C3A Cells-Inoculated Affinity Membrane for Bilirubin Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation and Modification
2.3. Characterization of Membranes
2.4. Permeability of Membranes
2.5. Blood Coagulation Time Tests
2.6. Hemolysis
2.7. Cell Viability
2.7.1. MTT
2.7.2. C3A Cells Inoculation
2.8. Bilirubin Adsorption Experiments
2.9. Determination of Albumin and Urea
2.10. Bilirubin Clearance
3. Results and Discussion
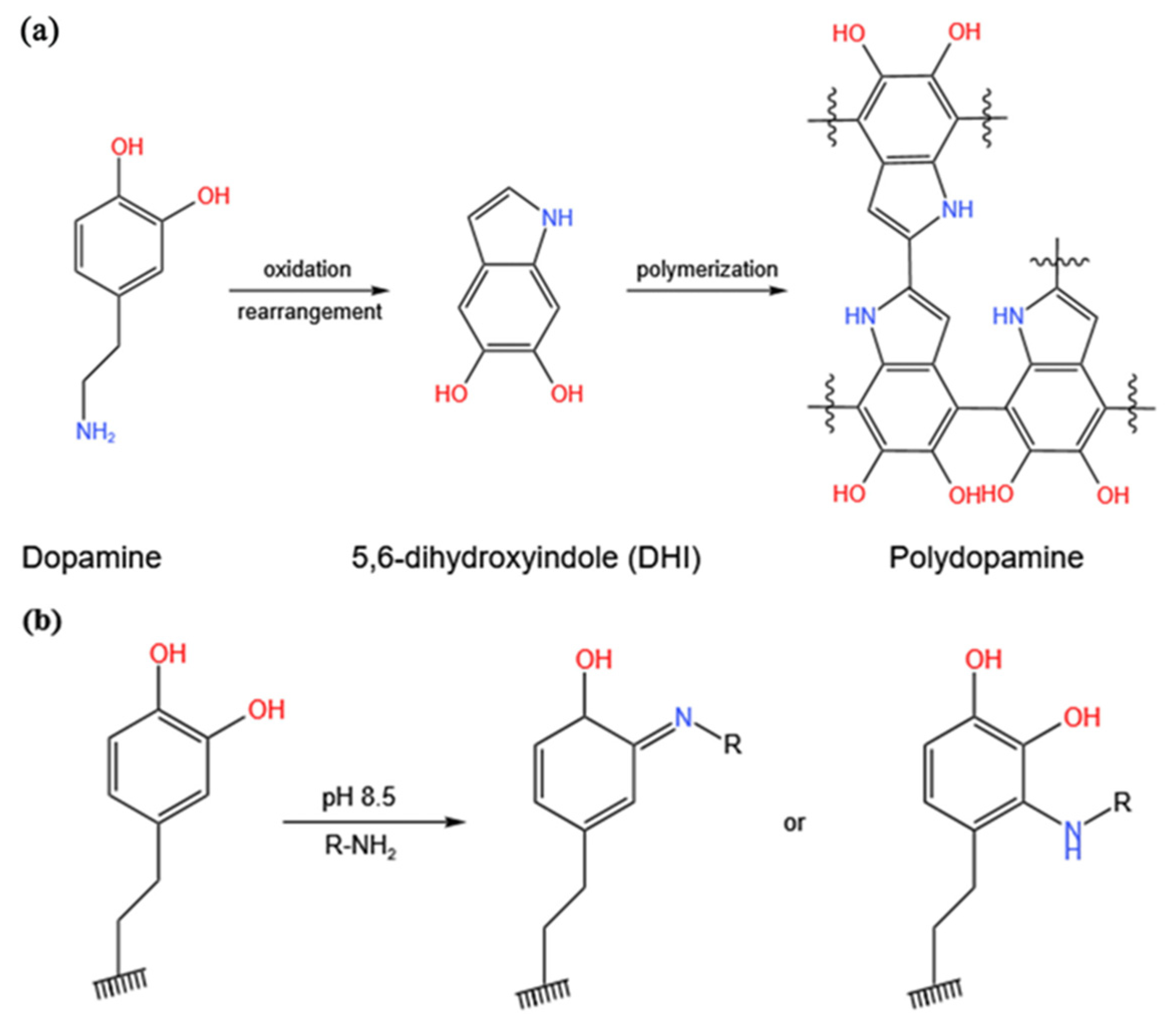
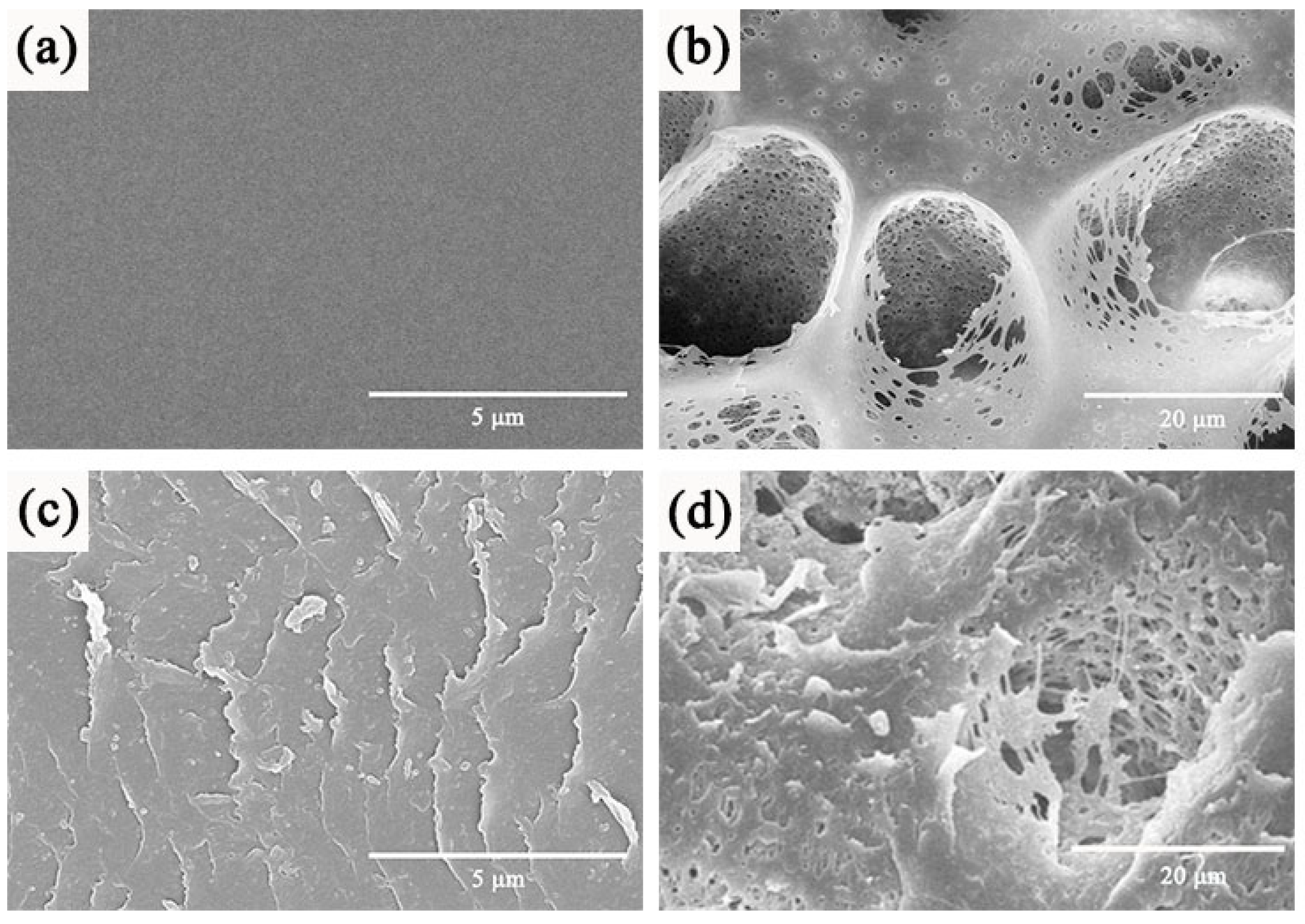
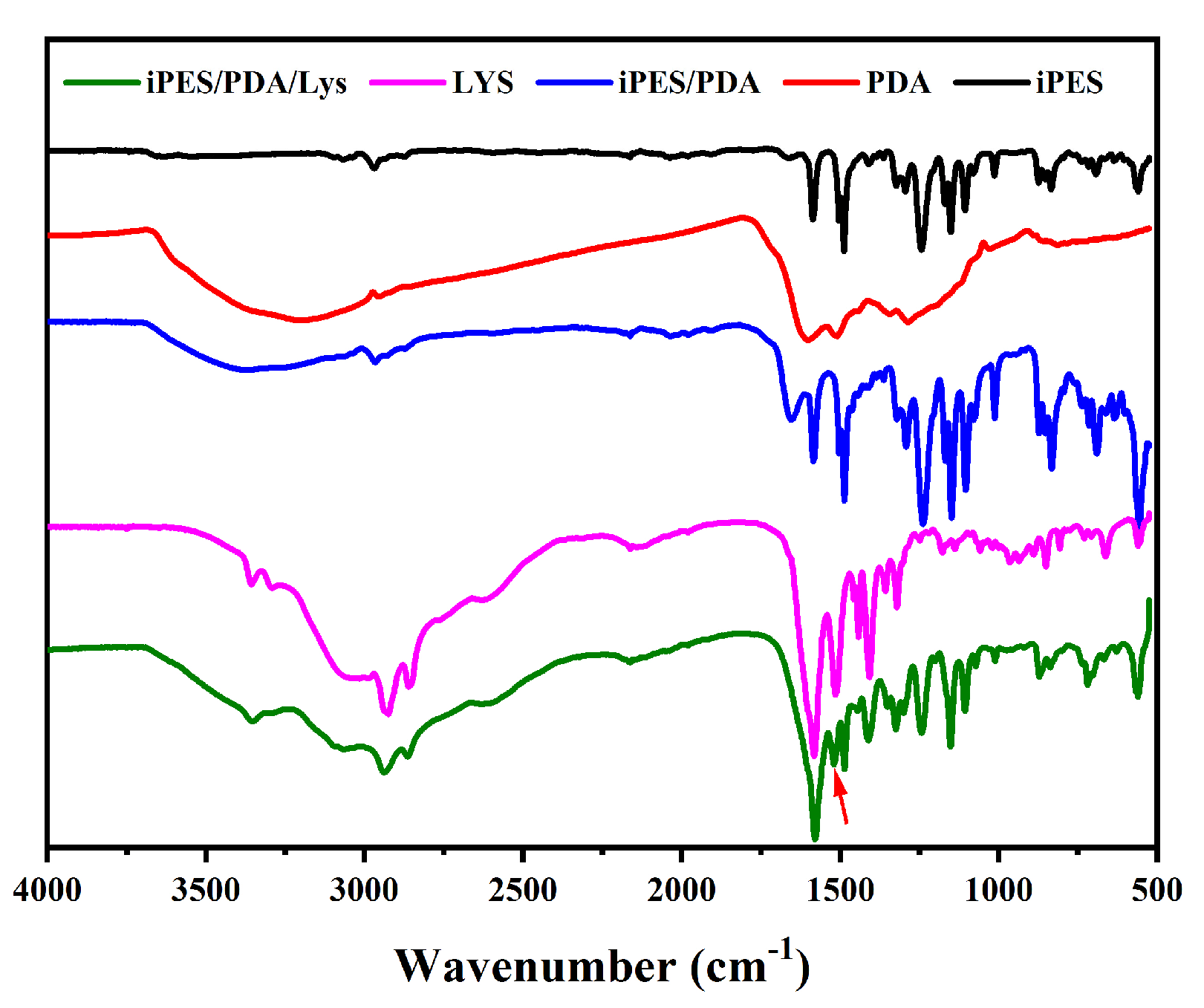
3.1. Characterization
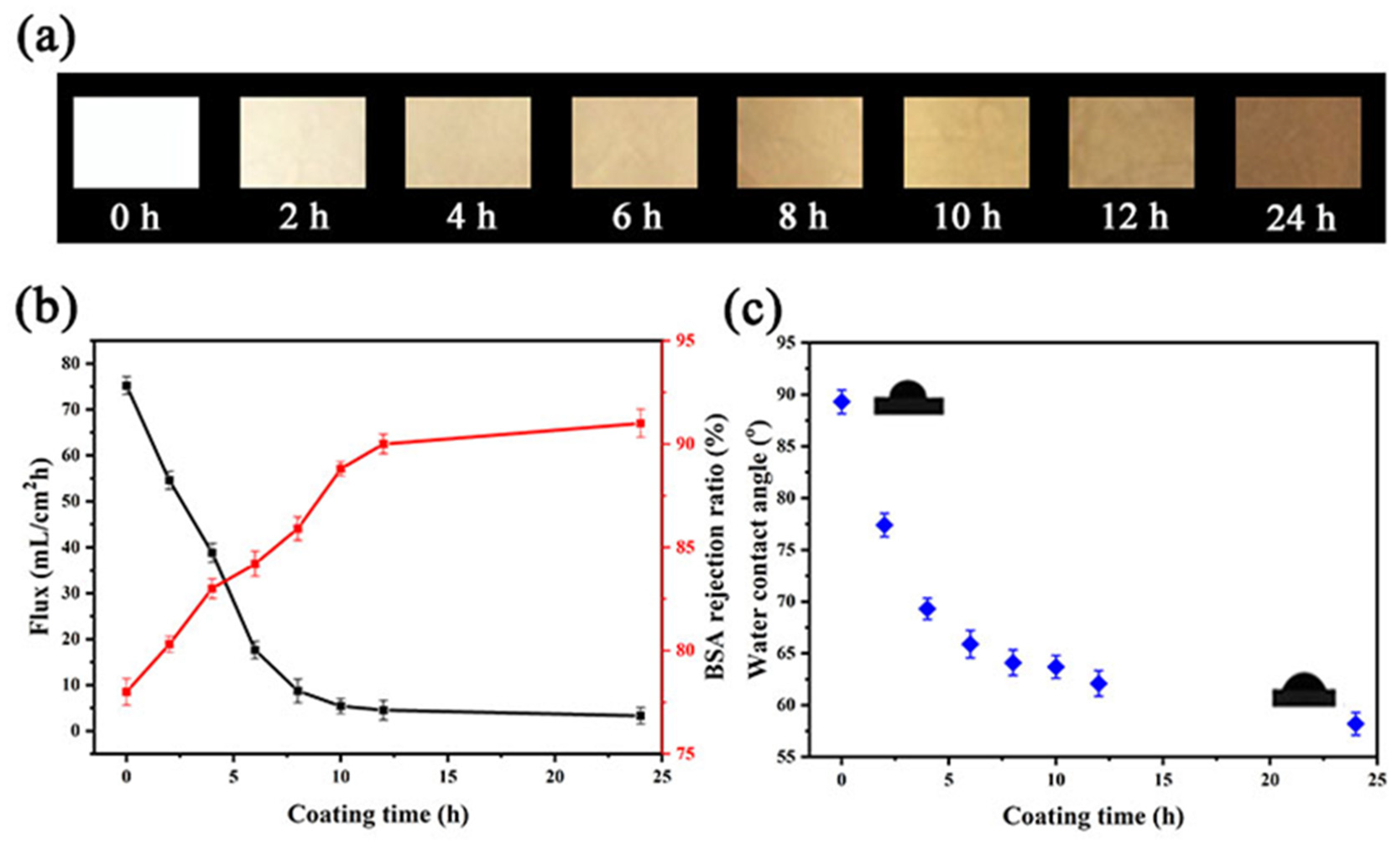
3.2. Membrane Performance
3.3. Hemocompatibility Studies
3.3.1. Blood Contact Angle
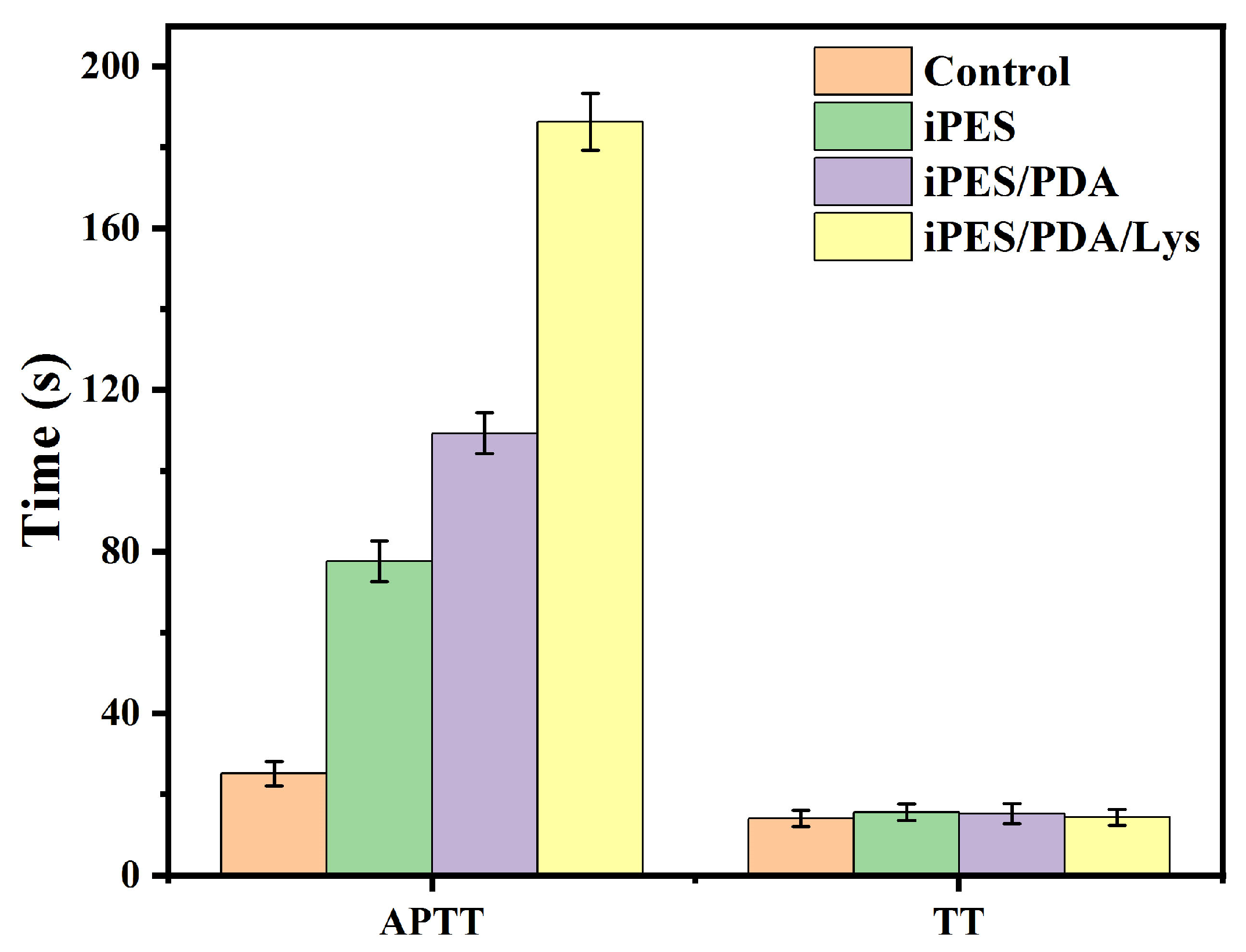
3.3.2. Blood Coagulation
3.3.3. Hemolysis
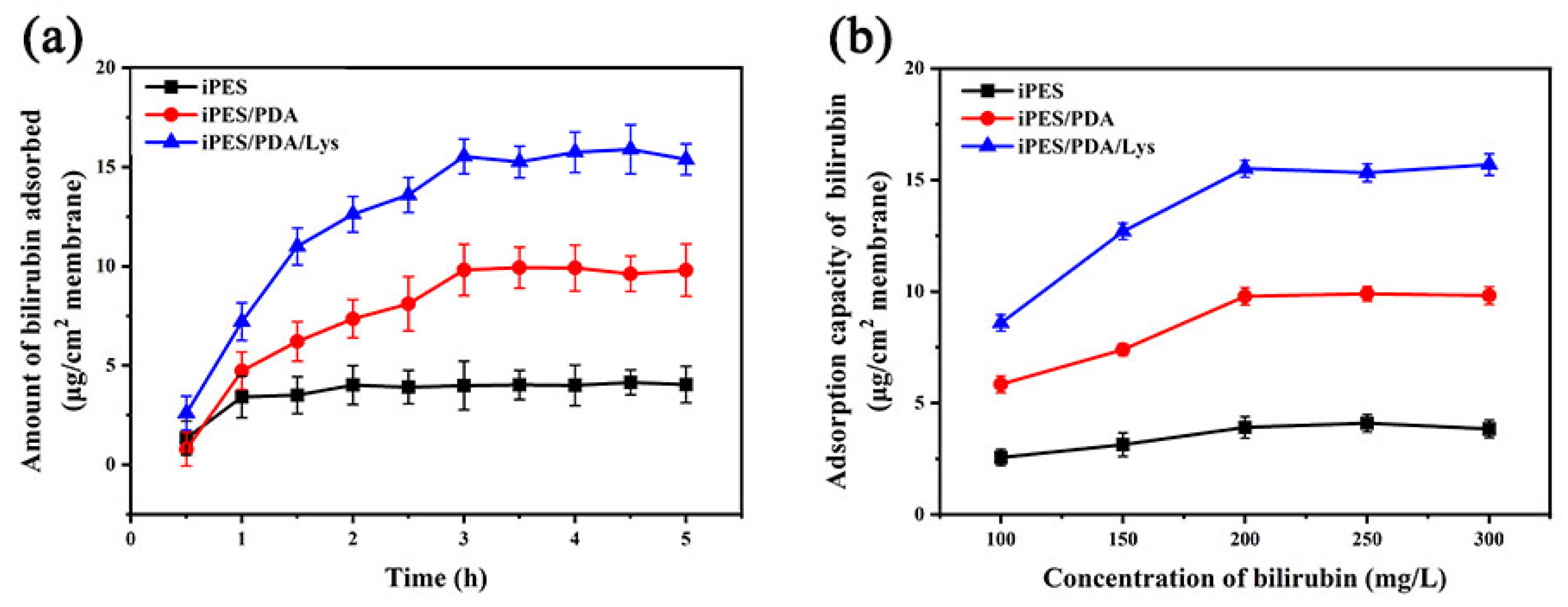
3.4. Adsorption of Bilirubin
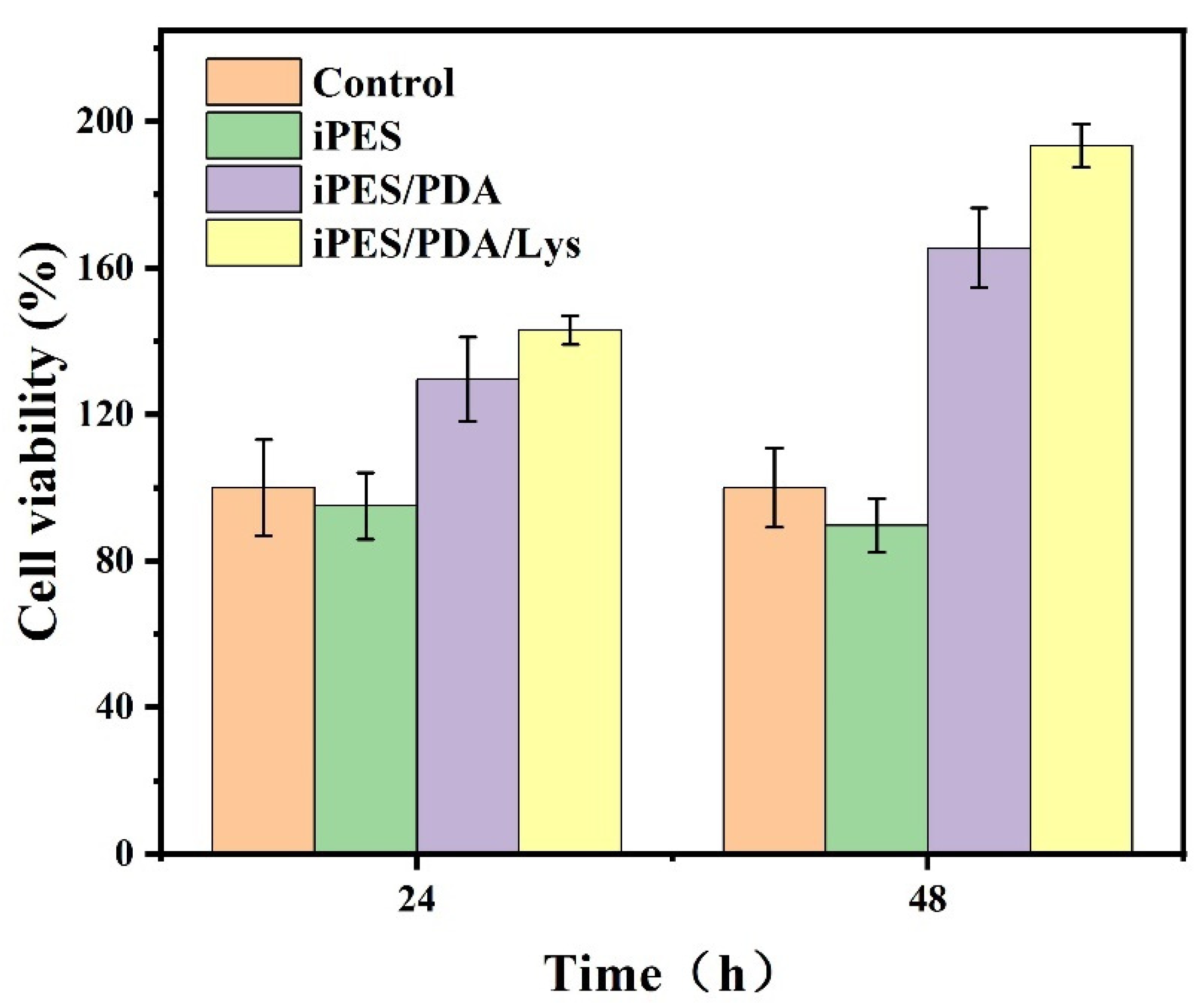
3.5. In Vitro Cytotoxicity
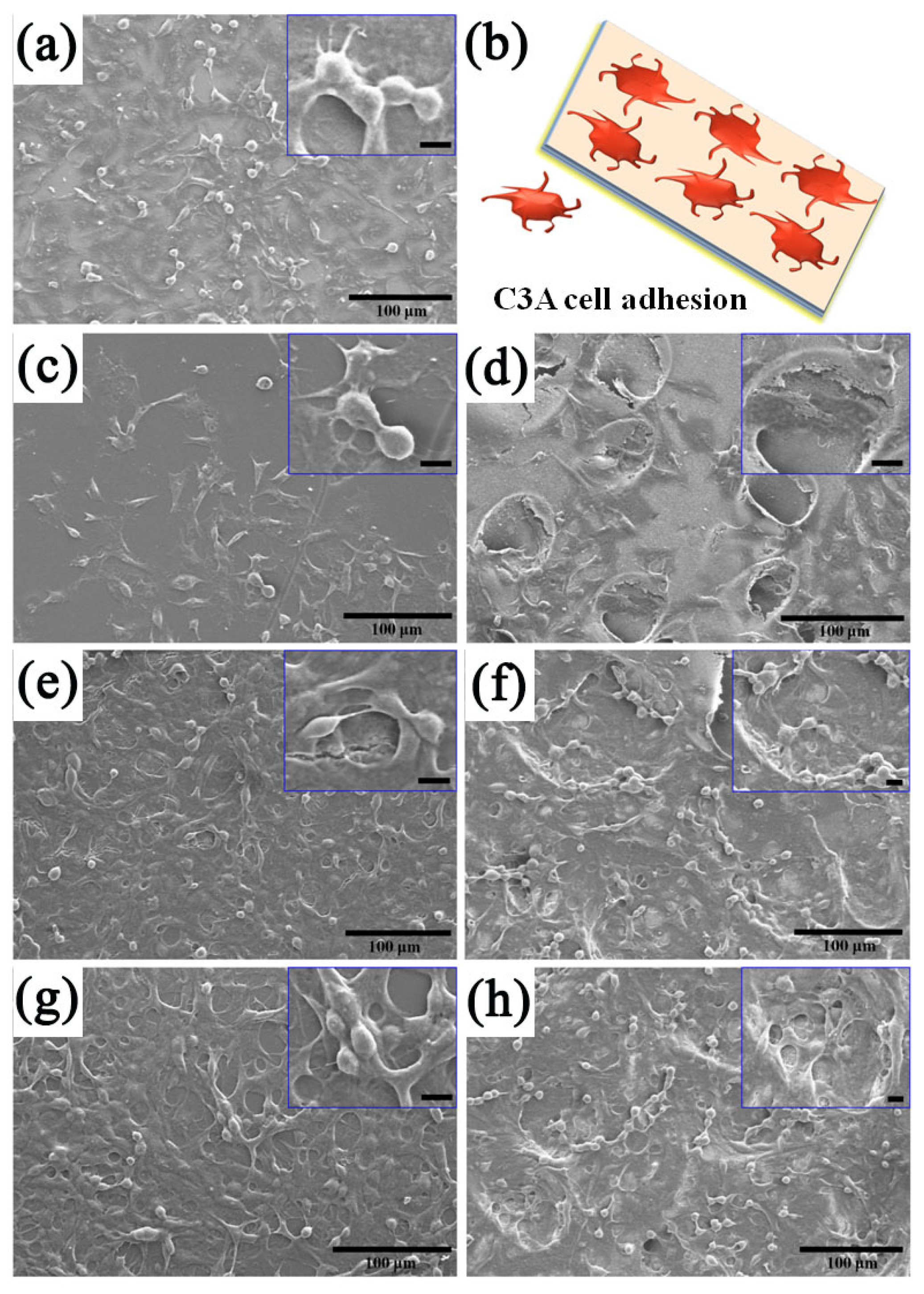
3.6. Cell Functionality and Bilirubin Clearance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, H.; Luo, Q.; Zhou, D. Affinity membrane chromatography for the analysis and purification of proteins. J. Biochem. Biophys. Methods 2001, 49, 199–240. [Google Scholar] [CrossRef]
- Xue, M.Q.; Ling, Y.S.; Wu, G.S.; Liu, X.; Ge, D.T.; Shi, W. Surface-modified anodic aluminum oxide membrane with hydroxyethyl celluloses as a matrix for bilirubin removal. J. Chromatogr. B 2013, 912, 1–7. [Google Scholar] [CrossRef]
- Charcosset, C. Membrane Processes in biotechnology: An overview. Biotechnol. Adv. 2006, 24, 482–492. [Google Scholar] [CrossRef]
- Shi, W.; Cao, H.-H.; Song, C.F.; Jiang, H.R.; Wang, J.; Jiang, S.H.; Tu, J.; Ge, D.T. Poly(pyrrole-3-carboxylic acid)-alumina composite membrane for affinity adsorption of bilirubin. J. Membr. Sci. 2010, 353, 151–158. [Google Scholar] [CrossRef]
- Anand, A.; Mallick, S.P.; Singh, B.N.; Kumari, S.; Suman, D.K.; Tripathi, S.; Singh, D.; Srivastava, P. A critical aspect of bioreactor designing and its application for the generation of tissue engineered construct: Emphasis on clinical translation of bioreactor. Biotechnol. Bioprocess Eng. 2022, 27, 494–514. [Google Scholar] [CrossRef]
- Tuerxun, K.; He, J.Y.; Ibrahim, I.; Yusupu, Z.; Yasheng, A.; Xu, Q.L.; Tang, R.H.; Aikebaier, A.; Wu, Y.Q.; Tuerdi, M. Bioartificial livers: A review of their design and manufacture. Biofabrication 2022, 14, 032003. [Google Scholar] [CrossRef]
- Wang, J.L.; Ren, H.Z.; Liu, Y.X.; Sun, L.Y.; Zhang, Z.H.; Zhao, Y.J.; Shi, X.L. Bioinspired artificial liver system with hiPSC-derived hepatocytes for acute liver failure treatment. Adv. Healthc. Mater. 2021, 10, 2101580. [Google Scholar] [CrossRef]
- Soto-Gutierrez, A.; Kobayashi, N.; Rivas-Carrillo, J.D.; Navarro-Alvarez, N.; Zhao, D.B.; Okitsu, T.; Noguchi, H.; Basma, H.; Tabata, Y.; Chen, Y.; et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat. Biotechnol. 2006, 24, 1412–1419. [Google Scholar] [CrossRef] [Green Version]
- Kulig, K.M.; Vacanti, J.P. Hepatic tissue engineering. Transpl. Immunol. 2004, 12, 303–310. [Google Scholar] [CrossRef]
- Maringka, M.; Giri, S.; Bader, A. Preclinical characterization of primary porcine hepatocytes in a clinically relevant flat membrane bioreactor. Biomaterials 2010, 31, 156–172. [Google Scholar] [CrossRef]
- Kinasiewicz, A.; Smietanka, A.; Dudzinski, K.; Chwojnowski, A.; Gajkowska, B.; Werynski, A. Spongy polyethersulfone membrane for hepatocyte cultivation: Studies on human hepatoma C3A cells. Artif. Organs 2008, 32, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, X.L.; Han, B.; Gu, J.Y.; Chu, X.H.; Xiao, J.Q.; Ren, H.Z.; Tan, J.J.; Ding, Y.T. The influence of membrane molecular weight cutoff on a novel bioartificial liver. Artif. Organs 2012, 36, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.B.; Shin, Y.M.; Lee, J.H.; Jun, I.D.; Kang, J.K.; Park, J.C.; Shin, H. Polydopamine-mediated immobilization of multiple bioactive molecules for the development of functional vascular graft materials. Biomaterials 2012, 33, 8343–8352. [Google Scholar] [CrossRef]
- Yang, K.; Lee, J.S.; Kim, J.; Lee, Y.B.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dai, L.F.; Zhang, X.M.; Sun, Y.N.; Shi, W.; Ge, D.T. The bioactive polypyrrole/polydopamine nanowire coating with enhanced osteogenic differentiation ability with electrical stimulation. Coatings 2020, 10, 1189. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, J.Y.; Zhou, H.; Liu, Q.; Jia, L.; Zhang, X.M.; Ge, D.T.; Shi, W.; Sun, Y.N. Polydopamine-based nanocomposite as a biomimetic antioxidant with a variety of enzymatic activities for Parkinson’s disease. ACS Appl. Mater. Interfaces 2022, 14, 32901–32913. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ai, K.L.; Liu, J.H.; Deng, M.; He, Y.Y.; Lu, L.H. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Z.; Shi, W.; He, Z.; Liu, Y.; Su, H.L.; Sun, Y.N.; Ge, D.T. Polynorepinephrine nanoparticles: A novel photothermal nanoagent for chemo-photothermal cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 19763–19773. [Google Scholar] [CrossRef]
- Chen, F.H.; Zhang, X.C.; Wang, Z.N.; Xu, C.S.; Hu, J.Z.; Liu, L.; Zhou, J.C.; Sun, B.W. Dual-responsive and NIR-driven free radical nanoamplifier with glutathione depletion for enhanced tumor-specific photothermal/thermodynamic/chemodynamic synergistic therapy. Biomater. Sci. 2022, 10, 5912–5924. [Google Scholar] [CrossRef]
- Ruan, C.Q.; Shi, W.; Jiang, H.R.; Sun, Y.N.; Liu, X.; Ge, D.T. One-pot preparation of glucose biosensor based on polydopamine-graphene composite film modified enzyme electrode. Sens. Actuators B Chem. 2013, 177, 826–832. [Google Scholar] [CrossRef]
- Li, Y.X.; Luo, L.X.; Nie, M.Y.; Davenport, A.; Li, Y.; Li, B.; Choy, K.L. A graphene nanoplatelet-polydopamine molecularly imprinted biosensor for ultratrace creatinine detection. Biosens. Bioelectron. 2022, 216, 114638. [Google Scholar] [CrossRef] [PubMed]
- Jaime, J.; Rangel, G.; Munoz-Bonilla, A.; Mayoral, A.; Herrasti, P. Magnetite as a platform material in the detection of glucose, ethanol and cholesterol. Sens. Actuators B Chem. 2017, 238, 693–701. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, F.B.; Zhang, G.L.; Jiang, L.Q.; Wang, S.L.; Xu, H. Facilitated transport of lipophilic toxins through polysulfone membrane using albumin as a carrier. Mol. Simulat. 2004, 30, 117–120. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Ma, L.; Qin, H.; Cheng, C.; Xia, Y.; He, C.; Nie, C.X.; Wang, L.R.; Zhao, C.S. Mussel-inspired self-coating at macro-interface with improved biocompatibility and bioactivity via dopamine grafted heparin-like polymers and heparin. J. Mater. Chem. B 2014, 2, 363–375. [Google Scholar] [CrossRef]
- Khan, W.; Kapoor, M.; Kumar, N. Covalent attachment of proteins to functionalized polypyrrole-coated metallic surfaces for improved biocompatibility. Acta Biomater. 2007, 3, 541–549. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Li, S.; Zhao, W.F.; Wei, Q.; Nie, S.Q.; Sun, S.D.; Zhao, C.S. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 417, 228–236. [Google Scholar] [CrossRef]
- Lin, W.C.; Liu, T.Y.; Yang, M.C. Hemocompatibility of polyacrylonitrile dialysis membrane immobilized with chitosan and heparin conjugate. Biomaterials 2004, 25, 1947–1957. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.Q.; Li, J.; Su, B.H.; Sun, S.D.; Zhao, C.S. Heparin-like macromolecules for the modification of anticoagulant biomaterials. Macromol. Biosci. 2012, 12, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.Q.; Tang, M.; Cheng, C.; Yin, Z.H.; Wang, L.R.; Sun, S.D.; Zhao, C.S. Biologically inspired membrane design with a heparin-like interface: Prolong blood coagulation, inhibited complement activation, and bio-artificial liver related cell proliferation. Biomater. Sci. 2014, 2, 98–109. [Google Scholar] [CrossRef] [PubMed]

| Membrane | Contact Angle (°) |
|---|---|
| iPES | 89 ± 7 |
| iPES/PDA | 58 ± 3 |
| iPES/PDA/Lys | 38 ± 5 |
| Sample | Optical Density | Hemolysis% |
|---|---|---|
| Positive control | 0.151 | 100 |
| Negative control | 0.005 | 0 |
| iPES | 0.015 | 6.9 |
| iPES/PDA | 0.011 | 4.1 |
| iPES/PDA/Lys | 0.009 | 2.8 |
| Sample | With Inoculation of Cells Removal Rate | Without Inoculation of Cells Removal Rate |
|---|---|---|
| iPES | 14.2% | 6.9% |
| iPES/PDA/Lys | 41.7% | 23.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Liu, H.; Luo, H.; Liu, X.; Sun, Y.; Ge, D.; Shi, W. C3A Cells-Inoculated Affinity Membrane for Bilirubin Removal. Coatings 2023, 13, 50. https://doi.org/10.3390/coatings13010050
Shen Y, Liu H, Luo H, Liu X, Sun Y, Ge D, Shi W. C3A Cells-Inoculated Affinity Membrane for Bilirubin Removal. Coatings. 2023; 13(1):50. https://doi.org/10.3390/coatings13010050
Chicago/Turabian StyleShen, Yuqing, Huijuan Liu, Huiling Luo, Xinxin Liu, Yanan Sun, Dongtao Ge, and Wei Shi. 2023. "C3A Cells-Inoculated Affinity Membrane for Bilirubin Removal" Coatings 13, no. 1: 50. https://doi.org/10.3390/coatings13010050
APA StyleShen, Y., Liu, H., Luo, H., Liu, X., Sun, Y., Ge, D., & Shi, W. (2023). C3A Cells-Inoculated Affinity Membrane for Bilirubin Removal. Coatings, 13(1), 50. https://doi.org/10.3390/coatings13010050






