
- Biczok, I. Concrete Corrosion Concrete Protection; Chemical Publishing Company: New York, NY, USA, 1967. [Google Scholar]
- Bellmann, F.; Möser, B.; Stark, J. Influence of sulfate solution concentration on the formation of gypsum in sulfate resistance test specimen. Cem. Concr. Res. 2006, 36, 358–363. [Google Scholar] [CrossRef]
- Ma, B.G.; Luo, Z.T.; Li, X.G.; Gao, X.J.; Zhang, M.X. Microscopic structure and growth mechanism of the corrosion products including thaumasite. J. Chin. Ceram. Soc. 2006, 34, 1503–1507. [Google Scholar]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate attack research—Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Bassuoni, M.T.; Nehdi, M.L. Durability of self-consolidating concrete to different exposure regimes of sodium sulfate attack. Mater. Struct. 2009, 42, 1039–1057. [Google Scholar] [CrossRef]
- Collepardi, M. Thaumasite formation and deterioration in historic buildings. Cem. Concr. Compos. 1999, 21, 147–154. [Google Scholar] [CrossRef]
- Bonen, D.; Cohen, M.D. Magnesium sulfate attack on Portland cement paste—II. Chemical and mineralogical analyses. Cem. Concr. Res. 1992, 22, 707–718. [Google Scholar] [CrossRef]
- Durgun, M.Y.; Sevinc, A.H. Determination of the effectiveness of various mineral additives against sodium and magnesium sulfate attack in concrete by Taguchi method. J. Build. Eng. 2022, 57, 104849. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, X.H.; Xiong, G.Q.; Zhang, M.T.; Deng, J.X.; Zhao, M.; Zhao, L. Will the magnesium sulfate attack of cement mortars always be inhibited by incorporating nanosilica. Constr. Build. Mater. 2021, 305, 124695. [Google Scholar] [CrossRef]
- Marchand, J.; Ivan Older Skalny, J.P. Sulfate Attack on Concrete (Morden Concrete Technology); Spon Press: London, UK; New York, NY, USA, 2002. [Google Scholar]
- Brown, P.W.; Taylor, H.F.W. The Role of Ettringite in External Sulfate Attack: Material Science of Concrete—Sulfate Attack Mechanism; American Ceramic Society: Westerville, OH, USA, 1999. [Google Scholar]
- Peng, J.H.; Lou, Z.H. Study Mech. Ettringite Formation. J. Chin. Ceram. Soc. 2000, 28, 511–515. [Google Scholar]
- Evju, C.; Hansen, S. The kinetics of ettringite formation and dilatation in a blended cement with β-hemihydrate and anhydrite as calcium sulfate. Cem. Concr. Res. 2005, 35, 2310–2321. [Google Scholar] [CrossRef]
- Silva, D.A.; Monteiro, P.J.M. Early Formation of Ettringite in Tricalcium Aluminate-Calcium Hydroxide-Gypsum Dispersions. J. Am. Ceram. Soc. 2007, 90, 614–617. [Google Scholar] [CrossRef]
- Skalny, J.; Marchand, J.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK, 2002. [Google Scholar]
- Schmidt, T. Sulfate Attack and the Role of Internal Carbonate on the Formation of Thaumasite; EPFL: Lausanne, Switzerland, 2007. [Google Scholar]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Souza, D.J.D.; Medeiros, M.H.F.D.; Filho, J.H. Evaluation of external sulfate attack (Na2SO4 and MgSO4): Portland cement mortars containing fillers. Rev. IBRACON Estrut. E Mater. 2020, 13, 644–655. [Google Scholar] [CrossRef]
- Collepardi, M. A state-of-the-art review on delayed ettringite attack on concrete. Cem. Concr. Compos. 2003, 25, 401–407. [Google Scholar] [CrossRef]
- Polivka, M. Factors influencing expansion of expansive cement concretes. Int. Concr. Abstr. Portal 1973, 38, 239–250. [Google Scholar]
- Cohen, M.D. Modeling of expansive cements. Cem. Concr. Res. 1983, 13, 519–528. [Google Scholar] [CrossRef]
- Cohen, M.D. Theories of expansion in sulfoaluminate—Type expansive cements: Schools of thought. Cem. Concr. Res. 1983, 13, 809–818. [Google Scholar] [CrossRef]
- Mehta, P.K. Mechanism of expansion associated with ettringite formation. Cem. Concr. Res. 1973, 3, 1–6. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Clifton, J.R.; Ponnersheim, J.M. Sulfate Attack of Cementitious Materials: Volumetric Relations and Expansions; Building and Fire Research, National Institute of Standards and Technology: Gaithersburg, MD, USA, 1994.
- Lothenbach, B.; Bary, B.; Bescop, P.L.; Schmidt, T.; Leterrier, N. Sulfate Ingress in Portland Cement. Cem. Concr. Res. 2010, 40, 1211–1225. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. On the relevance of volume increase for the length changes of mortar bars in sulfate solution s. Cem. Concr. Res. 2013, 46, 23–29. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Famy, C.; Scrivener, K.L. Delayed ettringite formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]
- Bizzpzero, J.; Gpsselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Metha, P.K. Evaluation of sulfate-resisting cements by a new test method. J. ACI 1975, 72, 573–575. [Google Scholar]
- Steiger, M. Crystal growth in porous materials—II: Influence of crystal size on the crystallization pressure. J. Cryst. Growth 2005, 282, 470–481. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Espinosa, R.M.; Franke, L.; Deckelmann, G. Model for the mechanical stress due to the salt crystallization in porous materials. Constr. Build. Mater. 2008, 22, 1350–1367. [Google Scholar] [CrossRef]
- Koniorczyk, M.; Gawin, D. Modelling of salt crystallization in building materials with microstructure—Poromechanical approach. Constr. Build. Mater. 2012, 36, 860–873. [Google Scholar] [CrossRef]
- Flatt, R.J. Salt damage in porous materials: How high supersaturations are generated. J. Cryst. Growth 2002, 242, 435–454. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Ju, X.D.; Feng, W.J.; Zhang, Y.J. Crystallization stress in brittle porous media. Chin. J. Geotech. Eng. 2016, 38, 1253–1264. [Google Scholar]
- Yin, G.J.; Shan, Z.Q.; Miao, L.; Tang, Y.J.; Zuo, X.B.; Wen, X.D. Finite element analysis on the diffusion-reaction-damage behavior in concrete subjected to sodium sulfate attack. Eng. Fail. Anal. 2022, 137, 106278. [Google Scholar] [CrossRef]
- Guan, B.W.; Liu, J.N.; Wu, J.W.; Chen, X.H.; Hu, Y.; Zhang, L.Q. Transport behavior of sulfate ions in concrete with attack damage. Bull. Chin. Ceram. Soc. 2020, 39, 3169–3174. [Google Scholar]
- Zhao, S.B.; Yang, X.M. Experimental study on regularity of sulfate-ion diffusion and distribution in concrete attacked by sulfate. China Harb. Eng. 2009, 161, 26–29. [Google Scholar]
- Ran, B.; Li, K.F.; Teddy, F.C.; Omikrine-Metalssi, O.; Dangka, P. Spalling rate of concretes subject to combined leaching and external sulfate attack. Cem. Concr. Res. 2022, 162, 106951. [Google Scholar] [CrossRef]
- Zou, D.J.; Qin, S.S.; Liu, T.J.; Jivkov, A. Experimental and numerical study of the effect of solution concentration and temperature on concrete under external sulfate attack. Cem. Concr. Res. 2021, 139, 106284. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.X. Numerical modelling of mechanical deterioration of cement mortar under sulfate attack. Constr. Build. Mater. 2018, 158, 490–502. [Google Scholar] [CrossRef]
- Shazail, M.A.; Baluch, M.H.; Al-Gadhib, A.H. Predicting residual strength in unsaturated concrete exposed to sulfate attack. J. Mater. Civil Eng. 2006, 18, 343–354. [Google Scholar] [CrossRef]
- Gospodinov, P.; Kazandjiev, R.; Mironova, M. The effect of sulfate ion diffusion on the structure of cement stone. Cem. Concr. Compos. 1996, 18, 401–407. [Google Scholar] [CrossRef]
- Gospodinov, P.N.; Kazandjiev, R.F.; Partalin, T.A.; Mironovac, M.K. Diffusion of sulfate ions into cement stone regarding simultaneous chemical reactions and resulting effects. Cem. Concr. Res. 1999, 29, 1591–1596. [Google Scholar] [CrossRef]
- Gospodinov, P.N. Numerical simulation of 3D sulfate ion diffusion and liquid push out of the material capillaries in cement composites. Cem. Concr. Res. 2005, 35, 520–526. [Google Scholar] [CrossRef]
- Marchand, J.; Samson, E.; Maltais, Y.; Beaudoin, J.J. Theoretical analysis of the effect of weak sodium sulfate solutions on the durability of concrete. Cem. Concr. Compos. 2002, 24, 317–329. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.W.; Wang, S.G.; Xu, F.; Han, J.D. Recent development on theoretical models of sulfate attack on concrete. Mater. Rev. 2014, 28, 89–95. [Google Scholar]
- Zuo, X.B.; Sun, W.; Li, H.; Zhao, Y.K. Modeling of diffusion-reaction behavior of sulfate ion in concrete under sulfate environments. Comput. Concr. 2012, 10, 47–51. [Google Scholar] [CrossRef]
- Sun, W.; Zuo, X.B. Numerical simulation of sulfate diffusivity in concrete under combination of mechanical loading and sulfate environments. J. Sustain. Cem.-Based Mater. 2012, 1, 46–55. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.K.; Zhu, J.; Zhang, M.H.; Ye, J. A new diffusion model of sulfate ions in concrete. Constr. Build. Mater. 2013, 39, 39–45. [Google Scholar] [CrossRef]
- Yang, C.C.; Su, J.K. Approximate migration coefficient of interfacial transition zone and the effect of aggregate content on the migration coefficient of mortar. Cem. Concr. Res. 2002, 32, 1559–1565. [Google Scholar] [CrossRef]
- Yang, C.C.; Cho, S.W. Approximate migration coefficient of percolated interfacial transition zone by using the accelerated chloride migration test. Cem. Concr. Res. 2005, 35, 344–350. [Google Scholar] [CrossRef]
- Huang, Q.H.; Zhou, C.Z.; Gu, X.L.; Zhang, W.P. Experimental study on moisture transport property of interfacial transition zone in concrete. J. Build. Struct. 2019, 40, 174–180. [Google Scholar]
- Atkinson, A.; Hearne, J.A. Mechanistic model for the durability of concrete barriers exposed to sulphate-bearing groundwaters. Mrs. Proc. 1989, 176, 149–156. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of Damage in cement-based materials subjected to external sulfate attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Basista, M.; Weglewski, W. Chemically Assisted Damage of Concrete: A Model of Expansion Under External Sulfate Attack. Int. J. Damage Mech. 2009, 18, 155–175. [Google Scholar] [CrossRef]
- Idiart, A.E.; López, C.M.; Carol, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cem. Concr. Compos. 2011, 33, 411–423. [Google Scholar] [CrossRef]
- Ikumi, T.; Cavalaro, S.H.P.; Segura, I.; Aguado, A. Alternative methodology to consider damage and expansions in external sulfate attack modeling. Cem. Concr. Res. 2014, 63, 105–116. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K.L. Degradation mechanism of slag blended mortars immersed in sodium sulfate solution. Cem. Concr. Res. 2015, 72, 37–47. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Sun, X.; Tang, Y.J. Macro-microscopically numerical analysis on expansion response of hardened cement paste under external sulfate attack. Constr. Build. Mater. 2019, 207, 600–615. [Google Scholar] [CrossRef]
- Bary, B. Simplified coupled chemo-mechanical modeling of cement pastes behavior subjected to combined leaching and external sulfate attack. Int. J. Numer. Anal. Methods Geomech. 2008, 32, 1791–1816. [Google Scholar] [CrossRef]
- Feng, P.; Bullard, J.W.; Garboczi, E.J. A multiscale microstructure model of cement paste sulfate attack by crystallization pressure. Modelling Simul. Mater. Sci. Eng. 2017, 25, 65013. [Google Scholar]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. II: Comparison with experiments. J. Mater. Civ. Eng. 2003, 15, 314–322. [Google Scholar] [CrossRef]
- Bary, B.; Leterrier, N.; Deville, E.; Bescop, P. Coupled chemo-transport-mechanical modelling and numerical simulation of external sulfate attack in mortar. Cem. Concr. Compos. 2014, 49, 70–83. [Google Scholar] [CrossRef]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; Sloot, H.V.D.; Kosson, D.S. Numerical simulation of cementitious materials degradation under external sulfate attack. Cem. Concr. Compos. 2010, 32, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; Sloot, S.V.D.; Kosson, D.S. Sensitivity Analysis of Damage in Cement Materials under Sulfate Attack and Calcium Leaching. J. Mater. Civ. Eng. 2012, 24, 430–440. [Google Scholar] [CrossRef]
- Ikumi, T.; Cavalaro, S.H.P.; Segura, I.; Fuente, A.D.L.; Aguado, A. Simplified methodology to evaluate the external sulfate attack in concrete structures. Mater. Des. 2016, 89, 1147–1160. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Damage modelling in concrete subject to sulfate attack. Frat. Integrità Strutt. 2014, 8, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Cefis, N.; Comi, C. Chemo-mechanical modelling of the external sulfate attack in concrete. Cem. Concr. Res. 2017, 93, 57–70. [Google Scholar] [CrossRef]
- Zuo, X.B.; Sun, W. Full process analysis of damage and failure of concrete subjected to external sulfate attack. J. Chin. Ceram. Soc. 2009, 37, 1063–1067. [Google Scholar]
- Zuo, X.B.; Sun, W.; Yu, C. Numerical investigation on expansive volume strain in concrete subjected to sulfate attac k. Constr. Build. Mater. 2012, 36, 404–410. [Google Scholar] [CrossRef]
- Nie, Q.; Zhou, C.; Li, H.; Sun, X.; Gong, H.; Huang, B. Numerical simulation of fly ash concrete under sulfate attack. Constr. Build. Mater. 2015, 84, 261–268. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Tang, Y.; Tang, Y.J. Numerical simulation on time-dependent mechanical behavior of concrete under coupled axial loading and sulfate attack. Ocean Eng. 2017, 142, 115–124. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Sun, X.; Tang, Y.J. Numerical investigation on ESA-induced expansion response of cement paste by using crystallization pressure. Model. Simul. Mater. Sci. Eng. 2019, 27, 25006. [Google Scholar] [CrossRef]
- Yu, Y.G.; Gao, W.; Feng, Y.; Gastel, A.; Chen, X.J.; Liu, A.R. On the competitive antagonism effect in combined chloride-sulfate attack A numerical exploration. Cem. Concr. Res. 2021, 144, 106406. [Google Scholar] [CrossRef]
- Yi, C.F.; Zheng, C.; Vivek, B. A non-homogeneous model to predict the service life of concrete subjected to external sulfate attack. Constr. Build. Mater. 2019, 212, 254–265. [Google Scholar] [CrossRef]
- Saetta, A.; Scotta, R.; Vitaliani, R. Mechanical Behavior of Concrete under Physical-Chemical Attacks. J. Eng. Mech. 1998, 124, 1100–1109. [Google Scholar] [CrossRef]
- Saetta, A.; Scotta, R.; Vitaliani, R. Coupled Environmental-Mechanical Damage Model of RC Structures. J. Eng. Mech. 1999, 125, 930–940. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Li, X.N.; Zou, Y.X. An integrated macro-microscopic model for concrete deterioration under external sulfate attack. Eng. Fract. Mech. 2020, 240, 107345. [Google Scholar] [CrossRef]
- Li, R.T.; Li, X.K. A coupled chemo-elastoplastic-damage constitutive model for plain concrete subjected to high temperature. Int. J. Damage Mech. 2010, 19, 971–1000. [Google Scholar]
- Yin, G.J.; Zuo, X.B.; Tang, Y.; Olawale, A.; Ding, D.N. Modeling of time-varying stress in concrete under axial loading and sulfate attack. Comput. Concr. 2017, 19, 143–152. [Google Scholar] [CrossRef]
- Yu, Y.G.; Gao, W.; Castel, A.; Liu, A.; Chen, X.J.; Liu, M. Assessing external sulfate attack on thin-shell artificial reef structures under uncertainty. Ocean Eng. 2020, 207, 107397. [Google Scholar] [CrossRef]
- Qin, S.; Zhou, D.; Liu, T.; Jiv, A. A chemo-transport-damage model for concrete under external attack. Cem. Concr. Res. 2020, 132, 106048. [Google Scholar] [CrossRef]
- Wang, P.; Mo, R.; Li, S.; Xu, J.; Jin, Z.Q.; Zhao, T.J.; Wang, D.Z. A chemo-damage-transport model for chloride ions diffusion in cement-based materials: Combined effects of sulfate attack and temperature. Constr. Build. Mater. 2021, 288, 123121. [Google Scholar] [CrossRef]
- Li, J.P.; Xie, F.; Zhao, G.W.; Li, L. Experimental and numerical investigation of cast-in-situ concrete under external sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118789. [Google Scholar] [CrossRef]
- Mazars, J.; Pyaudier-Cabot, G. Continuum damage theory-application to concrete. J. Eng. Mech. 1989, 115, 345–365. [Google Scholar] [CrossRef]
- Lubliner, J.; Oliver, J.; Oller, S.; Onate, E. A plastic-damage model for concrete. Int. J. Solids Struct. 1989, 25, 299–326. [Google Scholar] [CrossRef]
- Faria, R.; Oliver, J.; Cervera, M. A strain-based plastic viscous-damage model for massive concrete structures. Int. J. Solids Struct. 1998, 35, 1533–1558. [Google Scholar] [CrossRef]
- Grassl, P.; Jirásek, M. Damage-plastic model for concrete failure. Int. J. Solids Struct. 2006, 43, 7166–7196. [Google Scholar] [CrossRef] [Green Version]
- Grassl, P.; Xenos, D.; Nyström, U.; Rempling, R.; Gylltoft, K. CDPM2, A damage-plasticity approach to modelling the failure of concrete. Int. J. Solids Struct. 2013, 50, 3805–3816. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Li, J.; Faria, R. An energy release rate-based plastic-damage model for concrete. Int. J. Solids Struct. 2006, 43, 583–612. [Google Scholar] [CrossRef]
- Zhang, C.L.; Chen, W.K.; Mu, S.; Savija, B.; Liu, Q.F. Numerical investigation of external sulfate attack and its effect on chloride binding and diffusion in concrete. Constr. Build. Mater. 2021, 285, 122806. [Google Scholar] [CrossRef]
- Wang, H.L.; Chen, Z.; Li, H.; Sun, X.Y. Numerical simulation of external sulphate attack in concrete considering coupled chemo-diffusion-mechanical effect. Constr. Build. Mater. 2021, 292, 123325. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, Z.; Gu, C.; Bao, T.F.; Hu, J. A plastic damage model for concrete structure cracks with two damage variables. Sci. China Technol. Sci. 2012, 55, 2971–2980. [Google Scholar] [CrossRef]
- Silva, D.; Fajardo-San-Miguel, G.; Escadeillas, G.; Cruz-Moreno, D. Surface treatment with silicon-based nanoparticles in Portland cement specimens subjected to physical sulfate attack. Case Stud. Constr. Mater. 2023, 18, e01795. [Google Scholar] [CrossRef]
- Esselami, R.; Wilson, W.; Tagni-Hamou, A. An accelerated physical sulfate attack test using an induction period and heat drying: First applications to concrete with different binders including ground glass pozzolan and limestone filler. Constr. Build. Mater. 2022, 345, 128046. [Google Scholar] [CrossRef]
- Singh, A.; Liu, Q.; Xiao, J.Z.; Lyu, Q.F. Mechanical and macrostructural properties of 3D printed concrete dosed with steel fibers under different loading direction. Constr. Build. Mater. 2022, 323, 126616. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Haghighi, A. Large-scale automated additive construction: Overview, robotic solutions, sustainability, and future prospect. Sustainability 2022, 14, 9872. [Google Scholar] [CrossRef]
- Waseem, S.A.; Singh, B. An experimental study on shear capacity of interfaces in recycled aggregate concrete under multiaxial compression. Struct. Concr. 2018, 19, 230–245. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.H.; Kang, T.B.; Wang, F.C. Pore structure and strength of waste fiber recycled concrete. J. Eng. Fibers Fabr. 2019, 14, 1–10. [Google Scholar] [CrossRef]

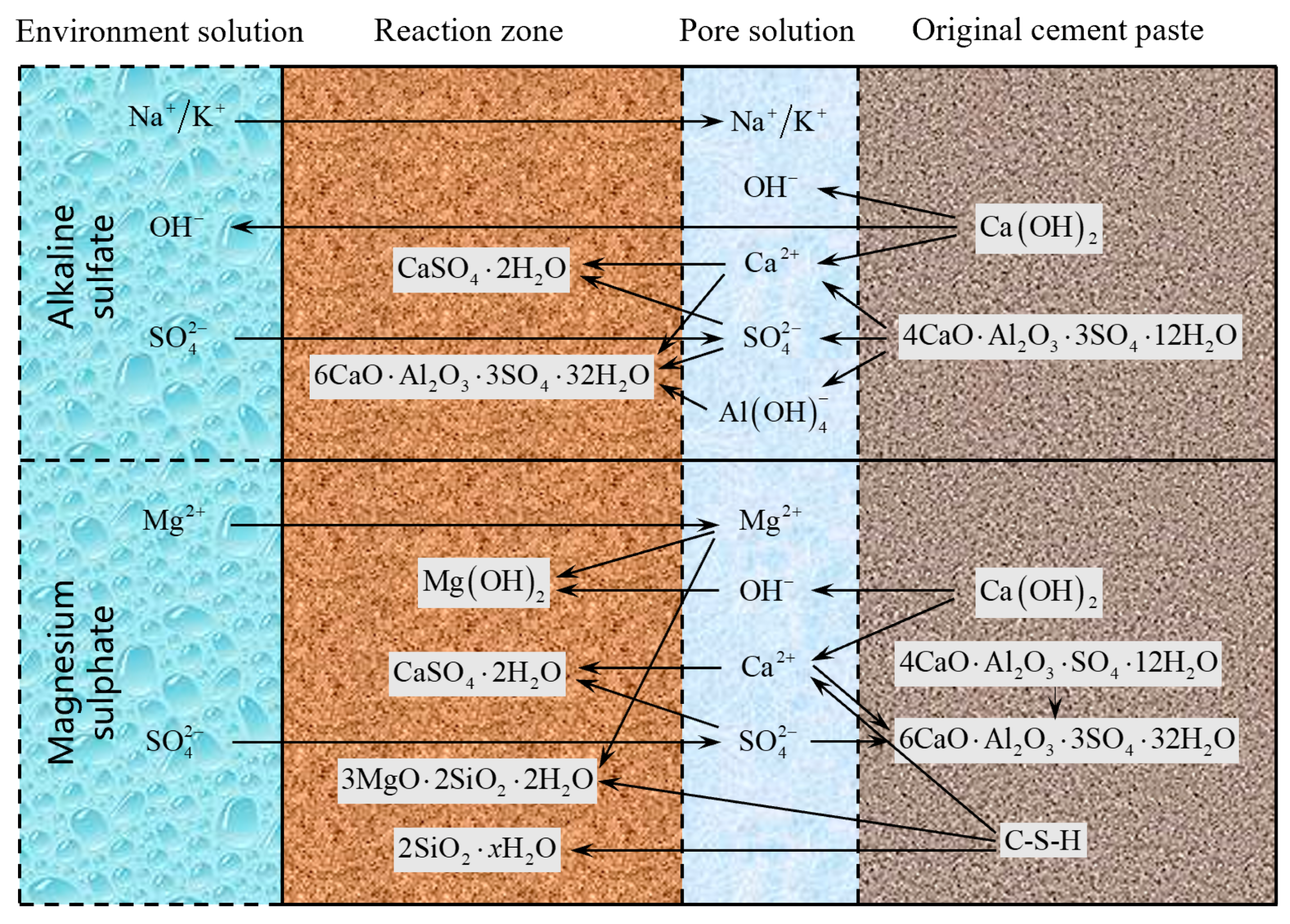
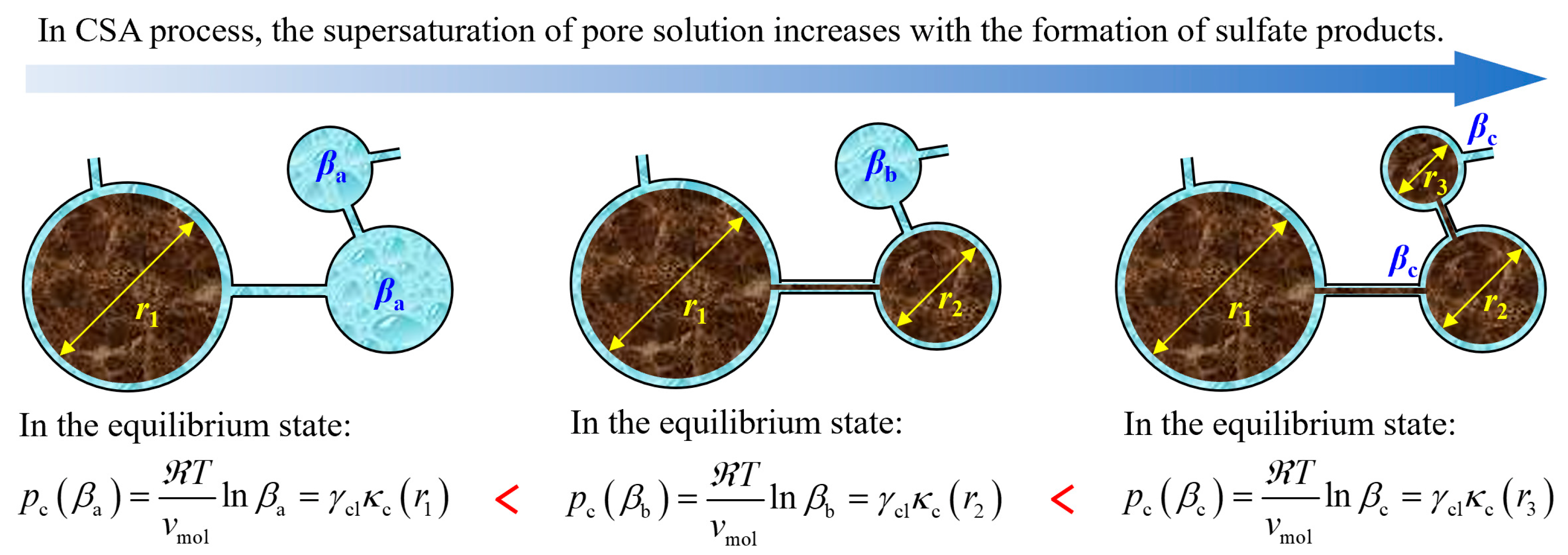

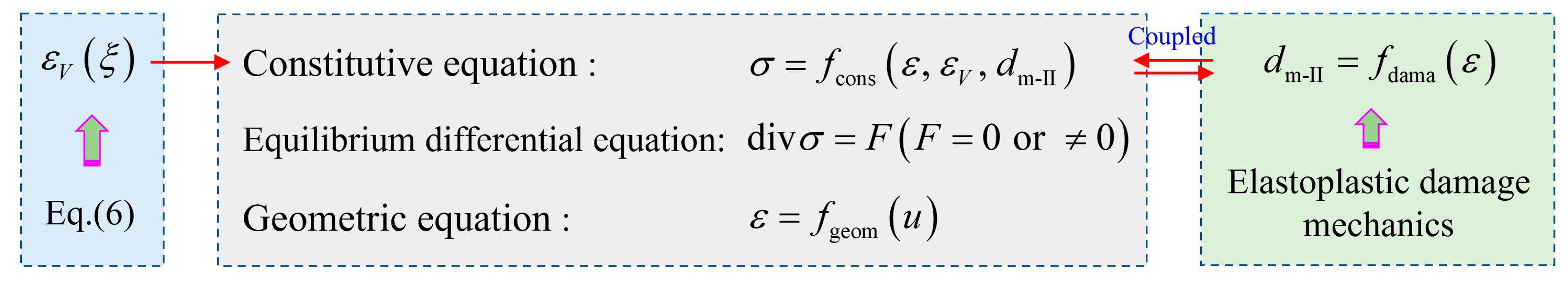
| Cation Type | Sulfate Product | Formation Mechanism | Chemical Reaction | |
|---|---|---|---|---|
| Na+/K+ | Gypsum | Ion–ion reaction | ||
| Ettringite | Topochemical mechanism (Solid–solid reaction) | |||
| Through-solution mechanism (Ion–ion reaction) | ||||
| Thaumasite | Direct reaction (Ion–ion reaction) | |||
| Indirect reaction (Solid–solid reaction) | ||||
| Mg2+ | Brucite | Ion–ion reaction | ||
| Cation Type | CSA Type/Sulfate Product | ||||
|---|---|---|---|---|---|
| Thaumasite | Gypsum | Ettringite | Brucite | ||
| Na+/K+ | √) | √ (main) | √ (main) | ||
| Mg2+ | √ | √ | √ (main) | ||
| Failure form | Cohesiveness | ◯ | ◯ | ||
| Softening | ◯ | ||||
| Volume expansion and its-induced cracking/spalling | ◯ | ◯ | |||
| Basic Form | ||
|---|---|---|
| Ion flow | Diffusion behavior | |
| Mutual restriction effect between charged ions | ||
| Influence of ionic chemical activity | ||
| Ion migration effect with solution convection | ||
| Temperature effect | ||
| Coupled by the above factors | ||
| Effective diffusivity | Temperature effect | |
| Sulfate products filling in pore | ||
| ESA-induced microcrack effect | ||
| Damage degree | ||
| Cement hydration | ||
| Author | Published Time | Degradation Cause | Degradation Mechanism |
|---|---|---|---|
| Tixier and Mobasher [78,86] | 2003 | Ettringite | Volume increase theory |
| Bary [84] | 2008 | Ettringite, Gypsum | Crystallization pressure theory |
| Bary et al. [87] | 2014 | Ettringite | Volume increase theory, crystallization pressure theory |
| Basista and Weglewski [79] | 2009 | Ettringite | Volume increase theory |
| Sarkar et al. [88] | 2010 | Ettringite | Volume increase theory |
| Sarkar et al. [89] | 2012 | Ettringite, Gypsum | Volume increase theory |
| Idiart et al. [80] | 2011 | Ettringite | Volume increase theory |
| Ikumi et al. [81] | 2014 | Ettringite | Volume increase theory |
| Ikumi et al. [90] | 2016 | Ettringite | Volume increase theory |
| Cefis and Comi [91] | 2014 | Ettringite | Volume increase theory |
| Cefis and Comi [92] | 2017 | Ettringite | Volume increase theory |
| Zuo et al. [93] | 2009 | Ettringite | Volume increase theory |
| Zuo et al. [94] | 2012 | Ettringite | Volume increase theory |
| Nie et al. [95] | 2015 | Ettringite | Volume increase theory |
| Yin et al. [96] | 2017 | Ettringite, Gypsum | Volume increase theory |
| Yin et al. [97] | 2019 | Ettringite | Crystallization pressure theory |
| Yu et al. [64] | 2018 | Ettringite | Volume increase theory |
| Yu et al. [98] | 2021 | Ettringite | Volume increase theory |
| Yi et al. [99] | 2019 | Ettringite | Volume increase theory |
| Authors | Basic Mechanical Equation | Number |
|---|---|---|
| Saetta et al. [100,101] | (8) | |
| dc is the CSA-induced chemical damage. is the loading damage caused by external loading. This model can be unsed to analyze the stress in concrete under the external load and environment corrosion. | ||
| Sarkar et al. [88] | (9) | |
| In sraker’s model, the is calclulated by the free expnasion and is caused by the growth of sulfate products. | ||
| Bary et al. [87] | (10) | |
| The effects of crystallization pressure and free expansion are considered together in the model. | ||
| Cefis and Comi [92] | (11) | |
| In this model, the CSA-induced mechanical and chemical damages are considered. Additionally, the hydrostatic pressure pw is introduced in the basic constitutive equation. So, this model is applicable to the case of sulfate attack on unsaturated concrete. | ||
| Ikumi et al. [81] | (12) | |
| Ikumi’s model is similar to that of Sarkar, but he considered the influence of pore size on . | ||
| Yin et al. [96] | (13) | |
| Yin et al. [102] | (14) | |
| In Yin’s models, the chemical damage dc is analyzed by using the elastoplastic damage mechanics, not determined by the empirical formula related to the content of sulfate ion or the reaction product. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.-J.; Wen, X.-D.; Miao, L.; Cui, D.; Zuo, X.-B.; Tang, Y.-J. A Review on the Transport-Chemo-Mechanical Behavior in Concrete under External Sulfate Attack. Coatings 2023, 13, 174. https://doi.org/10.3390/coatings13010174
Yin G-J, Wen X-D, Miao L, Cui D, Zuo X-B, Tang Y-J. A Review on the Transport-Chemo-Mechanical Behavior in Concrete under External Sulfate Attack. Coatings. 2023; 13(1):174. https://doi.org/10.3390/coatings13010174
Chicago/Turabian StyleYin, Guang-Ji, Xiao-Dong Wen, Ling Miao, Dong Cui, Xiao-Bao Zuo, and Yu-Juan Tang. 2023. "A Review on the Transport-Chemo-Mechanical Behavior in Concrete under External Sulfate Attack" Coatings 13, no. 1: 174. https://doi.org/10.3390/coatings13010174
APA StyleYin, G.-J., Wen, X.-D., Miao, L., Cui, D., Zuo, X.-B., & Tang, Y.-J. (2023). A Review on the Transport-Chemo-Mechanical Behavior in Concrete under External Sulfate Attack. Coatings, 13(1), 174. https://doi.org/10.3390/coatings13010174







