Annealing Treatment on Homogenous n-TiO2/ZnO Bilayer Thin Film Deposition as Window Layer for p-Cu2O-Based Heterostructure Thin Film
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Treatment on n-TiO2/ZnO Bilayer Window Layer
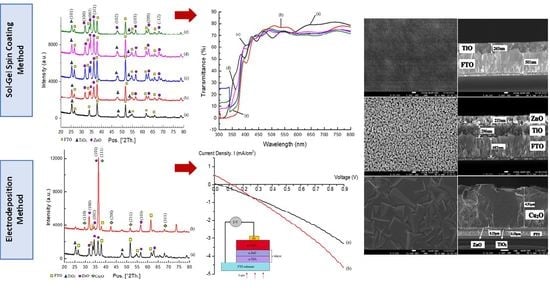
3.1.1. Structural Properties
3.1.2. Morphological Properties
3.1.3. Optical Properties
3.2. Fabrication of n-TiO2/ZnO Bilayer/p-Cu2O Heterostructured Thin Film
3.2.1. Structural Properties
3.2.2. Morphological Properties
3.2.3. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamad Arifin, N.; Mohamad, F.; Hui Ling, C.; Binti Zinal, N.; Binti Mohd Hanif, A.S.; Muhd Nor, N.H.B.; Izaki, M. Growth Mechanism of Copper Oxide Fabricaticated by Potentiostatic Electrodeposition Method. Mater. Sci. Forum 2017, 890, 303–307. [Google Scholar] [CrossRef]
- Ahmad Ramli, S.; Mohamad, F.; Anizam, A.G.A.; Ahmad, M.K.; Ahmad, N.; Mohd Ismail, A.Z.; Mohamad Arifin, N.; Maarof, N.A.S.; Nurhaziqah, A.M.S.; Saputri, D.G.; et al. Properties Enhancement of TiO2 Nanorod Thin Film Using Hydrochloric Acid Etching Treatment Method. J. Mater. Sci. Mater. Electron. 2022, 33, 16348–16356. [Google Scholar] [CrossRef]
- Mathur, A.S.; Singh, P.P.; Upadhyay, S.; Yadav, N.; Singh, K.S.; Singh, D.; Singh, B.P. Role of Absorber and Buffer Layer Thickness on Cu2O/TiO2 Heterojunction Solar Cells. Sol. Energy 2022, 233, 287–291. [Google Scholar] [CrossRef]
- Bin Rafiq, M.K.S.; Amin, N.; Alharbi, H.F.; Luqman, M.; Ayob, A.; Alharthi, Y.S.; Alharthi, N.H.; Bais, B.; Akhtaruzzaman, M. WS2: A New Window Layer Material for Solar Cell Application. Sci. Rep. 2020, 10, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussin, R.; Seng, G.H.; Zulkiflee, N.S.; Harun, Z.; Hatta, M.N.M.; Yunos, M.Z. ZnO/TiO2 Thin Films for Photocatalytic Application. AIP Conf. Proc. 2019, 2068, 020096. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Vaughan, B.; Holdsworth, J.; Belcher, W.; Zhou, X.; Dastoor, P. The Role of the Electron Transport Layer in the Degradation of Organic Photovoltaic Cells. Coatings 2022, 12, 1071. [Google Scholar] [CrossRef]
- Vitanov, P.; Ivanova, T.; Dikov, H.; Terziyska, P.; Ganchev, M.; Petkov, N.; Georgiev, Y.; Asenov, A. Effect of a Discontinuous Ag Layer on Optical and Electrical Properties of ZnO/Ag/ZnOStructures. Coatings 2022, 12, 1324. [Google Scholar] [CrossRef]
- Garcia, V.J.; Pelicano, C.M.; Yanagi, H. Low Temperature-Processed ZnO Nanorods-TiO2 Nanoparticles Composite as Electron Transporting Layer for Perovskite Solar Cells. Thin Solid Films 2018, 662, 70–75. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Shi, J.; Dong, J.; Luo, Y.; Li, D.; Meng, Q. Highly Efficient Planar Perovskite Solar Cells with a TiO2/ZnO Electron Transport Bilayer. J. Mater. Chem. A 2015, 3, 19288–19293. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. TiO2 Coated Zno Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 1339. [Google Scholar] [CrossRef]
- Saurdi, I.; Mamat, M.H.; Rusop, M. Electrical and Structural Properties of ZnO/TiO2 Nanocomposite Thin Films by RF Magnetron Co-Sputtering. Adv. Mater. Res. 2013, 667, 206–212. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Lu, H.L.; Geng, Y.; Ye, Z.Y.; Zhang, Y.; Sun, Q.Q.; Ding, S.J.; Zhang, D.W. Optical and Microstructural Properties of ZnO/TiO2 Nanolaminates Prepared by Atomic Layer Deposition. Nanoscale Res. Lett. 2013, 8, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.C. New Development of Atomic Layer Deposition: Processes, Methods and Applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareso, P.L.; Juarlin, E. Optical and Structural Characterization of ZnO/TiO2 Bilayer Thin Films Grown by Sol-Gel Spin Coating. J. Phys. Conf. Ser. 2018, 979, 012060. [Google Scholar] [CrossRef]
- Zulkiflee, N.S.; Hussin, R. Effect of Temperature on TiO2/ZnO Nanostructure Thin Films. Mater. Sci. Forum 2016, 840, 262–266. [Google Scholar] [CrossRef]
- Hacini, N.; Ghamnia, M.; Dahamni, M.A.; Boukhachem, A.; Pireaux, J.J.; Houssiau, L. Compositional, Structural, Morphological, and Optical Properties of ZnO Thin Films Prepared by PECVD Technique. Coatings 2021, 11, 202. [Google Scholar] [CrossRef]
- Malek, M.F.; Mamat, M.H.; Khusaimi, Z.; Sahdan, M.Z.; Musa, M.Z.; Zainun, A.R.; Suriani, A.B.; Sin, N.D.M.; Hamid, S.B.A.; Rusop, M. Sonicated Sol-Gel Preparation of Nanoparticulate ZnO Thin Films with Various Deposition Speeds: The Highly Preferred c-Axis (002) Orientation Enhances the Final Properties. J. Alloys Compd. 2014, 582, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Aydil, E.S. Heteroepitaxial Growth of Cu2O Thin Film on ZnO by Metal Organic Chemical Vapor Deposition. J. Cryst. Growth 2009, 311, 4188–4192. [Google Scholar] [CrossRef]
- Khan, M.I.; Bhatti, K.A.; Qindeel, R.; Bousiakou, L.G.; Alonizan, N. Fazal-e-Aleem Investigations of the Structural, Morphological and Electrical Properties of Multilayer ZnO/TiO2 Thin Films, Deposited by Sol-Gel Technique. Results Phys. 2016, 6, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Arifin, N.M.; Mohamad, F.; Hussin, R.; Ismail, A.Z.M.; Ramli, S.A.; Ahmad, N.; Nor, N.H.M.; Sahdan, M.Z.; Zain, M.Z.M.; Izaki, M. Development of Homogenous N-TiO2/ZnO Bilayer/p-Cu2O Heterostructure Thin Film. J. Sol-Gel Sci. Technol. 2021, 100, 224–231. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, F.; Zhao, N.; Yang, P. Influence of Annealing Temperature on Optical Properties of Sandwiched ZnO/Metal/ZnO Transparent Conductive Thin Films. Micromachines 2022, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Lahmar, H.; Seti, F.; Azizi, A.; Schmerber, G.; Dinia, A. On the Electrochemical Synthesis and Characterization of p-Cu2O/n-ZnO Heterojunction. J. Alloys Compd. 2017, 718, 36–45. [Google Scholar] [CrossRef]
- Khan, M.I.; Imran, S.; Shahnawaz; Saleem, M.; Ur Rehman, S. Annealing Effect on the Structural, Morphological and Electrical Properties of TiO2/ZnO Bilayer Thin Films. Results Phys. 2018, 8, 249–252. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kim, S.W.; Yoo, D.H.; Kim, E.J.; Hahn, S.H. Annealing Effect of ZnO Seed Layer on Enhancing Photocatalytic Activity of ZnO/TiO2 Nanostructure. Int. J. Photoenergy 2013, 2013, 130541. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Alagarasan, D.; Varadharajaperumal, S.; Ganesan, R.; Naik, R. Tuning the Nonlinear Susceptibility and Linear Parameters upon Annealing Ag60−xSe40Tex Nanostructured Films for Nonlinear and Photonic Applications. Mater. Adv. 2022, 3, 7640–7654. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Lai, J.D.; Feng, S.W.; Huang, C.J.; Chen, C.H.; Yang, F.W.; Wang, H.C.; Tu, L.W. Growth and Characterization of Textured Well-Faceted ZnO on Planar Si(100), Planar Si(111), and Textured Si(100) Substrates for Solar Cell Applications. Beilstein J. Nanotechnol. 2017, 8, 1939–1945. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.; Arifin, N.M.; Mohamad, F.; Sheng, L.Y.; Ismail, A.Z.; Ahmad, N.; Hisyamudin, N.; Nor, M.; Izaki, M. Construction of Nanorod-TiO2/p-Cu2O Heterostructure Thin Films for Solar Cell Application. Int. J. Adv. Trends Comput. Sci. Eng. 2020, 9, 304–310. [Google Scholar]
- Mohamad, F.; Arifin, N.M.; Ismail, A.Z.M.; Ahmad, N.; Hisyamudin, M.N.N.; Izaki, M. Cu2O-Based Homostructure Fabricated by Electrodeposition Method. Acta Phys. Pol. A 2019, 135, 911–914. [Google Scholar] [CrossRef]
- Wijesundera, R.P.; Gunawardhana, L.K.A.D.D.S.; Siripala, W. Solar Energy Materials & Solar Cells Electrodeposited Cu2O Homojunction Solar Cells: Fabrication of a Cell of High Short Circuit Photocurrent. Sol. Energy Mater. Sol. Cells 2016, 157, 881–886. [Google Scholar] [CrossRef]
- Septina, W.; Ikeda, S.; Khan, M.A.; Hirai, T.; Harada, T.; Matsumura, M.; Peter, L.M. Potentiostatic Electrodeposition of Cuprous Oxide Thin Films for Photovoltaic Applications. Electrochim. Acta 2011, 56, 4882–4888. [Google Scholar] [CrossRef]
- Ghrib, T.; AL-Saleem, N.K.; AL-Naghmaish, A.; Elshekhipy, A.A.; Brini, S.; Briki, K.; Elsayed, K.A. Annealing Effect on the Microstructural, Optical, Electrical, and Thermal Properties of Cu2O/TiO2/Cu2O/TiO2/Si Heterojunction Prepared by Sol-Gel Technique. Superlattices Microstruct. 2021, 107119. [Google Scholar] [CrossRef]
- Özdal, T.; Kavak, H. Fabrication and Characterization of ZnO/Cu2O Heterostructures for Solar Cells Applications. Superlattices Microstruct. 2020, 146, 106679. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arifin, N.M.; Mohamad, F.; Hussin, R.; Ismail, A.Z.M.; Ramli, S.A.; Ahmad, N.; Nor, N.H.M.; Sahdan, M.Z.; Zain, M.Z.M.; Izaki, M. Annealing Treatment on Homogenous n-TiO2/ZnO Bilayer Thin Film Deposition as Window Layer for p-Cu2O-Based Heterostructure Thin Film. Coatings 2023, 13, 206. https://doi.org/10.3390/coatings13010206
Arifin NM, Mohamad F, Hussin R, Ismail AZM, Ramli SA, Ahmad N, Nor NHM, Sahdan MZ, Zain MZM, Izaki M. Annealing Treatment on Homogenous n-TiO2/ZnO Bilayer Thin Film Deposition as Window Layer for p-Cu2O-Based Heterostructure Thin Film. Coatings. 2023; 13(1):206. https://doi.org/10.3390/coatings13010206
Chicago/Turabian StyleArifin, Nurliyana Mohamad, Fariza Mohamad, Rosniza Hussin, Anis Zafirah Mohd Ismail, Shazleen Ahmad Ramli, Norazlina Ahmad, Nik Hisyamudin Muhd Nor, Mohd Zainizan Sahdan, Mohd Zamzuri Mohammad Zain, and Masanobu Izaki. 2023. "Annealing Treatment on Homogenous n-TiO2/ZnO Bilayer Thin Film Deposition as Window Layer for p-Cu2O-Based Heterostructure Thin Film" Coatings 13, no. 1: 206. https://doi.org/10.3390/coatings13010206
APA StyleArifin, N. M., Mohamad, F., Hussin, R., Ismail, A. Z. M., Ramli, S. A., Ahmad, N., Nor, N. H. M., Sahdan, M. Z., Zain, M. Z. M., & Izaki, M. (2023). Annealing Treatment on Homogenous n-TiO2/ZnO Bilayer Thin Film Deposition as Window Layer for p-Cu2O-Based Heterostructure Thin Film. Coatings, 13(1), 206. https://doi.org/10.3390/coatings13010206