Evaluating the Electrochemical and In Vitro Degradation of an HA-Titania Nano-Channeled Coating for Effective Corrosion Resistance of Biodegradable Mg Alloy
Abstract
:1. Introduction
2. Material and Methods
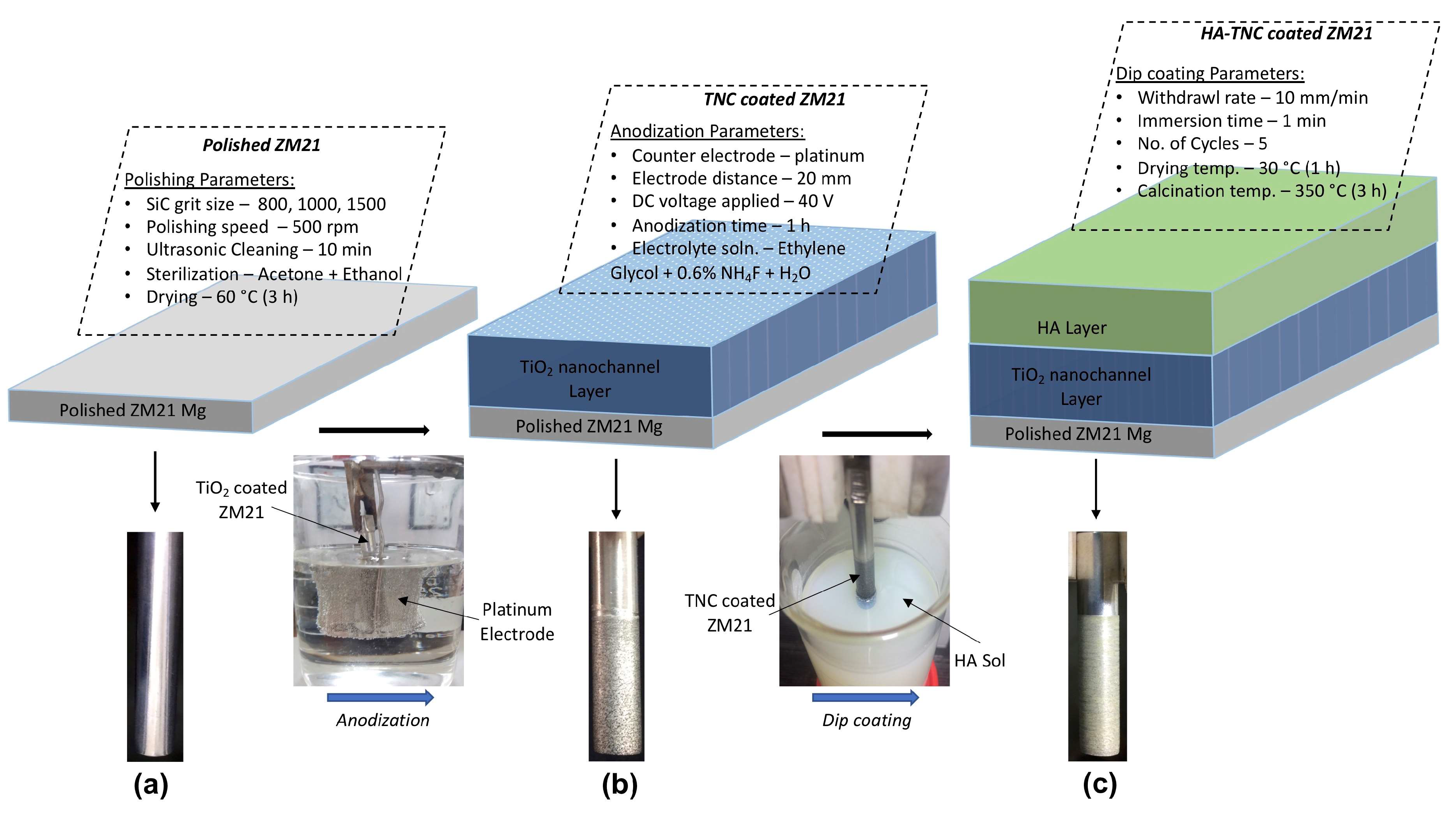
2.1. Substrate Preparation
2.2. Coatings Development
2.2.1. Development of TiO2 Nano-Channeled (TNC) Coating
2.2.2. Fabrication of Hydroxyapatite—TiO2 Nano-Channeled (HA−TNC) Coating
2.3. Analysis of the Surface Morphology
2.4. Electrochemical Corrosion Measurements
2.5. In Vitro Degradation Behavior
3. Results and Discussion
3.1. Analysis of Surface Morphology
3.2. Electrochemical Measurements
3.3. In Vitro Degradation Behavior
3.4. In Vitro Apatite Mineralization
4. Conclusions
- HA-TNC coatings with a HA layer at the top and TiO2 nano-channeled layer at the bottom were successfully developed on ZM21 Mg alloy by combining sol-gel dip coating and anodizing techniques.
- The polarization resistance (Rp) of 5.11 × 104 Ω·cm2 provided by the HA-TNC coatings was 26 and 82 times better than TNC-coated ZM21 and ZM21 Mg alloy, respectively. Therefore, the corrosion rate produced was 8.5 and 53.6 times lower compared to TNC-coated ZM21 and ZM21 Mg alloy.
- During in vitro immersion for up to 28 days in SBF, the HA-TNC coating maintained the lowest recorded degradation rate and hydrogen evolution rate (HER) of 1.10 ± 0.22 mg/cm2/day and 1.83 ± 0.41 mL/cm2/day, respectively. In contrast, the TNC coating and the polished ZM21 Mg failed to maintain such good corrosion resistance.
- A compact and structurally stable 2D plate-like HA (Ca/P:1.55), mineralized on HA-TNC-coated ZM21 samples, provided effective shielding against the penetration of aggressive ions with prolonged SBF immersion. Comparatively, the shielding ability of apatite mineralized on TNC-coated ZM21 samples was compromised by poor Ca/P ratios, due to excessive Mg substitution caused by Mg2+ ions leached from the ZM21 substrate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Fernández, M.J.; Hammoudeh, H.; Félix Lanao, R.P.; van Erk, M.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Bone-Adhesive Materials: Clinical Requirements, Mechanisms of Action, and Future Perspective. Adv. Mater. Interfaces 2019, 6, 1802021. [Google Scholar] [CrossRef]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility Fractures in Europe: Burden, Management and Opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Singh, N.; Batra, U.; Kumar, K.; Ahuja, N.; Mahapatro, A. Progress in Bioactive Surface Coatings on Biodegradable Mg Alloys: A Critical Review towards Clinical Translation. Bioact. Mater. 2023, 19, 717–757. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium-based Biomaterials: An Overview of Challenges and Opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium Degradation under Physiological Conditions—Best Practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Sekar, P.; Narendranath, S.; Desai, V. Recent Progress in in Vivo Studies and Clinical Applications of Magnesium Based Biodegradable Implants—A Review. J. Magnes. Alloys 2021, 9, 1147–1163. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in Functionalized Polymer Coatings on Biodegradable Magnesium Alloys—A Review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Singh, N.; Batra, U.; Kumar, K.; Mahapatro, A. Investigating TiO2–HA–PCL Hybrid Coating as an Efficient Corrosion Resistant Barrier of ZM21 Mg Alloy. J. Magnes. Alloys 2021, 9, 627–646. [Google Scholar] [CrossRef]
- Ali, M.; Elsherif, M.; Salih, A.E.; Ul-Hamid, A.; Hussein, M.A.; Park, S.; Yetisen, A.K.; Butt, H. Surface Modification and Cytotoxicity of Mg-Based Bio-Alloys: An Overview of Recent Advances. J. Alloys Compd. 2020, 825, 154140. [Google Scholar] [CrossRef]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Gunputh, U.F.; Le, H. A Review of In-Situ Grown Nanocomposite Coatings for Titanium Alloy Implants. J. Compos. Sci. 2020, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-Dimensional Titanium Dioxide Nanomaterials: Nanowires, Nanorods, and Nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium Alloys With Tunable Interfaces as Bone Implant Materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Batra, U.; Kumar, K.; Mahapatro, A. Evaluation of Corrosion Resistance, Mechanical Integrity Loss and Biocompatibility of PCL/HA/TiO2 Hybrid Coated Biodegradable ZM21 Mg Alloy. J. Magnes. Alloys 2021, 10, 3179–3204. [Google Scholar] [CrossRef]
- Mori, S.; Lamastra, F.R.; Kaciulis, S.; Soltani, P.; Montesperelli, G. Low-Temperature Titania Coatings for Aluminium Corrosion Protection. Corros. Eng. Sci. Technol. 2018, 53, 44–53. [Google Scholar] [CrossRef]
- Tamjid, E.; Bagheri, R.; Vossoughi, M.; Simchi, A. Effect of TiO2 Morphology on in Vitro Bioactivity of Polycaprolactone/TiO2 Nanocomposites. Mater. Lett. 2011, 65, 2530–2533. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Porous Structure of Bioceramics Carbonated Hydroxyapatite-Based Honeycomb Scaffold for Bone Tissue Engineering. Mater. Today Commun. 2021, 26, 102135. [Google Scholar] [CrossRef]
- Azari, R.; Rezaie, H.R.; Khavandi, A. Investigation of Functionally Graded HA-TiO2 Coating on Ti–6Al–4V Substrate Fabricated by Sol-Gel Method; Techna Group S.r.l.: Forlì, Italy, 2019; Volume 45, ISBN 9821772404. [Google Scholar]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V Implant Surfaces by Biocompatible TiO2/PCL Hybrid Layers Prepared via Sol-Gel Dip Coating: Structural Characterization, Mechanical and Corrosion Behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Characterization and Biological Properties of TiO2/PCL Hybrid Layers Prepared via Sol-Gel Dip Coating for Surface Modification of Titanium Implants. J. Non-Cryst. Solids 2015, 415, 9–15. [Google Scholar] [CrossRef]
- Ji, X.J.; Gao, L.; Liu, J.C.; Wang, J.; Cheng, Q.; Li, J.P.; Li, S.Q.; Zhi, K.Q.; Zeng, R.C.; Wang, Z.L. Corrosion Resistance and Antibacterial Properties of Hydroxyapatite Coating Induced by Gentamicin-Loaded Polymeric Multilayers on Magnesium Alloys. Colloids Surf. B Biointerfaces 2019, 179, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Gusmano, G. Thermalstability and Sintering Behaviour of Hydroxyapatite Nanopowders. J. Therm. Anal. Calorim. 2007, 88, 237–243. [Google Scholar] [CrossRef]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium Incorporation into Hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Abdul Kadir, M.R. Deposition of Nanostructured Fluorine-Doped Hydroxyapatite-Polycaprolactone Duplex Coating to Enhance the Mechanical Properties and Corrosion Resistance of Mg Alloy for Biomedical Applications. Mater. Sci. Eng. C 2016, 60, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 145–211. ISBN 9783319124605. [Google Scholar]
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Corrosion Behavior of Commercially Pure Mg and ZM21 Mg Alloy in Ringer’s Solution—Long Term Evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Kumar, K.; Gill, R.S.; Batra, U. Challenges and Opportunities for Biodegradable Magnesium Alloy Implants. Mater. Technol. 2018, 33, 153–172. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wen, C.; Wright, P.F.A.; Li, Y. Mechanical, Corrosion, and Biocompatibility Properties of Mg-Zr-Sr-Sc Alloys for Biodegradable Implant Applications. Acta Biomater. 2020, 102, 493–507. [Google Scholar] [CrossRef]
- Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Jia, S.; Qiao, L.; Su, Y.; Gu, R.; Li, G.; Lian, J. Enhanced Corrosion Resistance and Biocompatibility of Polydopamine/Dicalcium Phosphate Dihydrate/Collagen Composite Coating on Magnesium Alloy for Orthopedic Applications. J. Alloys Compd. 2020, 817, 152782. [Google Scholar] [CrossRef]
- Sofronia, A.M.; Baies, R.; Anghel, E.M.; Marinescu, C.A.; Tanasescu, S. Thermal and Structural Characterization of Synthetic and Natural Nanocrystalline Hydroxyapatite. Mater. Sci. Eng. C 2014, 43, 153–163. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A.; Lombardi, M.; Montanaro, L. Mg-Substituted Hydroxyapatite Nanopowders: Synthesis, Thermal Stability and Sintering Behaviour. J. Eur. Ceram. Soc. 2009, 29, 2969–2978. [Google Scholar] [CrossRef]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Magnesium Incorporated Hydroxyapatite: Synthesis and Structural Properties Characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef] [Green Version]
- Shaltout, A.A.; Allam, M.A.; Moharram, M.A. FTIR Spectroscopic, Thermal and XRD Characterization of Hydroxyapatite from New Natural Sources. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Preparation and Characterisation of Nanophase Sr, Mg, and Zn Substituted Hydroxyapatite by Aqueous Precipitation. Mater. Sci. Eng. C 2014, 35, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Ding, Y.; Leng, Y. Infrared Spectroscopic Characterization of Carbonated Apatite: A Combined Experimental and Computational Study. J. Biomed. Mater. Res.-Part A 2014, 102, 496–505. [Google Scholar] [CrossRef]
- Batra, U.; Kapoor, S.; Sharma, S. Influence of Magnesium Ion Substitution on Structural and Thermal Behavior of Nanodimensional Hydroxyapatite. J. Mater. Eng. Perform. 2013, 22, 1798–1806. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; Tenhuisen, K.S. Preparation of Magnesium-Substituted Hydroxyapatite Powders by the Mechanochemical-Hydrothermal Method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef]
- Kanasan, N.; Adzila, S.; Rahman, H.A.; Bano, N.; Panerselvan, G.; Hidayati, N.A. FTIR and XRD Evaluation of Magnesium Doped Hydroxyapatite/Sodium Alginate Powder by Precipitation Method. Key Eng. Mater. 2018, 791, 45–49. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J.; Zhu, Y.; Wu, W.; Cheng, G.; Zeng, Y.; Ruan, M. A Facile One-Step Surfactant-Free and Low-Temperature Hydrothermal Method to Prepare Uniform 3D Structured Carbonated Apatite Flowers. Cryst. Growth Des. 2009, 9, 177–181. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Lin, K.; Wu, C.; Chang, J. Advances in Synthesis of Calcium Phosphate Crystals with Controlled Size and Shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [CrossRef] [PubMed]










| Sample | Ecorr (V) | Icorr (A/cm²) | Polarization Resistance, Rp (Ω·cm2) | Corrosion Rate (mm/Year) |
|---|---|---|---|---|
| HA−TNC−coated ZM21 | −0.61 | 5.8 × 10−6 | 5.11 × 104 | 1.32 |
| TNC−coated ZM21 | −1.19 | 4.9 × 10−5 | 1.96 × 103 | 11.19 |
| ZM21 | −1.35 | 3.1 × 10−4 | 6.20 × 102 | 70.83 |
| Days | Sample | Vh (mL/cm2/Day) | W (mg/cm2/Day) | pH Change |
|---|---|---|---|---|
| 7 | HA-TNC-coated ZM21 | 1.41 ± 0.29 | 0.89 ± 0.16 | 0.25 ± 0.1 |
| TNC-coated ZM21 | 1.98 ± 0.24 | 1.24 ± 0.14 | 0.66 ± 0.1 | |
| ZM21 Mg | 2.90 ± 0.32 | 2.49 ± 0.31 | 1.28 ± 0.1 | |
| 14 | HA-TNC-coated ZM21 | 1.39 ± 0.13 | 0.54 ± 0.21 | 0.16 ± 0.1 |
| TNC-coated ZM21 | 1.76 ± 0.11 | 0.97 ± 0.15 | 0.45 ± 0.1 | |
| ZM21 Mg | 2.71 ± 0.33 | 2.34 ± 0.14 | 1.21 ± 0.2 | |
| 21 | HA-TNC-coated ZM21 | 1.68 ± 0.34 | 0.67 ± 0.15 | 0.28 ± 0.2 |
| TNC-coated ZM21 | 2.24 ± 0.26 | 1.33 ± 0.2 | 0.61 ± 0.1 | |
| ZM21 Mg | 3.12 ± 0.40 | 3.07 ± 0.35 | 1.41 ± 0.1 | |
| 28 | HA-TNC-coated ZM21 | 1.83 ± 0.41 | 1.10 ± 0.22 | 0.49 ± 0.1 |
| TNC-coated ZM21 | 2.82 ± 0.32 | 2.40 ± 0.25 | 1.91 ± 0.2 | |
| ZM21 Mg | 3.98 ± 0.34 | 3.84 ± 0.38 | 2.35 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Batra, U.; Kumar, K.; Siddiquee, A.N. Evaluating the Electrochemical and In Vitro Degradation of an HA-Titania Nano-Channeled Coating for Effective Corrosion Resistance of Biodegradable Mg Alloy. Coatings 2023, 13, 30. https://doi.org/10.3390/coatings13010030
Singh N, Batra U, Kumar K, Siddiquee AN. Evaluating the Electrochemical and In Vitro Degradation of an HA-Titania Nano-Channeled Coating for Effective Corrosion Resistance of Biodegradable Mg Alloy. Coatings. 2023; 13(1):30. https://doi.org/10.3390/coatings13010030
Chicago/Turabian StyleSingh, Navdeep, Uma Batra, Kamal Kumar, and Arshad Noor Siddiquee. 2023. "Evaluating the Electrochemical and In Vitro Degradation of an HA-Titania Nano-Channeled Coating for Effective Corrosion Resistance of Biodegradable Mg Alloy" Coatings 13, no. 1: 30. https://doi.org/10.3390/coatings13010030





